Abstract
There are concerns about the spread of heavy metals in the environment, and human activities are one of the most important factors in their spread. These agents have the high half-life resulting in their persistence in the environment. So, prevention of their spread is the first step. However, heavy metals are an inevitable part of modern and industrial life and they are applied in different fields. Cadmium is one of the heavy metals which has high carcinogenesis ability. Industrial waste, vehicle emissions, paints, and fertilizers are ways of exposing human to cadmium. This potentially toxic agent harmfully affects the various organs and systems of body such as the liver, kidney, brain, and cardiovascular system. Oxidative stress is one of the most important pathways of cadmium toxicity. So, improving the antioxidant defense system can be considered as a potential target. On the other hand, the Nrf2 signaling pathway involves improving the antioxidant capacity by promoting the activity of antioxidant enzymes such as catalase and superoxide dismutase. At the present review, we demonstrate how Nrf2 signaling pathway can be modulated to diminish the cadmium toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human activities are the key factors in spreading heavy metals in the environment. Arsenic, mercury, lead, chromium, and cadmium are potentially toxic heavy metals that negatively affect human body [35, 104, 126, 156]. It has been shown that heavy metals have a half-life more than the longevity of a person and when individuals are exposed to heavy metals, they are considered as permanent carriers [97]. Besides, these potentially harmful agents accumulate at the high level in the body, resulting in damages in various organs and systems [98]. To date, a number of rules have been set by government for controlling the spread of heavy metals. However, in some of the developing countries such as Iran, lead is added to gasoline to enhance the number of octanes and also to increase its power in inflaming [17]. Heavy metals are an inevitable part of modern life and human is exposed to these harmful agents daily [2]. Cadmium, a potentially toxic heavy metal, is extensively found in our surrounding environment due to the human activities including mining and agriculture works [3]. According to the evidence of Agency for Toxic Substance and Disease Registry (ATDSR) ranking, cadmium is at the place of seventh among the most hazardous heavy metals [49]. Industrial wastes, vehicle emissions, smoking, paints, fertilizers, and contaminated food are other ways of exposing human to cadmium. Besides, some plants such as tobacco are able to accumulate cadmium. Unfortunately, cadmium is colorless and tasteless leading to its high prevalence in the environment without being detectable. Furthermore, humans are exposed to cadmium by using vegetables. For instance, it has been demonstrated that some of the plants such as lettuce and peanuts have this ability to bioaccumulate cadmium [71]. In accordance to the data of the European Food Standards Authority, the weekly intake of cadmium as much as 2.5 μg/kg is tolerable [91]. Regardless of sources of cadmium and also its spread in the environment, this heavy metal harmfully affects a variety of organs and systems. The liver is one of the major organs in the body accounting for detoxification that is primarily affected by cadmium [46]. It appears that the affinity of heavy metals, particularly cadmium into the liver is due to the existence of metallothionein (MT) in liver [72]. Also, cadmium negatively affects the kidney, brain, and heart. Chronic kidney disease, decreased gain weight, steatohepatitis, and ischemia are a few of adverse effects of cadmium [63, 108].
A variety of studies have evaluated the harmful effects of cadmium on the various organs and systems of the body. One of the most common ways used by cadmium to exert neurotoxicity is stimulation of apoptotic cell death. Mitochondria are the powerhouse of cells. However, these important intracellular organelles are involved in intrinsic pathway of apoptosis [78]. It seems that cadmium induces apoptosis in the brain by disrupting mitochondrial membrane, releasing cytochrome C into cytosol, and stimulating of apoptosis cascade [85]. Endoplasmic reticulum (ER) is another intracellular organelle that triggers both autophagy and apoptosis to reduce stress. A high level of stress is associated with ER-mediated apoptosis [18]. Exposure to cadmium significantly enhances the level of oxidative stress resulting in ER-mediated apoptosis in brain [85]. Besides, cadmium induces neurotoxicity by reducing the activity of antioxidant enzymes leading to the sensitization of brain cells to oxidative damage [88]. Reproductive system is also one of the targets of cadmium. It appears that cadmium induces damages in testis via two main strategies: (A) enhancing the level of oxidative stress by upregulation of genes such as inducible nitric oxide synthase (iNOS) and (B) elevating the intensity of inflammation [33]. It is held that exposing to the cadmium not only affects the reproductive system of adults but also is associated with a number of adverse effects in their offspring such as neurotoxicity [152]. The same story occurs in the kidney and liver exposed to the cadmium. It seems that enhanced level of oxidative stress is responsible for stimulation of harmful effects of cadmium on both kidney and liver [28, 55, 56]. However, carcinogenesis activity has attracted much attention during past decades. Although novel anti-tumor drugs and updated technologies are extensively applied in treatment of cancer, this life-threatening condition is still one of the leading causes of death worldwide in spite of significant decrease in its incidence rate [19, 24, 82, 123]. Notably, exposure to the cadmium not only enhances the risk of cancer development, but also increases the proliferation and malignancy of tumor cells [50]. It has been reported that cadmium is capable of targeting a number of signaling pathways such as ERK to trigger proliferation and invasion of tumor cells [148].
The high spread of cadmium and its potential toxic impacts on the various organs and systems of body have led to the attention of scientists into this field. A number of studies have been directed to reduce the toxicity of cadmium after accumulation in the body. It is worth mentioning that reducing the level of oxidative stress using antioxidant agents is the most common strategy [1, 70]. According to the minimal toxicity and valuable pharmacological effects of plant-derived natural products [5, 15, 16, 20, 21, 117, 147], these compounds have been extensively applied in the amelioration of the harmful effects of cadmium with satisfactory results [7, 81, 111]. In the present study, we demonstrate that naturally occurring compounds applied for alleviation of cadmium toxicity target Nrf2 signaling pathway.
Nrf2 Signaling Pathway Regulation
The nuclear factor erythroid 2-related factor 2 (Nrf2) is a key member of Cap “n” Collar (CNC) subfamily of basic leucine zipper-type transcription factors which plays a remarkable role in preserving homeostasis [43]. In fact, Nrf2 signaling pathway is a defense system against oxidative stress damage, apoptosis, and inflammation and so on [109]. Nrf2 signaling pathway is mainly regulated by kelch-like ECH-associated protein 1 (keap1). During physiological conditions, there is no need for over-activation of antioxidant enzymes and Nrf2 signaling pathway is at the dormant form. Keap1 as a negative regulator of Nrf2 pathway, binds to the Nrf2, resulting in its proteosomal degradation. However, upon stress conditions, kead1 dissects from Nrf2, leading to the high accumulation of Nrf2 in the cytoplasm. Then, accumulated Nrf2 translocates to the nucleus and induces the activation of a number of genes containing antioxidant response element (ARE) region [124]. Heme-oxygenase 1 (HO-1), NADPH quinone oxidoreductase 1 (NQO1), catalase (CAT), and superoxide dismutase (SOD) are downstream mediators of Nrf2 signaling pathway that improve and reinforce antioxidant defense system [4].
Nrf2 Pathway in Pathological Conditions
The increased generation of reactive oxygen species (ROS) leads to the development of a condition known as oxidative stress [102, 143]. It has been demonstrated that oxidative stress plays a significant role in pathophysiology of disorders such as acute kidney injury, atherosclerosis, heart failure, cancer, diabetes, aging, Alzheimer’s disease (AD), and Parkinson’s disease (PD). The most important pathway which oxidative stress uses is negatively affecting the genetic material, lipids, and proteins, leading to the development of pathological conditions. Antioxidant defense system plays a pivotal role in neutralizing oxidative damage. However, when the load of oxidative stress exceeds from the capacity of this defense, complementary signaling pathways are stimulated to compensate and enhance the capability of antioxidant defense system. Nrf2 signaling pathway is one of these pathways which increases the ability of antioxidant defense system in combating with oxidative damage. This has resulted in modulation of Nrf2 signaling pathway in management of pathological conditions. It seems that oxidative stress is one of the key factors in the induction of AD, and reduction of ROS concentrations and inhibition of mitochondrial membrane potential loss are two important targets in AD. It has been suggested that using compounds with stimulatory impact on Nrf2 signaling pathway can diminish oxidative stress-mediated injury, resulting in alleviation of AD [105]. Notably, a similar story occurs in PD [58]. One of the most important mechanisms in the pathophysiology of PD is the neuron cell death mediated by mitochondrial dysfunction and subsequently, enhanced concentration of oxidative stress. It has been reported that targeting Nrf2/ARE signaling pathway can be considered as a potential candidate in PD therapy. However, the modulation of Nrf2 signaling pathway is a little different in cancer treatment. In order to diminish the viability and migration of tumor cells, the oxidative damage is induced in these malignant cells. It has been suggested that inhibition of Nrf2 signaling pathway is associated with decreased viability and invasion of breast cancer cells [153].
A growing body of evidence suggests that the Nrf2 signaling pathway plays a remarkable role in regulation of apoptotic cell death. Nuclear translocation of Nrf2 signaling pathway and enhanced transcriptional activity of ARE are associated with a decrease in the number of cells undergoing apoptosis [67]. However, it is held that protein kinase R-like ER kinase (PERK)/Nrf2 signaling pathway induces damages in cardiomyocytes by upregulation of ER stress and apoptosis [122]. These conflicting studies highlight dual role of Nrf2 pathway during apoptosis. More importantly, regulation of Nrf2 signaling pathway is of importance in attenuation of inflammation. For instance, formononetin as a naturally occurring compound reduces the intensity of inflammation in rats exposed to methotrexate by enhancing the expression of Nrf2 pathway leading to decreased concentrations of pro-inflammatory cytokines [8]. Furthermore, with respect to the potential role of Nrf2 signaling pathway in alleviation of inflammation, inhibition of Nrf2 predisposes cells into fibrosis [79].
Regulation of Nrf2 Signaling Pathway by Natural Antioxidants
At the previous sections, we described the several phases of the Nrf2 signaling pathway and its potential role in disease treatment. In order to direct further studies into this field, providing a brief discussion about the modulatory impact of naturally occurring antioxidants on Nrf2 signaling pathway is of importance. The impact of these plant-derived chemicals on the Nrf2 signaling pathway is limited to their modulatory impact on the upstream mediators of Nrf2 pathway and also their effect on the expression of Nrf2 and its nuclear translocation [4, 25]. For instance, microRNAs (miRs) and long non-coding RNAs (lncRNAs) function as the upstream modulators of Nrf2 pathway [139, 154]. Naturally occurring antioxidants are capable of affecting these upstream mediators to exert their therapeutic activities. Besides, these compounds are able to inhibit keap1 in stimulation of Nrf2 signaling pathway [59]. Upregulation/downregulation of nuclear translocation of Nrf2 and affecting the mRNA expression of Nrf2 are other strategies applied by naturally occurring antioxidants in regulation of Nrf2 signaling pathway [47, 66, 77].
Combating with Cadmium Toxicity Through Nrf2 Signaling Pathway
Nephrotoxicity
Notably, cadmium is considered as a potential disruptor of endocrine system [64]. The toxic impact of cadmium is mainly dependent on enhancing the level of oxidative stress [89]. Targeting Nrf2 signaling pathway and promoting the antioxidant balance are of importance in reducing the cadmium-mediated nephrotoxicity [56]. Pyracantha fortuneana is widely found in China and well-known due to its great antioxidant activity [120, 127, 145, 146]. Supplementation of Pyracantha fortuneana is suggested to be beneficial in attenuation of nephrotoxicity mediated by cadmium exposure. This renoprotective effect is induced by suppressing keap1 and subsequently, stimulation of Nrf2 pathway and downstream mediators such as HO-1 and NQO1 leading to the enhanced cell viability (upregulation of Bcl-2 and downregulation of Bax) and reduced inflammation (decreasing the concentration of tumor necrosis factor-α (TNF-α)) [68].
Royal jelly is secreted by the hypopharyngeal and mandibular glands of honey bees and contains a variety of proteins, monosaccharides, lipids, and fatty acids [38, 90, 121]. This compound has a number of therapeutic and biological activities such as antioxidant, anti-inflammatory, cardioprotective, and anti-tumor [14, 149]. Overall, exposure to cadmium enhances apoptotic cell death (Bcl-2 downregulation), elevates the levels of cytokines such as TNF-α and IL-1β, increases the expression of kidney injury molecular-1 (KIM-1), and reduces antioxidant defense system. Administration of royal jelly considerably alleviates these nephrotoxic impacts of cadmium via upregulation of the Nrf2/ARE signaling pathway and consequently, improving antioxidant defense system by enhancing the expression of HO-1 and NQO-1 [12].
The focus on using naturally occurring compounds is mainly due to their minimal side effects [6, 20, 26, 83]. Puerarin (PU) is an isoflavone glycoside derived from Pueraria lobata. PU has demonstrated great potential in decreasing cadmium toxicity [118]. Besides, PU is capable of targeting Nrf2 signaling pathway for inducing its therapeutic activities [36, 53]. Interestingly, PU follows a novel strategy in attenuation of cadmium-mediated oxidative damage. Administration of PU not only reduces the nuclear translocation of Nrf2, but also enhances the activity of keap1 to suppress Nrf2 signaling pathway leading to the protection of proximal tubular cells against cadmium toxicity [135]. Trehalose (Tr) enhances the expression of keap1 to inhibit Nrf2 signaling pathway leading to the reduced level of oxidative damage in proximal tubular cells [133].
Reproductive Toxicity
Sulforaphane is a naturally occurring compound isolated from cabbages, olives, and broccoli [29, 95, 103]. Sulforaphane is well-known due to its great anti-tumor activity against various cancer cell lines [116, 144]. Notably, this compound has demonstrated great antioxidant activity [42, 62] making it an appropriate option for reducing the adverse effects of cadmium. Exposing to cadmium significantly diminishes the antioxidant activity of testis, reduces the concentration of testosterone, and is associated with an enhanced level of MDA and lipid peroxidation. Importantly, sulforaphane treatment remarkably decreases the adverse impacts of cadmium on the leydig cells (in vitro) by stimulation of Nrf2/HO-1 signaling pathway [142].
Piceatannol (PT) is a hydroxylated analogue of resveratrol that is present in various plants and fruits such as grape, apple, and tea [128, 129]. Accumulating data demonstrates that PT is more efficient than resveratrol [94, 110]. PT is capable of targeting the Nrf2 signaling pathway in exerting its protective impacts [76, 130]. Administration of PT significantly enhances steroidogenesis and improves sperm parameters such as sperm motility, sperm count, and sperm viability by inhibition of keap1 and subsequently, activation of Nrf2 signaling pathway [112].
Hepatotoxicity
Although zinc (Zn) pollution is considered as an environmental problem, its interaction with cadmium is of interest in the field of toxicology [41]. It has been demonstrated that Zn considerably diminishes the cadmium-mediated toxicity by improving antioxidant capacity, reducing cadmium uptake, and stimulating immune system [31, 44, 45, 48, 51, 74]. A newly published article reveals that Zn activates the Nrf2 signaling pathway and subsequent targets to suppress inflammatory responses and enhance antioxidant defense system [136].
Trehalose (Tr) is a disaccharide exclusively found in yeast, fungi, and bacteria. Tr is capable of induction of autophagy through mammalian target of rapamycin (mTOR) showing the capability of this agent in affecting molecular signaling pathways [75]. Different studies have revealed the antioxidant capability of Tr. In case of reducing the harmful impacts of cadmium, Tr enhances the nuclear translocation of Nrf2 leading to the promoted activity of antioxidant enzymes of the liver and reduced number of apoptosis [57].
Royal jelly has a number of macromolecules such as glucose, lipid, protein, and minerals [40, 86]. It seems that these ingredients result in the great pharmacological impacts of royal jelly [119]. By enhancing the expression of Nrf2 signaling pathway, royal jelly inhibits oxidative and inflammatory reactions in liver exposed to the cadmium [9]. Noteworthy, exposure to cadmium enhances the generation of ROS. Enhanced level of ROS is associated with stimulation of apoptosis and autophagy [22, 87, 100, 137]. Selenium inhibits cadmium-mediated autophagy and apoptosis by upregulation of Nrf2 and consequently, reducing ROS production [150].
Neurotoxicity
Long half-life, high cytotoxicity, and capability of generation of pathological conditions have resulted in much attention to decreasing the cytotoxic impacts of cadmium [65]. Enhanced levels of oxidative stress, induction of DNA damage, stimulation of mitochondrial dysfunction, and changing molecular pathways are the results of exposing to cadmium [27, 80, 93]. Neurotoxicity is one of the common complications of cadmium exposure [131]. Naturally occurring compounds have demonstrated a promising profile in treatment of disorders [23]. Carvacrol (CVC) is a monoterpenoid phenol exclusively found in the species of Labiateae family including thyme and oregano [52]. This compound has a number of pharmacological impacts such as antioxidant, anti-inflammatory, anti-tumor, and anti-diabetic [61, 84, 125, 138]. Administration of CVC is associated with improvements in PC12 cell viability and glutathione level, and inhibition of DNA fragmentation and apoptosis by enhancing the expression of Nrf2 signaling pathway [30].
Exposure to cadmium incredibly diminishes the levels of detoxifying antioxidant enzymes such as CAT and SOD. By application of royal jelly, an increase occurs in the Nrf2 signaling pathway resulting in protection of cortical neurons [13].
Enhanced level of oxidative stress plays a remarkable role in generation of neurological disorders (NDs) such as AD and PD [39, 115]. So, with respect to the effect of cadmium on elevating the level of oxidative stress, exposure to this potentially toxic heavy metal can enhance the risk of developing NDs [37, 73]. α-Lipoic acid belongs to the organosulfur compounds exclusively found in plants and animals [92]. The efficacy of α-lipoic acid in penetration into blood-brain barrier (BBB) has made it suitable option for treatment of NDs [114]. Importantly, α-lipoic acid is able to reduce the neurotoxic activity of cadmium through its antioxidant, free radical scavenging, and chelating impacts. Investigation of molecular signaling pathways has exhibited that α-lipoic acid triggers Nrf2 signaling pathway by downregulation of keap1 resulting in improved antioxidant defense system [106].
Carcinogenesis
Notably, cadmium is able to predispose into cancer [34]. Exposure to cadmium is associated with the generation of cancer such as lung cancer [69]. There is controversial information about the molecular pathways involved in the carcinogenesis impact of cadmium. It is held that ROS is responsible for cancer development [132]. So, reducing the concentrations of ROS is of importance in inhibition of carcinogenesis effect of cadmium. Sulforaphane administration is related to the upregulation of Nrf2 signaling pathway leading to the decreased level of ROS and suppressing cadmium-carcinogenesis [134].
Cardiotoxicity
By stimulation of oxidative stress, cadmium predisposes to cardiovascular disorders such as hypertension, atherosclerosis, stroke, and myocardial infarction [107]. Carvedilol (CAR) is an efficient blocker of β-adrenoceptor that is clinically applied for treating cardiovascular disorders. Besides, CAR is able to diminish doxorubicin-mediated cardiotoxicity [155]. Administration of CAR is advantageous in suppressing cadmium-induced cardiotoxicity. It seems that 4-week treatment with CAR (1 and 10 mg/kg/day) is associated with a decrease in malondialdehyde (MDA), TNF-α, and caspase-3. These cardioprotective impacts are mediated by enhancing the expression of Nrf2 and subsequent targeting of HO-1 [101].
Spleenotoxicity
Although much emphasis was put on the potential role of Nrf2 signaling pathway activation in reducing the harmful effects of cadmium on organs and systems of the body, a study conducted by Qu and colleagues provides controversial results about the role of Nrf2 signaling pathway. This interesting experiment showed information on the interaction of the Nrf2 signaling pathway with apoptosis and autophagy. It seems that administration of trehalose remarkably reduces the nuclear translocation of Nrf2 to inhibit autophagy and apoptosis induced by cadmium [96]. More studies are needed to approve the findings of this study.
Conclusion: Current Challenges and Future Prospects
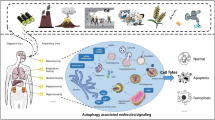
Nrf2 signaling pathway is considered as an important pathway in maintaining antioxidant balance. It has been suggested that any impairment in this signaling pathway is associated with pathological development. On the other hand, cadmium is one of the hazardous heavy metals which harmfully affects different organs and systems such as the liver, kidney, brain, and cardiovascular system. Stimulation of oxidative stress is one of the methods that cadmium uses to exert its adverse effects. So, targeting Nrf2 signaling pathway and, subsequently, improving antioxidant balance can be considered as a potential candidate in combating with cadmium toxicity. At the present review, we describe how the Nrf2 signaling pathway can be modulated to decrease cadmium toxicity. It was found that increased level of oxidative stress and inflammation can lead to malignant cell transformation. Using naturally occurring compounds such as sulforaphane can inhibit this malignant cell transformation by inhibition of oxidative stress via Nrf2 pathway upregulation. Inhibition of cell death, DNA damage, and ferroptosis are other results of Nrf2 pathway upregulation. Table 1 and Fig. 1 demonstrate the potential role of Nrf2 signaling pathway in overcoming to cadmium toxicity. However, Nrf2 signaling pathway is a novel target, and more studies are required to elucidate the role of this pathway in combating with cadmium toxicity. It was revealed that naturally occurring compound is able to target keap1, nuclear translocation of Nrf2, mRNA expression of Nrf2, and upstream modulators of Nrf2 to suppress the cytotoxic impacts of cadmium. Taking everything into account, it seems that regulation of Nrf2 signaling pathway is a promising strategy in combating cadmium toxicity. However, some changes can enhance the efficacy of naturally occurring antioxidants in regulation of Nrf2 signaling pathway and reducing cadmium toxicity. One of the most challenging difficulties faced in treatment of disorders using plant-derived chemicals is the low bioavailability of this valuable agent that considerably restricts their therapeutic activities. Notably, this problem is higher in the treatment of neurotoxicity caused by cadmium exposure compared to the other toxicities that is due to the BBB that prevents the entering of agents into the brain. Importantly, nanoparticles (NPs) have demonstrated great potential in crossing over BBB and enhancing the bioavailability of naturally occurring antioxidants. There is no study related to naturally occurring antioxidant-loaded NPs for reducing the toxicity of cadmium. These nanocarriers can be considered in future studies.
Combating with cadmium toxicity through Nrf2 signaling pathway and related molecular pathways. Nrf2, nuclear factor erythroid 2-related factor 2; NO, nitric oxide; KIM-1, kidney injury molecule-1; TNF-α, tumor necrosis factor-α; IL, interleukin; ALK, activin receptor-like kinase; PI3K, phosphatidylinositide 3-kinase; Akt, protein kinase-B; ROS, reactive oxygen species; NF-kB, nuclear factor-kB; COX-2, cyclooxygenase-2, GSP, grape seed proanthocyanidin
Abbreviations
- ATDSR:
-
Agency for Toxic Substance and Disease Registry
- MT:
-
Metallothionein
- ER:
-
Endoplasmic reticulum
- iNOS:
-
Inducible nitric oxide synthase
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- CNC:
-
Cap “n” Collar
- keap1:
-
Kelch-like ECH-associated protein 1
- ARE:
-
Antioxidant response element
- HO-1:
-
Heme oxygenase-1
- NQO1:
-
NADPH quinone oxidoreductase 1
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- ROS:
-
Reactive oxygen species
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- PERK:
-
Protein kinase R-like ER kinase
- miR:
-
MicroRNA
- lncRNA:
-
Long non-coding RNA
- TNF-α:
-
Tumor necrosis factor-α
- KIM:
-
Kidney injury molecule-1
- PU:
-
Puerarin
- PT:
-
Piceatannol
- Zn:
-
Zinc
- Tr:
-
Trehalose
- mTOR:
-
Mammalian target of rapamycin
- CVC:
-
Carvacrol
- NDs:
-
Neurological disorders
- BBB:
-
Blood-brain barrier
- CAR:
-
Carvedilol
- MDA:
-
Malondialdehyde
- NPs:
-
Nanoparticles
References
Ahmad M, Taweel GMA, Hidayathulla S (2018) Nano-composites chitosan-curcumin synergistically inhibits the oxidative stress induced by toxic metal cadmium. Int J Biol Macromol 108:591–597
Ahmadi Z, Ashrafizadeh M (2018) Downregulation of osteocalcin gene in chickens treated with lead acetate II. Int Biol Biomed J 4(4):0–0
Ahmadi Z, Ashrafizadeh M (2019) Down regulation of osteocalcin gene in chickens treated with cadmium. Iranian Journal of Toxicology 13(1):1–4
Ahmadi Z, Ashrafizadeh M (2019) Melatonin as a potential modulator of Nrf2. Fundam Clin Pharmacol
Ahmadi Z et al (2019) The targeting of autophagy and endoplasmic reticulum stress mechanisms by honokiol therapy. Rev Clin Med 6(2):66–73
Ahmadi Z, Mohammadinejad R, Ashrafizadeh M (2019) Drug delivery systems for resveratrol, a non-flavonoid polyphenol: emerging evidence in last decades. J Drug Deliv Sci Technol 51:591–604
Akinyemi AJ, Onyebueke N, Faboya OA, Onikanni SA, Fadaka A, Olayide I (2017) Curcumin inhibits adenosine deaminase and arginase activities in cadmium-induced renal toxicity in rat kidney. J Food Drug Anal 25(2):438–446
Aladaileh SH et al (2019) Formononetin upregulates Nrf2/HO-1 signaling and prevents oxidative stress, inflammation, and kidney injury in methotrexate-induced rats. Antioxidants (Basel) 8(10). https://doi.org/10.3390/antiox8100430
Almeer RS, Alarifi S, Alkahtani S, Ibrahim SR, Ali D, Moneim A (2018) The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed Pharmacother 106:1490–1498
Almeer R et al (2018) Royal jelly abrogates cadmium-induced oxidative challenge in mouse testes: involvement of the Nrf2 pathway. Int J Mol Sci 19(12):3979
Almeer RS et al (2018) Royal jelly mitigates cadmium-induced neuronal damage in mouse cortex. Mol Biol Rep 46(1):119–131
Almeer RS, AlBasher G, Alarifi S, Alkahtani S, Ali D, Abdel Moneim AE (2019) Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci Rep 9(1):5825
Almeer RS, Kassab RB, AlBasher G, Alarifi S, Alkahtani S, Ali D, Abdel Moneim AE (2019) Royal jelly mitigates cadmium-induced neuronal damage in mouse cortex. Mol Biol Rep 46(1):119–131
Asadi N, Kheradmand A, Gholami M, Saidi SH, Mirhadi SA (2019) Effect of royal jelly on testicular antioxidant enzymes activity, MDA level and spermatogenesis in rat experimental Varicocele model. Tissue Cell 57:70–77
Ashrafizadeh M, Ahmadi Z (2019) Effects of statins on gut microbiota (microbiome). Rev Clin Med 6(2):55–59
Ashrafizadeh M, Ahmadi Z (2019) Effect of astaxanthin treatment on the sperm quality of the mice treated with nicotine. Rev Clin Med 6(1):1–5
Ashrafizadeh M, Rafiei H, Ahmadi Z (2018) Histological changes in the liver and biochemical parameters of chickens treated with lead acetate II. Iran J Toxicol 12(6):1–5
Ashrafizadeh M et al (2019) Autophagy, anoikis, ferroptosis, necroptosis, and endoplasmic reticulum stress: potential applications in melanoma therapy. J Cell Physiol 234(11):19471–19479
Ashrafizadeh M et al (2019) Nanoparticles targeting STATs in cancer therapy. Cells 8(10):1158
Ashrafizadeh M et al (2019) Modulatory effects of statins on the autophagy: a therapeutic perspective. J Cell Physiol. https://doi.org/10.1002/jcp.29227
Ashrafizadeh M et al (2019) Autophagy as a molecular target of quercetin underlying its protective effects in human diseases. Arch Physiol Biochem:1–9. https://doi.org/10.1080/13813455.2019.1671458
Ashrafizadeh M et al (2019) Effects of newly introduced antidiabetic drugs on autophagy. Diabetes Metab Syndr 13(4):2445–2449
Ashrafizadeh M et al (2019) MicroRNAs mediate the anti-tumor and protective effects of ginsenosides. Nutr Cancer:1–12. https://doi.org/10.1080/01635581.2019.1675722
Ashrafizadeh M et al Monoterpenes modulating autophagy: a review study. Basic Clin Pharmacol Toxicol https://doi.org/10.1111/bcpt.13282
Ashrafizadeh M et al Therapeutic and biological activities of berberine: the involvement of Nrf2 signaling pathway. J Cell Biochem 0(0). https://doi.org/10.1002/jcb.29392
Samarghandian S et al Catechin treatment ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Dose-Response 15(1):1559325817691158
Badisa VL et al (2007) Mechanism of DNA damage by cadmium and interplay of antioxidant enzymes and agents. Environ Toxicol 22(2):144–151
Bahri S, Kaddour H, Karoui D, Bouraoui S, Amri M, Mokni M (2019) Protective role of vitamin E against cadmium induced oxidative stress into the rat liver. Tunis Med 97(1):100–105
Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, Chen Q, Tan Y, Cui T, Zheng Y, Cai L (2013) Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol 57:82–95
Banik S et al (2019) Carvacrol inhibits cadmium toxicity through combating against caspase dependent/independent apoptosis in PC12 cells. Food Chem Toxicol 134:110835. https://doi.org/10.1016/j.fct.2019.110835
Banni M, Chouchene L, Said K, Kerkeni A, Messaoudi I (2011) Mechanisms underlying the protective effect of zinc and selenium against cadmium-induced oxidative stress in zebrafish Danio rerio. Biometals 24(6):981–992
Bashir N et al (2019) The molecular and biochemical insight view of grape seed proanthocyanidins in ameliorating cadmium-induced testes-toxicity in rat model: implication of PI3K/Akt/Nrf-2 signaling. Biosci Rep 39(1):BSR20180515
Benvenga S et al (2019) Effects of Myo-inositol alone and in combination with Seleno-L-methionine on cadmium-induced testicular damage in mice. Curr Mol Pharmacol 12(4):311–323
Beryllium I (1993) Cadmium, mercury, and exposures in the glass manufacturing industry. Working group views and expert opinions, Lyon, 9–16 February 1993. IARC Monogr Eval Carcinog Risks Hum 58:1–415
Branca JJV, Morucci G, Pacini A (2018) Cadmium-induced neurotoxicity: still much ado. Neural Regen Res 13(11):1879–1882
Cai S-A et al (2018) Nrf2 is a key regulator on puerarin preventing cardiac fibrosis and upregulating metabolic enzymes UGT1A1 in rats. Front Pharmacol 9:540
Cai Y et al (2019) Cadmium exposure affects growth performance, energy metabolism, and neuropeptide expression in Carassius auratus gibelio. Fish Physiol Biochem. https://doi.org/10.1007/s10695-019-00709-3
Caixeta DC, Teixeira RR, Peixoto LG, Machado HL, Baptista NB, de Souza AV, Vilela DD, Franci CR, Salmen Espindola F (2018) Adaptogenic potential of royal jelly in liver of rats exposed to chronic stress. PLoS One 13(1):e0191889
Calderon-Garciduenas L, Reynoso-Robles R, Gonzalez-Maciel A (2019) Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: the culprit of Alzheimer and Parkinson’s diseases. Environ Res 176:108574
Çavuşoğlu K, Yapar K, Yalçin E (2009) Royal jelly (honey bee) is a potential antioxidant against cadmium-induced genotoxicity and oxidative stress in albino mice. J Med Food 12(6):1286–1292
Chen W-Y, Chen TY, Hsieh NH, Ju YT (2016) Site-specific water quality criteria for lethal/sublethal protection of freshwater fish exposed to zinc in southern Taiwan. Chemosphere 159:412–419
Chhunchha B, Kubo E, Singh DP (2019) Sulforaphane-induced Klf9/Prdx6 Axis acts as a molecular switch to control redox signaling and determines fate of \cells. Cells 8(10). https://doi.org/10.3390/cells8101159
Cho H-Y (2013) Genomic structure and variation of nuclear factor (erythroid-derived 2)-like 2. Oxidative Med Cell Longev 2013:286524. https://doi.org/10.1155/2013/286524
Chouchene L, Banni M, Kerkeni A, Saïd K, Messaoudi I (2011) Cadmium-induced ovarian pathophysiology is mediated by change in gene expression pattern of zinc transporters in zebrafish (Danio rerio). Chem Biol Interact 193(2):172–179
Chouchene L, Pellegrini E, Gueguen MM, Hinfray N, Brion F, Piccini B, Kah O, Saïd K, Messaoudi I, Pakdel F (2016) Inhibitory effect of cadmium on estrogen signaling in zebrafish brain and protection by zinc. J Appl Toxicol 36(6):863–871
Cobb-Abdullah A et al (2019) Diallyl disulfide attenuation effect on transcriptome in rat liver cells against cadmium chloride toxicity. Environ Toxicol 34(8):950–957
Deng Y, Tang K, Chen R, Nie H, Liang S, Zhang J, Zhang Y, Yang Q (2019) Berberine attenuates hepatic oxidative stress in rats with non-alcoholic fatty liver disease via the Nrf2/ARE signalling pathway. Exp Ther Med 17(3):2091–2098
Driessnack MK, Jamwal A, Niyogi S (2017) Effects of chronic waterborne cadmium and zinc interactions on tissue-specific metal accumulation and reproduction in fathead minnow (Pimephales promelas). Ecotoxicol Environ Saf 140:65–75
Faroon O, et al (2012) Toxicological profile for cadmium.
Filippini T, Malagoli C, Wise LA, Malavolti M, Pellacani G, Vinceti M (2019) Dietary cadmium intake and risk of cutaneous melanoma: an Italian population-based case-control study. J Trace Elem Med Biol 56:100–106
Fırat Ö, Kargın F (2010) Effects of zinc and cadmium on erythrocyte antioxidant systems of a freshwater fish Oreochromis niloticus. J Biochem Mol Toxicol 24(4):223–229
Fonseca LM, Cruxen CEDS, Bruni GP, Fiorentini ÂM, Zavareze EDR, Lim LT, Dias ARG (2019) Development of antimicrobial and antioxidant electrospun soluble potato starch nanofibers loaded with carvacrol. Int J Biol Macromol 139:1182–1190
Fu C, Chen B, Jin X, Liu X, Wang F, Guo R, Chen Z, Zheng H, Wang L, Zhang Y (2018) Puerarin protects endothelial progenitor cells from damage of angiotensin II via activation of ERK1/2-Nrf2 signaling pathway. Mol Med Rep 17(3):3877–3883
Fujiki K et al (2019) Blockade of ALK4/5 signaling suppresses cadmium-and erastin-induced cell death in renal proximal tubular epithelial cells via distinct signaling mechanisms. Cell Death Differ 26(11):2371–2385
Gabr SA, Alghadir AH, Ghoniem GA (2019) Biological activities of ginger against cadmium-induced renal toxicity. Saudi J Biol Sci 26(2):382–389
Ge J, Zhang C, Sun YC, Zhang Q, Lv MW, Guo K, Li JL (2019) Cadmium exposure triggers mitochondrial dysfunction and oxidative stress in chicken (Gallus gallus) kidney via mitochondrial UPR inhibition and Nrf2-mediated antioxidant defense activation. Sci Total Environ 689:1160–1171
Gong Z-G, Wang XY, Wang JH, Fan RF, Wang L (2019) Trehalose prevents cadmium-induced hepatotoxicity by blocking Nrf2 pathway, restoring autophagy and inhibiting apoptosis. J Inorg Biochem 192:62–71
Gureev AP, Popov VN (2019) Nrf2/ARE pathway as a therapeutic target for the treatment of Parkinson diseases. Neurochem Res 157:84–104
Hassanein EHM, Shalkami AS, Khalaf MM, Mohamed WR, Hemeida RAM (2019) The impact of Keap1/Nrf2, P38MAPK/NF-kappaB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed Pharmacother 109:47–56
He L, Li P, Yu LH, Li L, Zhang Y, Guo Y, Long M, He JB, Yang SH (2018) Protective effects of proanthocyanidins against cadmium-induced testicular injury through the modification of Nrf2-Keap1 signal path in rats. Environ Toxicol Pharmacol 57:1–8
Hou N et al (2019) Carvacrol attenuates diabetic cardiomyopathy by modulating the PI3K/AKT/GLUT4 pathway in diabetic mice. Front Pharmacol 10:998
Huo L et al (2019) Sulforaphane protects the male reproductive system of mice from obesity-induced damage: involvement of oxidative stress and autophagy. Int J Environ Res Public Health 16(19). https://doi.org/10.3390/ijerph16193759
Hyder O, Chung M, Cosgrove D, Herman JM, Li Z, Firoozmand A, Gurakar A, Koteish A, Pawlik TM (2013) Cadmium exposure and liver disease among US adults. J Gastrointest Surg 17(7):1265–1273
Järup L, Åkesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238(3):201–208
Jiang C, Yuan Y, Hu F, Wang Q, Zhang K, Wang Y, Gu J, Liu X, Bian J, Liu Z (2014) Cadmium induces PC12 cells apoptosis via an extracellular signal-regulated kinase and c-Jun N-terminal kinase-mediated mitochondrial apoptotic pathway. Biol Trace Elem Res 158(2):249–258
Jiang W, Li S, Chen X, Zhang W, Chang Y, He Y, Zhang S, Su X, Gao T, Li C, Jian Z (2019) Berberine protects immortalized line of human melanocytes from H2O2-induced oxidative stress via activation of Nrf2 and Mitf signaling pathway. J Dermatol Sci 94(1):236–243
Jin A et al (2019) PHLPP2 downregulation protects cardiomyocytes against hypoxia-induced injury through reinforcing Nrf2/ARE antioxidant signaling. Chem Biol Interact 314:108848
Ke Y et al (2019) Protective roles of Pyracantha fortuneana extract on acute renal toxicity induced by cadmium chloride in rats. Acta Cir Bras 34(7):e201900706
Kim J, Song H, Heo HR, Kim JW, Kim HR, Hong Y, Yang SR, Han SS, Lee SJ, Kim WJ, Hong SH (2017) Cadmium-induced ER stress and inflammation are mediated through C/EBP–DDIT3 signaling in human bronchial epithelial cells. Exp Mol Med 49(9):e372
Kim KS, Lim HJ, Lim JS, Son JY, Lee J, Lee BM, Chang SC, Kim HS (2018) Curcumin ameliorates cadmium-induced nephrotoxicity in Sprague-Dawley rats. Food Chem Toxicol 114:34–40
Kirkham M (2006) Cadmium in plants on polluted soils: effects of soil factors, hyperaccumulation, and amendments. Geoderma 137(1–2):19–32
Klaassen CD, Liu J, Choudhuri S (1999) METALLOTHIONEIN: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol 39(1):267–294
Kocovic DM et al (2019) Cadmium versus lanthanum effects on spontaneous electrical activity and expression of connexin isoforms Cx26, Cx36, and Cx45 in the human fetal cortex. Cereb Cortex. https://doi.org/10.1093/cercor/bhz163
Lanctôt CM, Cresswell T, Melvin SD (2017) Uptake and tissue distributions of cadmium, selenium and zinc in striped marsh frog tadpoles exposed during early post-embryonic development. Ecotoxicol Environ Saf 144:291–299
Lee H-J, Yoon Y-S, Lee S-J (2018) Mechanism of neuroprotection by trehalose: controversy surrounding autophagy induction. Cell Death Dis 9(7):712
Li H et al (2019) Piceatannol alleviates inflammation and oxidative stress via modulation of the Nrf2/HO-1 and NF-kappaB pathways in diabetic cardiomyopathy. Chem Biol Interact 310:108754
Liang Y, Fan C, Yan X, Lu X, Jiang H, di S, Ma Z, Feng Y, Zhang Z, Feng P, Feng X, Feng J, Jin F (2019) Berberine ameliorates lipopolysaccharide-induced acute lung injury via the PERK-mediated Nrf2/HO-1 signaling axis. Phytother Res 33(1):130–148
Liu Y, Zou J, Liu X, Zhang Q (2019) MicroRNA-138 attenuates myocardial ischemia reperfusion injury through inhibiting mitochondria-mediated apoptosis by targeting HIF1-alpha. Exp Ther Med 18(5):3325–3332
Mahmoud AM et al (2019) Mesoporous silica nanoparticles trigger liver and kidney injury and fibrosis via altering TLR4/NF-kappaB, JAK2/STAT3 and Nrf2/HO-1 signaling in rats. Biomolecules 9(10):528. https://doi.org/10.3390/biom9100528
Mao W, Zhang NN, Zhou FY, Li WX, Liu HY, Feng J, Zhou L, Wei CJ, Pan YB, He ZJ (2011) Cadmium directly induced mitochondrial dysfunction of human embryonic kidney cells. Hum Exp Toxicol 30(8):920–929
Mohajeri M, Rezaee M, Sahebkar A (2017) Cadmium-induced toxicity is rescued by curcumin: a review. Biofactors 43(5):645–661
Mohammadinejad R et al (2019) Shedding light on gene therapy: carbon dots for the minimally invasive image-guided delivery of plasmids and noncoding RNAs. J Adv Res 18:81–93
Mohammadinejad R et al (2019) Berberine as a potential autophagy modulator. J Cell Physiol. https://doi.org/10.1002/jcp.28325
Mohebbati R et al (2019) Zataria multiflora and its main ingredient, carvacrol, affect on the renal function, histopathological, biochemical and antioxidant parameters in adriamycin-induced nephrotic rats. Arch Physiol Biochem:1–9. https://doi.org/10.1080/13813455.2019.1650069
Mostafa DG, Khaleel EF, Badi RM, Abdel-Aleem GA, Abdeen HM (2019) Rutin hydrate inhibits apoptosis in the brains of cadmium chloride-treated rats via preserving the mitochondrial integrity and inhibiting endoplasmic reticulum stress. Neurol Res 41(7):594–608
Nakajima Y et al (2009) Comparison of bee products based on assays of antioxidant capacities. BMC Complement Altern Med 9(1):4
Nasheed Hamad Almohammed Z et al (2020) The effect of melatonin on mitochondrial function and autophagy in in vitro matured oocytes of aged mice. Cell J 22(1):9–16
Oboh G, Adebayo AA, Ademosun AO, Olowokere OG (2019) Rutin alleviates cadmium-induced neurotoxicity in Wistar rats: involvement of modulation of nucleotide-degrading enzymes and monoamine oxidase. Metab Brain Dis 34(4):1181–1190
Omotosho IO (2019) Oxidative stress indices as markers of lead and cadmium exposure toxicity in auto technicians in Ibadan, Nigeria. Oxidative Med Cell Longev 2019:3030614
Pan Y et al (2018) Royal jelly reduces cholesterol levels, ameliorates Aβ pathology and enhances neuronal metabolic activities in a rabbit model of Alzheimer’s disease. Front Aging Neurosci 10:50
Panel EC (2011) Scientific opinion on tolerable weekly intake for cadmium. EFSA J 9(2):19
Patel MS, Packer L (2008) Lipoic acid: energy production, antioxidant activity and health effects: CRC Press, 1st Edition: 556. https://doi.org/10.1201/9781420045390
Patra R, Rautray AK, Swarup D (2011) Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int 2011:457327. https://doi.org/10.4061/2011/457327
Piotrowska H, Kucinska M, Murias M (2012) Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat Res 750(1):60–82
Qu H (2014) Effects of sulforaphane combined with tea polyphenols on expression of protein kinase A anchorage protein 95 and cyclin E2 in lung cancer tissues. Mod Pharm Clin 10:1092–1095
Qu K-C, Wang ZY, Tang KK, Zhu YS, Fan RF (2019) Trehalose suppresses cadmium-activated Nrf2 signaling pathway to protect against spleen injury. Ecotoxicol Environ Saf 181:224–230
Rafiei H, Ashrafizadeh M (2018) Expression of collagen type II and osteocalcin genes in mesenchymal stem cells from rats treated with lead acetate II. Iranian Journal of Toxicology 12(5):35–40
Rafiei H, Ahmadi Z, Ashrafizadeh M (2018) Effects of orally administered lead acetate II on rat femur histology, mineralization properties and expression of osteocalcin gene. Int Biol Biomed J 4(3):149–155
Rathi VK et al (2017) Naringin abates adverse effects of cadmium-mediated hepatotoxicity: an experimental study using HepG2 cells. J Biochem Mol Toxicol 31(8):e21915
Raut GK et al (2019) Glucose starvation induced upregulation of Prohibitin 1 via ROS generation causes mitochondrial dysfunction and apoptosis in breast cancer cells. Free Radic Biol Med. https://doi.org/10.4061/2011/457327
Refaie MM et al (2019) Mechanisms mediating the cardioprotective effect of carvedilol in cadmium induced cardiotoxicity. Role of eNOS and HO1/Nrf2 pathway. Environ Toxicol Pharmacol:103198. https://doi.org/10.1016/j.etap.2019.103198
Roumeliotis S, Eleftheriadis T, Liakopoulos V (2019) Is oxidative stress an issue in peritoneal dialysis? In Seminars in dialysis. Semin Dial 32(5):463–466
Rudolf E, Červinka M (2011) Sulforaphane induces cytotoxicity and lysosome-and mitochondria-dependent cell death in colon cancer cells with deleted p53. Toxicol in Vitro 25(7):1302–1309
Sabir S et al (2019) Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: inserting the association into perspectives. Biomed Pharmacother 114:108802
Sajjad N et al (2019) Artemisia amygdalina Upregulates Nrf2 and Protects Neurons Against Oxidative Stress in Alzheimer Disease. Cell Mol Neurobiol 39(3):387–399
Saleh HM, el-Sayed YS, Naser SM, Eltahawy AS, Onoda A, Umezawa M (2017) Efficacy of α-lipoic acid against cadmium toxicity on metal ion and oxidative imbalance, and expression of metallothionein and antioxidant genes in rabbit brain. Environ Sci Pollut Res 24(31):24593–24601
Sarmiento-Ortega V et al (2018) The NOAEL metformin dose is ineffective against metabolic disruption induced by chronic cadmium exposure in Wistar rats. Toxics 6(3):55
Satarug S (2018) Dietary cadmium intake and its effects on kidneys. Toxics 6(1):15
Schmidt A, Bekeschus S (2018) Redox for repair: cold physical plasmas and nrf2 signaling promoting wound healing. Antioxidants 7(10):146
Setoguchi Y, Oritani Y, Ito R, Inagaki H, Maruki-Uchida H, Ichiyanagi T, Ito T (2014) Absorption and metabolism of piceatannol in rats. J Agric Food Chem 62(12):2541–2548
Shati AA (2019) Resveratrol protects against cadmium chloride-induced hippocampal neurotoxicity by inhibiting ER stress and GAAD 153 and activating sirtuin 1/AMPK/Akt. Environ Toxicol 34(12):1340–1353
Shi X, Fu L (2019) Piceatannol inhibits oxidative stress through modification of Nrf2-signaling pathway in testes and attenuates spermatogenesis and steroidogenesis in rats exposed to cadmium during adulthood. Drug Des Dev Ther 13:2811
Shi C, Zhou X, Zhang J, Wang J, Xie H, Wu Z (2016) α-Lipoic acid protects against the cytotoxicity and oxidative stress induced by cadmium in HepG2 cells through regeneration of glutathione by glutathione reductase via Nrf2/ARE signaling pathway. Environ Toxicol Pharmacol 45:274–281
Shila S, Kokilavani V, Subathra M, Panneerselvam C (2005) Brain regional responses in antioxidant system to α-lipoic acid in arsenic intoxicated rat. Toxicology 210(1):25–36
Shin JH, Park SJ, Jo DS, Park NY, Kim JB, Bae JE, Jo YK, Hwang JJ, Lee JA, Jo DG, Kim JC, Jung YK, Koh JY, Cho DH (2019) Down-regulated TMED10 in Alzheimer disease induces autophagy via ATG4B activation. Autophagy 15(9):1495–1505
Singh KB et al (2019) Reversal of the Warburg phenomenon in chemoprevention of prostate cancer by sulforaphane. Carcinogenesis. https://doi.org/10.1093/carcin/bgz155
Sobhani B et al (2019) Histopathological analysis of testis: effects of astaxanthin treatment against nicotine toxicity. Iranian Journal of Toxicology 13(1):41–44
Song X-B, Liu G, Wang ZY, Wang L (2016) Puerarin protects against cadmium-induced proximal tubular cell apoptosis by restoring mitochondrial function. Chem Biol Interact 260:219–231
Spannhoff A, Kim YK, Raynal NJ, Gharibyan V, Su MB, Zhou YY, Li J, Castellano S, Sbardella G, Issa JP, Bedford MT (2011) Histone deacetylase inhibitor activity in royal jelly might facilitate caste switching in bees. EMBO Rep 12(3):238–243
Sun Q, Dong M, Wang Z, Wang C, Sheng D, Li Z, Huang D, Yuan C (2016) Selenium-enriched polysaccharides from Pyracantha fortuneana (Se-PFPs) inhibit the growth and invasive potential of ovarian cancer cells through inhibiting β-catenin signaling. Oncotarget 7(19):28369–28383
Sun Y, Han M, Shen Z, Huang H, Miao X (2018) Anti-hypertensive and cardioprotective effects of a novel apitherapy formulation via upregulation of peroxisome proliferator-activated receptor-α and-γ in spontaneous hypertensive rats. Saudi J Biol Sci 25(2):213–219
Tao T et al (2019) The PERK/Nrf2 pathway mediates endoplasmic reticulum stress-induced injury by upregulating endoplasmic reticulophagy in H9c2 cardiomyoblasts. Life Sci 237:116944. https://doi.org/10.1016/j.lfs.2019.116944
Tavakol S et al (2019) Autophagy modulators: mechanistic aspects and drug delivery systems. Biomolecules 9(10):530
Theodore M, Kawai Y, Yang J, Kleshchenko Y, Reddy SP, Villalta F, Arinze IJ (2008) Multiple nuclear localization signals function in the nuclear import of the transcription factor Nrf2. J Biol Chem 283(14):8984–8994
Trindade GGG, Thrivikraman G, Menezes PP, França CM, Lima BS, Carvalho YMBG, Souza EPBSS, Duarte MC, Shanmugam S, Quintans-Júnior LJ, Bezerra DP, Bertassoni LE, Araújo AAS (2019) Carvacrol/beta-cyclodextrin inclusion complex inhibits cell proliferation and migration of prostate cancer cells. Food Chem Toxicol 125:198–209
Turner A (2018) Cadmium pigments in consumer products and their health risks. Sci Total Environ 657:1409–1418
Van Gelder CW, Flurkey WH, Wichers HJ (1997) Sequence and structural features of plant and fungal tyrosinases. Phytochemistry 45(7):1309–1323
Vinas P et al (2009) Solid-phase microextraction on-fiber derivatization for the analysis of some polyphenols in wine and grapes using gas chromatography–mass spectrometry. J Chromatogr A 1216(9):1279–1284
Viñas P, Martínez-Castillo N, Campillo N, Hernández-Córdoba M (2011) Directly suspended droplet microextraction with in injection-port derivatization coupled to gas chromatography–mass spectrometry for the analysis of polyphenols in herbal infusions, fruits and functional foods. J Chromatogr A 1218(5):639–646
Wahdan SA et al (2019) Piceatannol protects against cisplatin nephrotoxicity via activation of Nrf2/HO-1 pathway and hindering NF-kappaB inflammatory cascade. Naunyn Schmiedebergs Arch Pharmacol 392(11):1331–1345
Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxidative Med Cell Longev 2013:898034. https://doi.org/10.1155/2013/898034
Wang L, Wise JT, Zhang Z, Shi X (2016) Progress and prospects of reactive oxygen species in metal carcinogenesis. Curr Pharmacol Rep 2(4):178–186
Wang XY et al (2018) Alleviation of cadmium-induced oxidative stress by trehalose via inhibiting the Nrf2-Keap1 signaling pathway in primary rat proximal tubular cells. J Biochem Mol Toxicol 32(1):e22011
Wang Y, Mandal AK, Son YO, Pratheeshkumar P, Wise JTF, Wang L, Zhang Z, Shi X, Chen Z (2018) Roles of ROS, Nrf2, and autophagy in cadmium-carcinogenesis and its prevention by sulforaphane. Toxicol Appl Pharmacol 353:23–30
Wang L-Y, Fan RF, Yang DB, Zhang D, Wang L (2019) Puerarin reverses cadmium-induced lysosomal dysfunction in primary rat proximal tubular cells via inhibiting Nrf2 pathway. Biochem Pharmacol 162:132–141
Wang C-C, Si LF, Guo SN, Zheng JL (2019) Negative effects of acute cadmium on stress defense, immunity, and metal homeostasis in liver of zebrafish: the protective role of environmental zinc dpre-exposure. Chemosphere 222:91–97
Wang Y et al (2019) Autophagy suppression accelerates apoptosis induced by Norcantharidin in cholangiocarcinoma. Pathol Oncol Res. https://doi.org/10.1007/s12253-019-00719-9
Xiao Y, Li B, Liu J, Ma X (2018) Carvacrol ameliorates inflammatory response in interleukin 1beta-stimulated human chondrocytes. Mol Med Rep 17(3):3987–3992
Xu XZ, Tang Y, Cheng LB, Yao J, Jiang Q, Li KR, Zhen YF (2019) Targeting Keap1 by miR-626 protects retinal pigment epithelium cells from oxidative injury by activating Nrf2 signaling. Free Radic Biol Med 143:387–396
Yang S-H, Long M, Yu LH, Li L, Li P, Zhang Y, Guo Y, Gao F, Liu MD, He JB (2016) Sulforaphane prevents testicular damage in Kunming mice exposed to cadmium via activation of Nrf2/ARE signaling pathways. Int J Mol Sci 17(10):1703
Yang S-H et al (2018) Protective mechanism of sulforaphane on cadmium-induced sertoli cell injury in mice testis via nrf2/are signaling pathway. Molecules 23(7):1774
Yang S-H et al (2019) Sulforaphane protect against cadmium-induced oxidative damage in mouse Leydigs cells by activating Nrf2/ARE signaling pathway. Int J Mol Sci 20(3):630
Yaribeygi H, Farrokhi FR, Rezaee R, Sahebkar A (2018) Oxidative stress induces renal failure: a review of possible molecular pathways. J Cell Biochem 119(4):2990–2998
Yasuda S, Horinaka M, Sakai T (2019) Sulforaphane enhances apoptosis induced by Lactobacillus pentosus strain S-PT84 via the TNFalpha pathway in human colon cancer cells. Oncol Lett 18(4):4253–4261
Yuan C, Wang C, Bu Y, Xiang T, Huang X, Wang Z, Yi F, Ren G, Liu G, Song F (2010) Antioxidative and immunoprotective effects of Pyracantha fortuneana (Maxim.) Li polysaccharides in mice. Immunol Lett 133(1):14–18
Yuan C, Li Z, Yi M, Wang X, Peng F, Xiao F, Chen T, Wang C, Mushtaq G, Kamal MA (2015) Effects of polysaccharides from selenium-enriched Pyracantha fortuneana on mice liver injury. Med Chem 11(8):780–788
Zarif Najafi P et al (2019) The protective effect of Zataria Multiflora on the embryotoxicity induced by bisphenol A in the brain of chicken embryos. Biointerface Res Appl Chem 9(5):4239–4242
Zhai H, Pan T, Yang H, Wang H, Wang Y (2019) Cadmium induces A549 cell migration and invasion by activating ERK. Exp Ther Med 18(3):1793–1799
Zhang S et al (2017) The effect of royal jelly on the growth of breast cancer in mice. Oncol Lett 14(6):7615–7621
Zhang C, Lin J, Ge J, Wang LL, Li N, Sun XT, Cao HB, Li JL (2017) Selenium triggers Nrf2-mediated protection against cadmium-induced chicken hepatocyte autophagy and apoptosis. Toxicol in Vitro 44:349–356
Zhang J et al (2017) Regeneration of glutathione by alpha-lipoic acid via Nrf2/ARE signaling pathway alleviates cadmium-induced HepG2 cell toxicity. Environ Toxicol Pharmacol 51:30–37
Zhang T et al (2019) The effects of long-term exposure to low doses of cadmium on the health of the next generation of mice. Chem Biol Interact 312:108792
Zhang HS et al (2019) Nrf2 promotes breast cancer cell migration via up-regulation of G6PD/HIF-1α/Notch1 axis. J Cell Mol Med 23(5):3451–3463
Zhang Z et al (2019) LncRNA TUG1 promotes cisplatin resistance in esophageal squamous cell carcinoma cells by regulating Nrf2. Acta Biochim Biophys Sin Shanghai 51(8):826–833
Zheng W, Li D, Gao X, Zhang W, Robinson BO (2019) Carvedilol alleviates diabetic cardiomyopathy in diabetic rats. Exp Ther Med 17(1):479–487
Zwolak I (2019) The role of selenium in arsenic and cadmium toxicity: an updated review of scientific literature. Biol Trace Elem Res:1–20. https://doi.org/10.1007/s12011-019-01691-w
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ashrafizadeh, M., Ahmadi, Z., Farkhondeh, T. et al. Back to Nucleus: Combating with Cadmium Toxicity Using Nrf2 Signaling Pathway as a Promising Therapeutic Target. Biol Trace Elem Res 197, 52–62 (2020). https://doi.org/10.1007/s12011-019-01980-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01980-4