Abstract
The objective of this study was to compare the effects of nanoselenium (NS) and selenium yeast (SY) on the performance, egg selenium (Se) concentration, and anti-oxidative capacity of hens. A total of 216 Brown Hy-line hens (29-week old) were randomly allocated into three treatments (6 replicate/treatment, 12 hens/replicate). The pre-trial period lasted 7 days, and the experimental period lasted 35 days. Dietary treatments included corn-soybean meal basal diet (containing 0.16 μg Se/g, as control group), and basal diet supplemented with 0.3 mg Se/kg diet (Se was from NS or SY), called as SY group or NS group, respectively. At the end of the experiment, one hen per replicate from each treatment was slaughtered. Liver, spleen, and kidney tissues were sampled for the determination of Se concentrations. The results showed that NS or SY supplement significantly improved feed conversion ratio (P < 0.05), soft broken egg rate (P < 0.05), and the serum T-AOC value (P < 0.05) when compared with control group. Remarkably, the deposition of Se increased significantly (P < 0.05) and equivalently in egg, liver, and kidney of hens supplemented with both NS and SY. Interestingly, SY supplement also enhanced the serum CAT and SOD activities (P < 0.05), NS but not SY significantly reduced serum MDA (P < 0.05), whereas RT-PCR results did not show significant differences in the mRNA levels of antioxidant genes among three groups (P > 0.05). Taken together, dietary supplemented with SY or NS improved the Se deposition in eggs, liver and kidney of laying hens, increased antioxidant activity, and NS supplement had greater Se deposition in the kidney tissue than SY supplement. SY or NS supplement could be considered to be applied for Se-enriched egg production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that selenium (Se) is an essential trace element exerting many functions in animal biological processes, such as antioxidant defense [7], immune function [11, 28], reproduction [33], and thyroid hormone metabolism [13]. To date, glutathione peroxidases 1 (GPX1) and at least seven selenoproteins have been reported playing important role in protecting against oxidative stress and the elimination of reactive oxygen species toxins [46]. In fact, Se supplement is a common practice in the poultry industry. Traditionally, sodium selenite (SS) is the most common source of Se used in animal feeds, whereas organic forms, such as Se yeast (SY), are also used in recent years [7, 14].
Recently, many studies have shown that SY has higher absorption [5], higher antioxidant activity [1, 7], and bioavailability [14] in birds as compared with inorganic forms, and its supplementation results in more Se are deposited into body tissues and eggs [10, 16, 24]. With the increasing interest in the physiological function of Se in recent years, it has been found that nano-Se (NS), with low toxicity and acceptable bioavailability, could increase selenoprotein expression, scavenge free radicals, and prevent oxidative DNA damage [32, 41, 44]. NS is red elemental Se, and Se nanoparticles are typically 10–45 nm (nm) with specific surface area (SSA) in the 30–50 m2/g range and also available with an average particle size of 75–100 nm range with a specific surface area of approximately 2–10 m2/g. Concurrently, NS supplementation could enhance tissue Se contents, GPX1 activity, and GPx-1 mRNA expression [34]. Both the dietary concentration and source of Se have been demonstrated to affect antioxidant system and Se status [4, 21, 36]. However, there were few studies about the functional comparison between organic or nano-Se supplement in the poultry industry. Therefore, SY was used as the source of organic Se in this study, to compare the functional effects of NS and SY on Se deposition in tissues and eggs, the antioxidant capacity, and productivity of hens.
Materials and Methods
Birds and Management
A total of 216 29-week-old Brown Hy-line laying hens with similar body size were selected from a commercial flock (Changsha County, Changsha City, China) and divided into 3 equal groups (6 replicate/treatment, 12 hens/replicate). Dietary treatments were a corn-soybean meal basal diet containing 0.16 μg Se/g from feed ingredients as the control group, and basal diet supplemented with 0.3 mg Se/kg (Se was from NS or SY, provided by Xing-Jia bio-engineering Co., Ltd., 410300, Changsha), which was called as SY group or NS group, respectively. The average egg production of each treatment was around 94% during 1-week statistics before the formal experiment (P = 0.989). The trial lasted for 35 days, from 30 to 35 weeks (March to April). Two birds were housed in a 39 × 35 × 38-cm wire cage with three ladders and no bedding, and then, six wire cages formed an experimental unit that was randomly distributed in the shed. The lighting regimen used was a 16 h light and 8 h darkness cycle with lights beginning at 06:00 h local time. All the birds were fed twice a day (07:30 h and 15:30 h) and allowed ad libitum access to water and treatment diets during the experiment period.
Diets
The basal diet was formulated to meet nutrient requirements suggested in the National Research Council (1994) and feeding standard of chickens (NY/T 33-2004) for Brown laying hens with no additional Se supplementation. The ingredient composition and the nutrient contents of the basal diet are shown in Table 1. All the hens received the basal layer diet during the 1-week adaptation period, and then, SY or NS group received basal diet supplemented with Se supplement diet. The total analyzed Se concentrations of the control, SY, and NS diets were 0.16, 0.45, and 0.43 mg Se/kg diet, respectively.
Sample Collection and Analytical Determination
Sample Collection
During the experiment, 12 eggs per group (2 eggs per replicate) were collected for Se analyses. At the end of the experiment, one hen per replicate from each group was slaughtered; then, liver, spleen, and kidney were sampled for the determination of Se concentrations.
Laying Performance
Daily egg production and egg weight of each replicate were monitored every day. Feed intake was recorded on a replicate basis every day. The feed conversion ratio is expressed as grams of feed consumed per grams of eggs produced.
Serum Biochemical Analyses
The concentrations of serum total protein (TP), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), uric acid (UA), glucose (GLU), total cholesterol (TCHOL), and high density lipoprotein (HDL) were determined by an Automated Biochemistry Analyzer (Synchron CX Pro, Beckman Coulter, Fullerton, CA) according to the commercial kits (Beijing Chemlin Biotech Co., Ltd., Beijing, China) and manufacturer’s instructions.
The Concentrations of Se Analyses
Two representative eggs per replicate were selected at 9 days, 18 days, 27 days, and 35 days of the experimental period for Se content assay, respectively. The samples of liver, spleen, and kidney were collected from one hen per replicate at 36 days and stored in − 20 °C for further analyses. The concentrations of Se in liver, spleen, kidney, and egg of hens were measured by a fluorescence spectrophotometer (AFS 830, Titan., Beijing, China) as described by Meng et al. [26].
Measurement of Antioxidant Enzyme Activity and Product of Oxidative Injury
Superoxide dismutase (SOD), GPX1, total antioxidant capability (T-AOC), catalase (CAT), and malondialdehyde (MDA) in the serum and liver were determined by assay kits (Nanjing Jiancheng Biotechnology Institute, China) according to the manufacturer’s instructions.
cDNA Synthesis and mRNA Quantification
Approximately 100 mg of liver tissue was pulverized in liquid nitrogen. Total RNA was isolated from homogenate using the TRIzol reagent (Beyotime Biotechnology, Shanghai, China), and then treated with DNase I according to the manufacturer’s instructions. The RNA purity and concentration of samples were determined by NanoDrop spectrophotometer (Thermo fisher scientific, New York, USA). First-strand cDNA was synthesized with reverse transcriptase kit (Takara Biomedical Technology, Japan). Primers were designed in NCBI according to the gene sequence of chick (http://www.ncbi.nlm.nih.gov/pubmed/) to produce an amplification product (Table 2). Real-time PCR was performed as previous study [22]. The relative level of mRNA expression was calculated using the 2−ΔΔCt method after normalization with β-actin as a housekeeping gene.
Statistical Analyses
This experiment was performed at a completely randomized design. All statistical analyses were performed using SPSS 17.0 software (SPSS 17.0 for Windows; IBM-SPSS Inc., Chicago, IL, USA). All data in this experiment were shown as mean ± standard error of mean (SEM). Significant differences among treatment means were determined by one-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test (SPSS, 17.0). The differences were considered significant when P < 0.05.
Results
Laying Performance
The laying performances of hens are presented in Table 3. The NS and SY groups improved feed conversion ratio and soft broken egg rate when compared with control group (P < 0.05). The results showed no significant differences in egg production, egg weight, and egg mass of hens (P > 0.05).
Serum Biochemistry
The NS and SY supplement markedly decreased serum ALT when compared with control group (P < 0.05) (Table 4). No significant differences were found in serum GLU, TP, ALB, AST, UA, TCHOL, and HDL among three groups (P > 0.05).
Se Depositions
The results of Se concentrations in the liver, kidney, and spleen are presented in Table 5. NS or SY supplement in hens increased the Se concentration in liver by 48.00% and 40.00%, respectively (P < 0.05), and kidney by 34.29% and 21.43%, respectively (P < 0.05), whereas no significant difference in spleen.
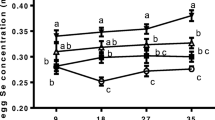
Eggs from SY or NS group had higher (P < 0.01) Se contents than those from the control group at 9 days, 18 days, 27 days, and 35 days of the experimental period (Fig. 1). Additionally, egg Se concentration was higher in SY group than that in NS group on day 9 (P < 0.05).
Effects of dietary supplementation with different Se sources on egg Se deposition with different sampling time (9 days, 18 days, 27 days, and 35 days of the experimental period). Data are means of 12 eggs per dietary treatment. Lines represent the means ± SEM, lines with different letters are statistically significant in different treatments (P < 0.05). Control = the diet supplemented with 0 mg/kg Se, SY = the diet supplemented with 0.3 mg/kg Se from selenium yeast, and NS = the diet supplemented with 0.3 mg/kg Se from nano-selenium
Antioxidant Capacity in Serum and Liver
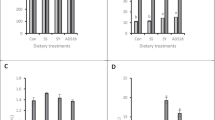
The effects of dietary supplementation with different Se sources on GPX1 activity, catalase (CAT) activity, total antioxidant capacity (T-AOC), SOD, and MDA in serum and liver are shown in Figs. 2 and 3, respectively. The serum T-AOC value in NS or SY groups was increased markedly than control group (P < 0.05). Meanwhile, SY supplement also enhanced the serum CAT and SOD activities (P < 0.05), NS but not SY significantly reduced serum MDA (P < 0.05).
Effects of dietary supplementation with different Se sources on antioxidant status in serum. Data are means of 6 replicates of 12 hens per dietary treatment. Bars represent the means ± SEM, bars with different letters are statistically significant in different treatments (P < 0.05). Control = the diet supplemented with 0 mg/kg Se, SY = the diet supplemented with 0.3 mg/kg Se from selenium yeast, and NS = the diet supplemented with 0.3 mg/kg Se from nano-selenium
Effects of dietary supplementation with different Se sources on antioxidant status in liver. Data are means of 6 replicates of 12 hens per dietary treatment. Bars represent the means ± SEM, bars with different letters are statistically significant in different treatments (P < 0.05). Control = the diet supplemented with 0 mg/kg Se, SY = the diet supplemented with 0.3 mg/kg Se from selenium yeast, and NS = the diet supplemented with 0.3 mg/kg Se from nano-selenium
NS or SY supplement had the trend to increase the hepatic T-AOC than control group (0.05 < P < 0.1). However, NS or SY increased the MDA content in the liver (P < 0.05). There was no effect of either Se supplementation on hepatic GPX1 activity.
The mRNA Levels of Antioxidant Genes in Liver
On the basis of above results, the relative mRNA expressions of Cu/Zn-SOD, CAT, GPX-1, and GPX-4 in the liver were determined (Table 6). However, we did not see significant differences among three groups (P > 0.05).
Discussion
Enhancing the Se concentration of eggs by appropriate Se supplementation of the hens’ diet can be an effective approach to improve the Se status of humans in a controlled manner. The requirement of Se for laying hens is recommended at 0.1-mg/kg diet [47]. Most studies demonstrated that the maximum Se supplementation in the diet is 0.2 to 0.5 mg/kg [6, 20, 23, 30]. Dietary Se concentration in the present experiment ranged from 0.16 to 0.43 mg/kg, indicating that laying hens received adequate Se. It has been shown that NS supplement improved feed conversion ratio in the Guangxi Yellow chicken [43], SS + SY supplement increased egg production of Jing Hong layers [10], and SY supplement increased egg production [23], which were consistent with present study. And yet, there was still another view that egg production, egg mass, and feed intake were not affected by supplement with up to 0.3 mg Se/kg [6, 20, 25, 39]. Previous reports about the effects of Se on animal growth performance are not always consistent. Actually, the effect of Se on performance might relate to the duration of the experiments, animal species, feedstuff, environment, and the quality of SY products etc. Meanwhile, the result of soft broken egg rate was decreased in Se supplement groups, which could partly relate to the fallopian tube inflammation of laying hens.
Alanine aminotransferase is an enzyme that catalyzes the transfer of amino groups to form the hepatic metabolite oxaloacetate. ALT is found abundantly in the cytosol of the hepatocyte. Liver is the primary sources of serum ALT, and ALT activity in the liver is about 3000 times that of serum activity. Thus, in the case of hepatocellular injury or death, release of ALT from damaged liver cells increases measured ALT activity in the serum [17]. In this experiment, serum ALT activities in SY and NS groups reduced, indicating that dietary supplement with Se benefits improving the health status (especially the health of the liver) of the hens.
It has been demonstrated that the addition of SY increased Se deposition in the liver and kidney of laying hens [10, 14, 18, 29]. Selenomethionine (SeMet) is the predominant form of Se in SY, SeMet metabolism is closely related to its sulfur homolog and can be incorporated into proteins in the place of methionine nonspecifically, and the incorporation of SeMet into proteins is reversible, allowing SeMet to be re-used [35, 38, 40]. With the SS or SY supplementation, the order of Se distribution in tissue and egg is liver > kidney > spleen > cardiac muscle > egg > blood > breast muscle [29]. On the other hand, Se supplement from NS in bird feeds also increased hepatic Se deposition [43]. It was reported that chicks fed with NS showed high Se contents in different tissues including liver, breast muscle, pancreas, and kidney, but showed low Se contents in spleen [9, 27]. Consistently, the present study showed that both NS and SY supplementation improved the Se concentrations in liver and kidney in hens, whereas no significant effects in spleen. However, there were few comparative studies about the effects of NS and SY on Se tissue deposition in hens. In the present study, the equivalent high deposition of Se in kidney, liver, and egg from NS as compared with SY is remarkable because NS is inorganic Se and thus cannot be metabolized directly to SeMet in monogastric animals [38]. Because hepatic GPX1 and GPX4 activities are the same in control hens and in hens supplemented with NS or SY, the increased tissue and egg Se do not appear to be present as selenoproteins. This strongly suggests that the equivalent deposition from NS as from SY may have occurred because NS was metabolized to SeMet by microbes in the gut or bedding and recycled to be deposited in protein as SeMet. Alternatively, a recent metabolomics study in turkeys reported that more hepatic Se is present as low molecular weight and high molecular weight selenosugars than was present as selenoproteins in turkeys supplemented with 0.4 or 5 μg Se/g diet as selenite [15]. Thus, the hens in this study supplemented with 0.30 μg Se/g additional Se as NS or SY may be readily metabolizing and depositing the supplemental Se as selenosugars in eggs, liver, and kidney.
It is well known that there are two assumptions about the absorption mechanism of organic mineral complexes [2]. One is that “complexed” or “chelated” trace minerals are absorbed in intact form, and the metal atoms remain safely bound or protected within organic molecular structures or “ligands” during absorption. The other is that organic ligands can prevent the harmful effect of competitive ligands such as phosphate, phytate, and other compounds, which can bind free metal ions and render the trace minerals unavailable for absorption. Maintaining a dietary mineral in solution allows maximum opportunity for contact with intestinal mucosa. SeMet is the predominant form of Se in SY and is metabolized along with methionine (Met) by the same enzymes [35]. Our previous study found that SY affects Met metabolism gene glycine N-methyltranserfase expression in the liver [26]. Meanwhile, NS exhibits high specific surface area, small particle size, and good intestinal absorption due to the formation of nanoemulsion droplets [12]. However, molecular mechanisms of NS action were not clear.
Actually, it has been demonstrated that eggs were suitable to study the absorption of Se compounds because egg Se deposition was closely related to dosage and source of Se [8]. In the present study, Se supplementation with SY or NS markedly increased Se concentrations in eggs, which was consistent with other works [29,30,31]. Moreover, it is reported that SY group had an approximate 3-fold increase in egg Se concentration over the control group at 28 days [39]. In addition, Lu et al. reported that egg Se concentrations of different SY level treatments increased as time advanced [25], whereas our result did not show that the egg Se concentration increased as time advanced. Our study showed that Se deposition in liver, kidney, and spleen of hens fed NS were higher than those of hens fed SY, whereas the opposite in egg was observed, which might suggest the different absorption mechanism between NS and SY.
As a Se-dependent enzyme that catalyzes, GPX1 could reduce hydrogen peroxide and organic peroxides to water and the corresponding stable alcohol, thus inhibiting the formation of free radicals [3]. It has been reported that organic, inorganic, or nano-Se supplementation affected GPX1 activity [4, 14, 34]. Our other study showed that both SY and NS improved the serum GPX1 activity of hens, but SY exhibited a greater ability [21]. Another study showed that NS seems to be more effective in increasing serum GPX1 activity in growing male goats [36]. Differently, the present study showed that serum GPX1 activity had no significant difference in the NS group or SY group, while hepatic GPX1 activity was higher in the NS group than in the SY group. A potential reason for such result may be due to different quality of SY and NS products.
Meanwhile, our results also found that the Se supplement (ignore NS or SY) increased T-AOC values in serum and liver. The T-AOC value reflects the total antioxidant capacity of the body [42]. Low T-AOC could be an indication of oxidative stress or higher susceptibility to oxidative damage. Therefore, NS or SY supplementation can improve the antioxidant capacity of serum in laying hens, thereby ensuring the maintenance of egg laying performance. Furthermore, SY supplementation enhanced the serum CAT and SOD activities; NS supplementation lowered the serum MDA content. Both the CAT and SOD enzymes are not Se dependent for their activities. CAT is a heme-containing enzyme that catalyzes the decomposition of H2O2 to give water and oxygen molecules [45]. SOD is an important antioxidant enzyme in organisms and catalyzes the dismutation of superoxide anion to H2O2 and molecular oxygen [37]. MDA is one of the metabolic products of lipid peroxides [1]. Unexpectedly, RT-PCR results did not show significant differences in the mRNA levels of antioxidant genes among three groups. Most studies reported that SeMet incorporated nonspecifically into proteins in place of methionine, increasing tissue Se concentrations but without further increases in mRNA levels of catalytic selenoproteins in Se adequate birds [19, 40]. The equivalent ability of NS as compared with SY in this study to provide deposition of Se into eggs, liver, and kidney suggests that dietary Se supplemented as NS above concentrations needed to maintain selenoprotein levels is readily metabolized to intermediates common to both inorganic and organic Se metabolism.
Conclusions
Taken together, it was concluded that the supplementation with NS or SY tends to improve feed conversion ratio and soft broken egg rate, to increase the serum T-AOC value, and the Se depositions in egg, liver, and kidney in laying hens. SY or NS supplement could be considered to be applied for Se-enriched egg production.
References
Ahmad H, Tian J, Wang J, Khan MA, Wang Y, Zhang L, Wang T (2012) Effects of dietary sodium selenite and selenium yeast on antioxidant enzyme activities and oxidative stability of chicken breast meat. J Agric Food Chem 60:7111–7120
Ashmead HD (1991) Comparative intestinal absorption and subsequent metabolism of metal amino acid chelates and inorganic metal salts. Biol Trace Elem Res:306–319
Behne D, Kyriakopoulos A (2001) Mammalian selenium-containing proteins. Annu Rev Nutr 21:453–473
Boostani A, Sadeghi AA, Mousavi SN, Chamani M, Kashan N (2015) Effects of organic, inorganic, and nano-Se on growth performance, antioxidant capacity, cellular and humoral immune responses in broiler chickens exposed to oxidative stress. Livest Sci 178:330–336
Bugel S, Larsen EH, Sloth JJ, Flytlie K, Overvad K, Steenberg LC et al (2008) Absorption, excretion, and retention of selenium from a high selenium yeast in men with a high intake of selenium. Food Nutr Res 52:1642
Cai SJ, Wu CX, Gong LM, Song T, Wu H, Zhang LY (2012) Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult Sci 91:2532–2539
Cao C, Zhao X, Fan R, Zhao J, Luan Y, Zhang Z, Xu S (2016) Dietary selenium increases the antioxidant levels and ATPase activity in the arteries and veins of poultry. Biol Trace Elem Res 172:222–227
Delezie E, Rovers M, Van der Aa A, Ruttens A, Wittocx S, Segers L (2014) Comparing responses to different selenium sources and dosages in laying hens. Poult Sci 93:3083–3090
Gangadoo S, Dinev I, Willson NL, Moore RJ, Chapman J, Stanley D (2020) Nanoparticles of selenium as high bioavailable and non-toxic supplement alternatives for broiler chickens. Environ Sci Pollut Res Int 27(14):16159–16166
Han XJ, Qin P, Li WX, Ma QG, Ji C, Zhang JY, Zhao LH (2017) Effect of sodium selenite and selenium yeast on performance, egg quality, antioxidant capacity, and selenium deposition of laying hens. Poult Sci 96:3973–3980
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52:1273–1280
Hu CH, Li YL, Xiong L, Zhang HM, Song J, Xia MS (2012) Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim Feed Sci Technol 177:204–210
Huang Y, Li W, Xu D, Li B, Tian Y, Zan L (2016) Effect of dietary selenium deficiency on the cell apoptosis and the level of thyroid hormones in chicken. Biol Trace Elem Res 171:445–452
Jing CL, Dong XF, Wang ZM, Liu S, Tong JM (2015) Comparative study of DL-selenomethionine vs sodium selenite and seleno-yeast on antioxidant activity and selenium status in laying hens. Poult Sci 94:965–975
Katarzyna B, Taylor RM, Szpunar J, Lobinski R, Sunde RA (2020) Identification and determination of selenocysteine, selenosugar, and other selenometabolites in turkey liver. Metallomics 12(5):758–766
Khan MT, Mahmud A, Zahoor I, Javed K (2017) Organic and inorganic selenium in Aseel chicken diets: effect on hatching traits. Poult Sci 96:1466–1472
Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC, Public Policy Committee of the American Association for the Study of Liver D (2008) Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 47:1363–1370
Leeson S, Namkung H, Caston L, Durosoy S, Schlegel P (2008) Comparison of selenium levels and sources and dietary fat quality in diets for broiler breeders and layer hens. Poult Sci 87:2605–2612
Li JL, Sunde RA (2016) Selenoprotein transcript level and enzyme activity as biomarkers for selenium status and selenium requirements of chickens (Gallus gallus). PLoS One 11(4):e0152392
Li JL, Zhang L, Yang ZY, Zhang ZY, Jiang Y, Gao F, Zhou GH (2018) Effects of different selenium sources on growth performance, antioxidant capacity and meat quality of local Chinese Subei chickens. Biol Trace Elem Res 181:340–346
Lin X, Yang T, Li H, Ji Y, Zhao Y, He J (2020) Interactions between different selenium compounds and essential trace elements involved in the antioxidant system of laying hens. Biol Trace Elem Res 193:252–260
Liu Y, Wan D, Zhou X, Ruan Z, Zhang T, Wu X, Yin Y (2019) Effects of dynamic feeding low- and high-methionine diets on the variation of glucose and lipid metabolism-related genes in the liver of laying hens. Poult Sci 98:2231–2240
Liu H, Yu Q, Fang C, Chen S, Tang X, Ajuwon KM, Fang R (2020) Effect of selenium source and level on performance, egg quality, egg selenium content, and serum biochemical parameters in laying hens. Foods. 9(1):68. https://doi.org/10.3390/foods9010068
Lu J, Qu L, Shen MM, Hu YP, Guo J, Dou TC, Wang KH (2018) Comparison of dynamic change of egg selenium deposition after feeding sodium selenite or selenium-enriched yeast. Poult Sci 97:3102–3108
Lu J, Qu L, Shen MM, Wang XG, Guo J, Hu YP, Dou TC, Wang KH (2019) Effects of high-dose selenium-enriched yeast on laying performance, egg quality, clinical blood parameters, organ development, and selenium deposition in laying hens. Poult Sci 98(6):2522–2530
Meng T, Liu YL, Xie CY, Zhang B, Huang YQ, Zhang YW, Yao Y, Huang R, Wu X (2019) Effects of different selenium sources on laying performance, egg selenium concentration, and antioxidant capacity in laying hens. Biol Trace Elem Res 189:548–555
Mohapatra P, Swain RK, Mishra SK, Behera T, Swain P, Mishra SS et al (2014) Effects of dietary nano-selenium on tissue selenium deposition, antioxidant status and immune functions in layer chicks. Int J Pharmacol 10:160–167
Nettleford SK, Prabhu KS (2018) Selenium and selenoproteins in gut inflammation-a review. Antioxidants (Basel) 7
Pan C, Huang K, Zhao Y, Qin S, Chen F, Hu Q (2007) Effect of selenium source and level in hen's diet on tissue selenium deposition and egg selenium concentrations. J Agric Food Chem 55:1027–1032
Paton ND, Cantor AH, Pescatore AJ, Ford MJ, Smith CA (2002) The effect of dietary selenium source and level on the uptake of selenium by developing chick embryos. Poult Sci 81:1548–1554
Payne RL, Lavergne TK, Southern LL (2005) Effect of inorganic versus organic selenium on hen production and egg selenium concentration. Poult Sci 84:232–237
Peng D, Zhang J, Liu Q, Taylor EW (2007) Size effect of elemental selenium nanoparticles (nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activity. J Inorg Biochem 101:1457–1463
Qazi IH, Angel C, Yang H, Pan B, Zoidis E, Zeng CJ et al (2018) Selenium, selenoproteins, and female reproduction: a review. Molecules 23
Qin SY, Chen F, Guo YG, Huang BX, Zhang JB, Ma JF (2014) Effects of nano-selenium on kidney selenium contents, glutathione peroxidase activities and GPx-1 mRNA expression in mice. Adv Mater Res 1051:383–387
Schrauzer GN (2003) The nutritional significance, metabolism and toxicology of selenomethionine. 47:73–112
Shi L, Xun W, Yue W, Zhang C, Ren Y, Shi L, Wang Q, Yang R, Lei F (2011) Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Ruminant Res 96:49–52
Surai PF, Kochish II, Fisinin VI, Kidd MT (2019) Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants (Basel):8
Sunde RA, Li J-L, Taylor RM (2016) Insights for setting of nutrient requirements, gleaned by comparison of selenium status biomarkers in turkeys and chickens versus rats, mice, and lambs. Adv Nutr: An Int Rev J 7(6):1129–1138
Utterback PL, Parsons CM, Yoon I, Butler J (2005) Effect of supplementing selenium yeast in diets of laying hens on egg selenium content. Poult Sci 84:1900–1901
Zhao L, Sun L-H, Huang J-Q, Briens M, Qi D-S, Xu S-W, Lei XG (2017) A novel organic selenium compound exerts unique regulation of selenium speciation, selenogenome, and selenoproteins in broiler chicks. J Nutr 147(5):789–797
Zhang J, Wang X, Xu T (2008) Elemental selenium at nano size (nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol Sci 101:22–31
Zhang Y, Zhu S, Wang X, Wang C, Li F (2011) The effect of dietary selenium levels on growth performance, antioxidant capacity and glutathione peroxidase 1 (GSHPx1) mRNA expression in growing meat rabbits. Anim Feed Sci Tech 169:259–264
Zhou X, Wang Y (2011) Influence of dietary nano elemental selenium on growth performance, tissue selenium distribution, meat quality, and glutathione peroxidase activity in Guangxi Yellow chicken. Poult Sci 90:680–686
Zhu C, Zhang S, Song C, Zhang Y, Ling Q, Hoffmann PR, Li J, Chen T, Zheng W, Huang Z (2017) Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-kappaB mediated hyper inflammation. J Nanobiotechnol 15:20
Zoidis E, Pappas AC, Georgiou CA, Komaitis E, Feggeros K (2010) Selenium affects the expression of GPx4 and catalase in the liver of chicken. Comp Biochem Physiol B Biochem Mol Biol 155:294–300
Yang Z, Liu C, Zheng W, Teng X, and Li S (2016) The functions of antioxidants and heat shock proteins are altered in the immune organs of selenium-deficient broiler chickens. Biol Trace Elem Res 169:341-351.
NRC (1994) Nutrient requirements of poultry, 9th edn. National Academy Press, Washington, DC.
Funding
This research received financial support from national key research and development program of China (2016YFD0200900); Poverty Alleviation through Agricultural Projects from the Agricultural Office of Chinese Academy of Sciences; Agricultural innovation project of Hunan Province (2019TD01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Statement
Animal experiments were approved by the Animal Care Committee of the Institute of Subtropical Agriculture, Chinese Academy of Science.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, TT., Lin, X., Xie, CY. et al. Nanoselenium and Selenium Yeast Have Minimal Differences on Egg Production and Se Deposition in Laying Hens. Biol Trace Elem Res 199, 2295–2302 (2021). https://doi.org/10.1007/s12011-020-02349-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02349-8