Abstract
Selenium (Se) is an essential trace element to maintain homeostasis in humans and animals. The aim of the present study was to clarify the mechanism of Se deficiency-induced inflammation in the pig’s brain. Twenty-four healthy pigs were randomly divided into two groups (n = 12/group): control group (group C) was fed diet with 0.3 mg/kg inorganic Se, and Se-deficient group (group L) was fed diet with 0.007 mg/kg inorganic Se. At the 90th day of the experiment, the histology in the pig’s brain was observed by the microscope, the NO levels and iNOS activity were assayed, and the mRNA and protein expression levels of inflammatory cytokines (iNOS, COX-2, NF-κB, and PTGEs) and HSPs (HSP27, HSP40, HSP60, HSP70, and HSP90) were detected by real-time quantitative PCR and Western blot. Compared with group C, both of NO levels and iNOS activity were increased in group L, and the mRNA and protein expression levels of inflammatory cytokines (iNOS, COX-2, NF-κB, and PTGEs) and HSPs (HSP27, HSP40, HSP60, HSP70, and HSP90) were also upregulated; histological observation displayed inflammatory response in the brain of pig. In summary, diet with Se deficiency can activate the iNOS/NF-κB pathway to upregulate the expression of inflammatory cytokines, thereby leading to inflammatory lesions in the pig’s brain, and HSPs are involved in the compensatory regulation of inflammation. This study provides a reference for the prevention of pig brain inflammation from the perspective of nutrition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential trace element to maintain homeostasis in humans and animals and has functions of regulating endocrine and immunity [1,2,3]. White muscle disease, mulberry heart, pancreatic atrophy, and exudative diathesis can be caused by Se deficiency in animals [4,5,6,7]. Brain tissue is one of the target organs for Se deficiency [8, 9], and previous studies had found that Se levels in the brain of Alzheimer’s patients were reduced [10, 11]. Nutritional brain softening could be induced by Se deficiency in chicken brain [12], and Se deficiency could increase the expression of inflammatory cytokines to induce inflammation [13, 14]. Inflammatory cytokines play a key role in most inflammation [15, 16]. For example, the expression of prostaglandin E synthase (PTGEs), cyclooxygenase-2 (COX-2), nuclear factor-kappa B (NF-κB), and inducible nitric oxide synthase (iNOS) could be increased by Se deficiency in the gastrointestinal tract of chickens, leading to inflammatory lesions of the digestive tract [17]. Interestingly, diet with Se deficiency could also increase the expression of inflammatory cytokines in peripheral blood lymphocyte in pigs and induce inflammation [18]. Additionally, previous study had shown that supplementation with Se significantly reduced the expression of NF-κB and COX-2 induced by bacterial endotoxin lipopolysaccharides [19]. It has also been reported that HSP60 and HSP90 had protective effects during erythrocyte injury induced by Se deficiency [20], and Se deficiency activated the expression of HSPs in the spleen and thymus [21, 22].
Nitric oxide (NO) is an autocrine and paracrine signaling pathway molecule that can diffuse freely in biofilms. In normal physiological state, the main physiological function of NO is to maintain blood vessel homeostasis [23, 24]. NO plays an important role in host defense, including regulation of inflammation [25]. NO is a molecular aggressor in inflammation, especially chronic inflammation [26]. NF-κB pathway is an important way to regulate the expression of inflammatory cytokines [27], and COX-2 and iNOS are two important target genes of NF-κB [28]. For example, small heterodimer partners inhibited the expression of COX-2 and iNOS by inhibiting the activity of NF-κB promoter in renal tubular injury [29], and iNOS expression and inflammation were inhibited via the NF-κB signaling pathway in acute renal ischemia reperfusion injury rats [30]. INOS could be activated via the NF-κB signal pathway following inflammatory stimulation such as meningitis in mouse choroid plexus cells [31], and inflammation in mucosa of rats could be ameliorated by NF-kB signaling pathway inhibition [32]. The activation of NF-κB pathway could be suppressed by heat shock treatment in heart inflammation of rats [33], and HSP70 could afford protection by the inhibition of NF-κB-mediated inflammation in ischemic acute renal failure of rats [34]. And HSP70 also could protect the brain from a variety of damage by the inhibition of iNOS and NO in the ischemia-reperfusion model [35].
The above studies suggested that Se deficiency could induce inflammation, which was closely related to iNOS/NF-κB signaling pathway, but the mechanism was still unclear. The aim of our experiment was to clarify the mechanism of Se deficiency-induced inflammation in the brain of pigs via the iNOS/NF-κB signaling pathway.
Materials and Methods
Establishment of Se Deficiency Animal Model and Grouping
All procedures used in this experiment were administered in accordance with the animal welfare guidelines and the Institutional Animal Care and Use Committee of Northeast Agricultural University. Twenty-four healthy 42-day-old pigs were randomly divided into two groups of 12 animals each. Group C was fed with 0.3 mg/kg inorganic Se, and group L was fed with 0.007 mg/kg inorganic Se. The diet was prepared according to NRC 2012 pig nutrition requirements, and the content of Se in control diet was 0.3 mg/kg by adding SeMet. The basic composition of the diet was shown in Table 1. At the 90th day of the experiment, the pigs were euthanized, brain tissue was extracted and partially fixated in 10% formalin, and left part was in liquid nitrogen for further use.
Histological Observation of Pig Brain Tissue
After being fixed in 10% neutral formalin solution for fixation for at least 24 h, the tissues were embedded in paraffin, and sectioned and stained with hematoxylin and eosin (HE). The section was dehydrated in ethanol, and dried and sealed with neutral resin; it could be for the light microscopic observation when dried.
Assay of NO Levels and iNOS Activity
NO levels and iNOS activity were assayed using NO and iNOS assay kits (Nanjing Institute of Bioengineering, Nanjing, China).
Real-Time Quantitative PCR Analysis
Total RNA was extracted from brain tissues by using Trizol reagent according to the manufacturer’s instructions, and the reverse transcription steps of cDNA were also based on the manufacturer’s instructions (Roche, Shanghai, China). The primers used in our experiment were shown in Table 2. qRT-PCR was performed using the Light Cycler® 480 System (Roche, Basel, Switzerland) and Fast Universal SYBR Green Master Mix (Roche, Basel, Switzerland). Only one peak for each PCR product was shown in the melting curve analysis. The genes’ relative abundance of mRNA was calculated by using the 2-ΔΔCT method, accounting for gene-specific efficiencies and was normalized to the mean expression of the above mentioned indexes.
Western Blot Analysis
After total protein being subjected to 12% SDS-polyacrylamide gel electrophoresis, separated proteins were transferred to nitrocellulose membranes in Tris-glycine buffer containing 20% methanol at 4 °C. Membranes were blocked with 5% skim milk at 37 °C for 2 h and then incubated overnight with diluted primary antibodies against rabbit at 4 °C, and the diluted concentration was shown in Table 3. And then, it was incubated with peroxidase-conjugated secondary antibodies against rabbit IgG (1:5000, Santa Cruz, USA) for 50 min at 37 °C. Finally, the signal was detected with X-ray films (TransGen Biotech Co., Beijing, China).
Statistical Analysis
The data were analyzed with t tests, using GraphPad Prism software (version 7.0, GraphPad Software Inc., San Diego, CA, USA). The data for each group are reported as mean ± standard (SD) deviation. The difference is considered statistically significant at P < 0.05.
Results
Detection Results of Histological Changes in Brain Tissue
As shown in Fig. 1, compared with group C, the number of nerve cells was decreased in group L, the cell morphology was irregular, the structure was incomplete, the nucleus was dissolved and fragmented, and the cytoplasm staining deepened; the number of glial cells was increased irregularly, and the nucleus condensed and darkened. Moreover, there were small vessel congestion and inflammatory cell infiltration in group L. It indicated that inflammation occurred in the brain tissue of pigs due to Se deficiency.
Effects of Se deficiency on histological in the brain of pigs. a 20× group C. The yellow arrow represents normal nerve cells, and the blue arrow represents glial cells in the stroma. (b) 40× group C. The yellow arrow represents normal nerve cells, and the blue arrow represents glial cells in the stroma. (c) 20× group L. Red arrows denote damage of nerve cells—neurotropic, blue arrows denote damage of glial cells, and yellow arrow denotes the small vessel congestion. (d) 40× group L. The red arrow denotes damage to nerve cells—neurotropic, the yellow arrow denotes infiltration of inflammatory cell, the blue arrow denotes damage of nerve cell, and the black arrow denotes damage of glial cells
Detection Results of NO Levels and iNOS Activity in Brain Tissue
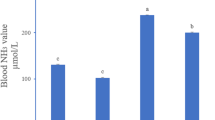
As shown in Fig. 2, compared with group C, iNOS activity and NO levels were increased by 20% and 97% in group L, with significant differences (P < 0.05). It indicated that Se deficiency decreased the ability of brain tissue to clear RNS, induced inflammatory lesions.
Detection Results of HSP Expression in Brain Tissue
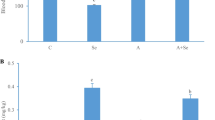
The mRNA and protein expression levels of HSPs were shown in Fig. 3. Compared with group C, the mRNA expression levels of HSP27, HSP40, HSP60, HSP70, and HSP90 were increased by 133%, 51%, 17%, 266%, and 147% in group L, and the protein expression levels of HSP70 and HSP90 were increased by 750% and 235%, with significant differences (P < 0.05). These results provided evidence that HSPs were involved in the compensatory regulation of inflammation induced by Se deficiency.
The mRNA and protein levels of HSP27, HSP40, HSP60, HSP70, and HSP90. a The mRNA levels of HSPs (HSP27, HSP40, HSP60, HSP70, and HSP90) in the brain of pigs. Each value represents the mean ± SD of 5 individuals. *Significant differences (P < 0.05) between the L and the C groups. b The protein levels of HSP70 and HSP90. Each value represents the mean ± SD of 5 individuals. *Significant differences (P < 0.05) between the L and the C groups
Detection Results of Inflammatory Cytokine Expression in Brain Tissue
The mRNA and protein expression levels of inflammatory cytokines were shown in Fig. 4. Compared with group C, the mRNA expression levels of PTGEs, COX-2, NF-κB, and iNOS were increased by 144%, 252%, 62%, and 280% in group L, and the protein expression levels of NF-κB and iNOS were increased by 500% and 22%, with significant differences (P < 0.05). These results indicated that Se deficiency upregulated the expression of inflammatory cytokines in pig brain tissue.
The mRNA and protein levels of PTGEs, COX-2, NF-κB, and iNOS. a The mRNA levels of inflammatory cytokines (PTGEs, COX-2, NF-κB, iNOS) in the brain of pigs. Each value represents the mean ± SD of 5 individuals. *Significant differences (P < 0.05) between the L and the C groups. b The protein levels of inflammatory cytokines iNOS and inflammatory cytokines NF-κB. Each value represents the mean ± SD of 5 individuals. *Significant differences (P < 0.05) between the L and the C groups
Discussion
The trace element Se plays an important biological role in the body. Many studies had shown that Se regulated inflammatory response and maintained normal physiological function in humans and animals [36, 37]. A large number of data provided evidence that inflammatory cytokines and HSPs had cross talk in Se deficiency-induced inflammation in the brain. For example, the inflammatory lesions of chicken brain tissue could be induced by Se deficiency [12]. The results of our experiment proved that diet with Se deficiency activated the iNOS/NF-κB signaling pathway and induced inflammatory lesions in pig brain; the results of histological observation also displayed it. At the same time, HSPs were involved in the compensatory regulation of inflammation.
Lots of NO can be produced by the activation of iNOS, which in turn induces inflammation. An increase of NO levels and iNOS activity was observed in the intestinal tract of inflammatory injury in Se deficiency chickens [38]. Moreover, the intrinsic relationship between NO levels and iNOS activity in the chicken inflammatory lesion duodenum was also reported [39]. In the present study, the iNOS activity and the NO levels in group L were significantly upregulated compared with group C. It was consistent with the above; histological observation also displayed that Se deficiency could cause inflammatory lesions in pig brain. The activation of iNOS could increase NF-κB expression. Activation of NF-κB was closely related to inflammation [40] and could increase the expressions of iNOS, COX-2, and PTGEs. This mechanism was observed in chicken respiratory inflammation and pneumonia response [41, 42]. Many studies had shown that blocking the NF-κB signaling pathway in renal tubular and rat brains could inhibit inflammation by reducing the expression of inflammatory cytokines (PTGEs, COX-2, and iNOS) and NO levels [29, 43,44,45]. In our study, the mRNA levels of PTGEs, COX-2, NF-κB, and iNOS and the protein expression levels of NF-κB and iNOS in group L were upregulated, and it was consistent with the above studies.
HSPs are a series of highly conserved proteins that protect cells from stimuli, improve tolerance, and attenuate the adverse effects of stimuli. Many studies showed that large amounts of HSPs were induced to protect the cells and maintain normal metabolic function when a tissue was damaged [46,47,48], such as inflammation. Induction of HSP40, HSP60, and HSP70 could inhibit inflammatory response by inhibiting lymphocyte damage during Se deficiency-induced inflammation in peripheral blood lymphocytes of pigs [18]. Recent studies had found that Se deficiency could increase the expression of inflammatory cytokines to lead inflammation and was accompanied by high expression of HSP27, HSP40, HSP60, HSP70, and HSP90 in mouse skeletal muscle and chicken erythrocytes [20, 49]. Tao et al. showed that COX-2 and iNOS were approximate promoters of inflammatory bowel cancer, HSP70 could exert tumor suppressive effects by limiting inflammatory cytokines [50], and Helicobacter pylori promoted the apoptosis of gastric glandular epithelial cells by activating COX-2 and inhibiting HSP70 [51]. Our results showed that the mRNA expression of HSP27, HSP40, HSP60, and HSP70 and the protein expression of HSP70 were increased in group L, which was consistent with the above studies. Furthermore, previous study had shown that HSP90 was involved in the transcription of iNOS; inhibition of HSP90 expression could inhibit iNOS expression and indicated that HSP90 could promote inflammatory response [52]; the mRNA and protein expression levels of HSP90 were both increased in our experiment, and it was consistent with the above.
In summary, our present experiments provided evidence that Se deficiency can activate the iNOS/NF-κB pathway to upregulate the expression of inflammatory cytokines, thereby leading inflammatory lesions in the pig’s brain, and HSPs are involved in the compensatory regulation of inflammation.
References
Yang J, Zhang Y, Hamid S, Cai J et al (2017) Interplay between autophagy and apoptosis in selenium deficient cardiomyocytes in chicken. J Inorg Biochem 170:17–25
Chi Q, Luan Y, Zhang Y, Hu X et al (2019) The regulatory effects of miR-138-5p on selenium deficiency-induced chondrocyte apoptosis are mediated by targeting SelM. Metallomics 11(4):845–857
Sun Z, Xu Z, Wang D, Yao H et al (2018) Selenium deficiency inhibits differentiation and immune function and imbalances the Th1/Th2 of dendritic cells. Metallomics 10(5):759–767
Creech BG, Rahman MM, Reid BL, Couch JR (1958) Exudative diathesis in chicks. J Nutr 64(1):55–65
Yao H, Wu Q, Zhang Z, Zhang J et al (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of se-deficient chicks. J Nutr 143(5):613–619
Matsuda A, Kimura M, Itokawa Y (1998) Influence of selenium deficiency on vital functions in rats. Biol Trace Elem Res 61(3):287–301
Gudmundson J (1976) The clinicopathological findings of mulberry heart disease in a piglet. Can Vet J La Revue Vétérinaire Canadienne 17(2):45
Huang J, Ren F, Jiang Y, Lei X (2016) Characterization of selenoprotein M and its response to selenium deficiency in chicken brain. Biol Trace Elem Res 170(2):449–458
Sharma SK, Bansal MP, Sandhir R (2019) Altered dietary selenium influences brain iron content and behavioural outcomes. Behav Brain Res 372:112011
Pillai R, Uyehara-Lock JH, Bellinger FP (2014) Selenium and selenoprotein function in brain disorders. IUBMB Life 66(4):229–239
Varikasuvu SR, Prasad VS, Kothapalli J, Manne M (2019) Brain selenium in Alzheimer’s disease (BRAIN SEAD study): a systematic review and meta-analysis. Biol Trace Elem Res 189(2):361–369
Sheng P, Jiang Y, Zhang Z, Zhang J et al (2014) The effect of Se-deficient diet on gene expression of inflammatory cytokines in chicken brain. Biometals 27(1):33–43
Luan Y, Zhao J, Yao H, Zhao X et al (2015) Selenium deficiency influences the mRNA expression of selenoproteins and cytokines in chicken erythrocytes. Biol Trace Elem Res 171(2):427–436
Hu X, Chi Q, Liu Q, Wang D et al (2019) Atmospheric H2S triggers immune damage by activating the TLR-7/MyD88/NF-κB pathway and NLRP3 inflammasome in broiler thymus. Chemosphere 237:124427
Sun X, Li J, Zhao H, Wang Y et al (2018) Synergistic effect of copper and arsenic upon oxidative stress, inflammation and autophagy alterations in brain tissues of Gallus gallus. J Inorg Biochem 178:54
Jing H, Gao X, Xu L, Lin H et al (2019) H2S promotes a glycometabolism disorder by disturbing the Th1/Th2 balance during LPS-induced inflammation in the skeletal muscles of chickens. Chemosphere 222:124–131
Gao X, Zhang Z, Xing H, Yu J et al (2016) Selenium deficiency-induced inflammation and increased expression of regulating inflammatory cytokines in the chicken gastrointestinal tract. Biol Trace Elem Res 173(1):210–218
Liu T, Yang T, Pan T, Liu C et al (2017) Effect of low-selenium/high-fat diet on pig peripheral blood lymphocytes: perspectives from selenoproteins, heat shock proteins, and cytokines. Biol Trace Elem Res 183(3):1–12
Zamamiri-Davis F, Lu Y, Thompson JT, Prabhu KS et al (2002) Nuclear factor-κB mediates over-expression of cyclooxygenase-2 during activation of RAW 264.7 macrophages in selenium deficiency. Free Radic Biol Med 32(9):890–897
Zhao J, Xing H, Liu C, Zhang Z et al (2016) Effect of selenium deficiency on nitric oxide and heat shock proteins in chicken erythrocytes. Biol Trace Elem Res 171(1):208–213
Khoso PA, Liu C, Liu C, Khoso MH et al (2016) Selenium deficiency activates heat shock protein expression in chicken spleen and thymus. Biol Trace Elem Res 173(2):1–9
Chen X, Zhu Y, Cheng X, Zhang Z et al (2012) The protection of selenium against cadmium-induced cytotoxicity via the heat shock protein pathway in chicken splenic lymphocytes. Molecules 17(12):14565–14572
Hoffman RA, Mahidhara RS, Wolf-Johnston AS, Lu L et al (2002) Differential modulation of CD4 and CD8 T-cell proliferation by induction of nitric oxide synthesis in antigen presenting cells. Transplantation 74(6):836–845
Kim IY, Stadtman TC (1997) Inhibition of NF-kappaB DNA binding and nitric oxide induction in human T cells and lung adenocarcinoma cells by selenite treatment. Proc Natl Acad Sci U S A 94(24):12904–12907
Christopherson KS, Bredt DS (1997) Nitric oxide in excitable tissues: physiological roles and disease. J Clin Investig 100(10):2424–2429
Miller MJ, Grisham MB (1995) Nitric oxide as a mediator of inflammation?-You had better believe it. Mediat Inflamm 4(6):387–396
Abate A, Oberle S, Schröder H (1998) Lipopolysaccharide-induced expression of cyclooxygenase-2 in mouse macrophages is inhibited by chloromethylketones and a direct inhibitor of NF- κB translocation. Prostag Oth Lipid M 56(5-6):277
Lee YW, Han S, Lee M, Yang K et al (2000) 2-Amino-3-methylimidazo[4,5-f]quinoline inhibits nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 cells by blocking p38 kinase activation. Cancer Lett 156(2):133–139
Park JS, Choi HI, Bae EH, Ma SK et al (2017) Small heterodimer partner attenuates hydrogen peroxide-induced expression of cyclooxygenase-2 and inducible nitric oxide synthase by suppression of activator protein-1 and nuclear factor-κB in renal proximal tubule epithelial cells. Int J Mol Med 39(3):701–710
Gao D, Jing S, Zhang Q, Wu G (2018) Pterostilbene protects against acute renal ischemia reperfusion injury and inhibits oxidative stress, inducible nitric oxide synthase expression and inflammation in rats via the Toll-like receptor 4/nuclear factor-κB signaling pathway. Exp Ther Med 15(1):1029–1035
Takano M, Ohkusa M, Otani M, Min KS et al (2015) Lipid A-activated inducible nitric oxide synthase expression via nuclear factor-κB in mouse choroid plexus cells. Immunol Lett 167(2):57–62
Raish M, Ahmad A, Ansari MA, Alkharfy KM et al (2018) Momordica charantia polysaccharides ameliorate oxidative stress, inflammation, and apoptosis in ethanol-induced gastritis in mucosa through NF-kB signaling pathway inhibition. Int J Biol Macromol 111:193
Chen Y, Arrigo AP, Currie RW (2004) Heat shock treatment suppresses angiotensin II-induced activation of NF-kappaB pathway and heart inflammation: a role for IKK depletion by heat shock? Am J Physiol Heart Circ Physiol 287(3):1104–1114
Jo S-K, Ko GJ, Boo CS, Cho WY et al (2006) Heat preconditioning attenuates renal injury in ischemic ARF in rats: role of heat-shock protein 70 on NF-kappaB-mediated inflammation and on tubular cell injury. J Am Soc Nephrol 17(11):3082
Zheng Z, Kim JY, Ma H, Lee JE et al (2008) Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab 28(1):53–63
Yao H, Wu Q, Zhang Z, Li S et al (2013) Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim Biophys Acta 1830(4):3112–3120
Yang T, Liu T, Cao C, Xu S (2019) miR-200a-5p augments cardiomyocyte hypertrophy induced by glucose metabolism disorder via the regulation of selenoproteins. J Cell Physiol 234(4):4095–4103
Yu J, Yao H, Gao X, Zhang Z et al (2015) The role of nitric oxide and oxidative stress in intestinal damage induced by selenium deficiency in chickens. Biol Trace Elem Res 163(1-2):144–153
Jin X, Jia T, Liu R, Xu S (2018) The antagonistic effect of selenium on cadmium-induced apoptosis via PPAR-γ/PI3K/Akt pathway in chicken pancreas. J Hazard Mater 357:355–362
Zheng S, Jin X, Chen M, Shi Q et al (2019) Hydrogen sulfide exposure induces jejunum injury via CYP450s/ROS pathway in broilers. Chemosphere 214:25–34
Shi Q, Wang W, Chen M, Zhang H et al (2019) Ammonia induces Treg/Th1 imbalance with triggered NF-κB pathway leading to chicken respiratory inflammation response. Sci Total Environ 659:354–362
Wang W, Chen M, Jin X, Li X et al (2018) H 2 S induces Th1/Th2 imbalance with triggered NF-κB pathway to exacerbate LPS-induce chicken pneumonia response. Chemosphere 208:241–246
Jo MJ, Lee JR, Cho IJ, Kim YW et al (2013) Roots of Erigeron annuus attenuate acute inflammation as mediated with the inhibition of NF- κ B-associated nitric oxide and prostaglandin E2 production. Evid-Based Compl Alt Med 2013(1):297427
Hwangbo M, Jung JY, Ki SH, Park SM et al (2014) U-Bang-Haequi Tang: A herbal prescription that prevents acute inflammation through inhibition of NF-κB-mediated inducible nitric oxide synthase. Evid-Based Compl Alt Med 2014:542825
Hwang JW, Jeon YT, Lim YJ, Park HP (2017) Sevoflurane postconditioning-induced anti-inflammation via inhibition of the toll-like receptor-4/nuclear factor kappa B pathway contributes to neuroprotection against transient global cerebral ischemia in rats. Int J Mol Sci 18(11):2347
Zhao P, Guo Y, Zhang W, Chai H et al (2017) Neurotoxicity induced by arsenic in Gallus gallus: regulation of oxidative stress and heat shock protein response. Chemosphere 166:238–245
Chen M, Li X, Shi Q, Zhang Z et al (2019) Hydrogen sulfide exposure triggers chicken trachea inflammatory injury through oxidative stress-mediated FOS/IL8 signaling. J Hazard Mater 368:243–254
Su Y, Wei H, Bi Y, Wang Y et al (2019) Pre-cold acclimation improves the immune function of trachea and resistance to cold stress in broilers. J Cell Physiol 234(5):7198–7212
Liu Z, Yao X, Du J, Song B et al (2017) Selenium deficiency augments the levels of inflammatory factors and heat shock proteins via the redox regulatory pathway in the skeletal muscles of mice. Biol Trace Elem Res 182(13):1–8
Tao Y, Hart J, Lichtenstein L, Joseph LJ et al (2009) Inducible heat shock protein 70 prevents multifocal flat dysplastic lesions and invasive tumors in an inflammatory model of colon cancer. Carcinogenesis 30(1):175–182
Targosz A, Brzozowski T, Pierzchalski P, Szczyrk U et al (2012) Helicobacter pylori promotes apoptosis, activates cyclooxygenase (COX)-2 and inhibits heat shock protein HSP70 in gastric cancer epithelial cells. Inflamm Res 61(9):955–966
Luo S, Wang T, Qin H, Lei H et al (2011) Obligatory role of heat shock protein 90 in iNOS induction. Am J Physiol Cell Physiol 301(1):C227
Acknowledgments
The study was supported by the Earmarked Fund for China Agriculture Research System (no. CARS 35).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Cui, J., Lu, Y. et al. Selenium Deficiency Induces Inflammation via the iNOS/NF-κB Pathway in the Brain of Pigs. Biol Trace Elem Res 196, 103–109 (2020). https://doi.org/10.1007/s12011-019-01908-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01908-y