Abstract
The purpose of this study was to investigate the interactions between different selenium (Se) compounds including sodium selenite (SS), selenium-enriched yeast (SY), and nano-selenium (NS) and various essential trace elements involved in the antioxidant systems, and to evaluate the effects on laying performance and egg quality. A total of 288 21-week-old Hyline Sophie hens were allotted to four dietary treatments: (1) basal diet without Se supplementation; (2) basal diet supplemented with 0.3 mg/kg Se of SS; (3) basal diet supplemented with 0.3 mg/kg Se of SY; (4) basal diet supplemented with 0.3 mg/kg Se of NS. Each treatment had eight replicates with nine hens per replicate. The trial lasted for 35 days. Results demonstrated that NS supplementation decreased the egg production (EP) and increased the feed conversion rate (FCR) and eggshell thickness and that SY changed the egg shape index (p < 0.05). Supplementation with three Se compounds significantly increased serum Se concentration and glutathione peroxidase (GSH-Px) activity in all treatment groups, as well as total superoxide dismutase (T-SOD) activity in the SY and NS groups. Yolk iron (Fe) and copper (Cu) concentrations in the NS group were also increased with Se supplementation. While the serum zinc (Zn) concentration decreased in the NS and SY groups, as well as the yolk manganese (Mn) concentration in the SY group. And the total antioxidant capability (T-AOC) of yolk with 3 days of storage in the SY and NS groups, malondialdehyde (MDA) value in the NS group, and the T-SOD activity and MDA value of yolk with 10 days of storage in the SY group also decreased. Thus, the source of Se compounds may influence the balance between Se and other trace elements including Zn, Mn, Fe, and Cu, which is important for proper antioxidant defense in blood and egg yolk of laying hens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Essential trace elements are important minerals for the normal function of all basic biochemical processes; deficiency of which may lead to poor health and production [1]. Selenium (Se) is an essential trace element, and it exerts antioxidant defense, immune responses, resists the free radicals, and improves reproduction in humans and animals [2,3,4,5,6,7]. It was reviewed that the broiler chicks were susceptible to dietary Se deficiency [8], which could lead to oxidative injury and alteration in the immune organs of broiler chickens [9]. In animal diets, there are two Se sources: organic and inorganic Se; organic sources of Se include selenium-enriched yeast (SY), normally explored as an alternative to an inorganic source, and sodium selenite (SS) supplementation, because of its higher rates of absorption, tissues deposition, and antioxidant activities [2, 4, 10,11,12]. Nano-selenium (NS) appears to be more effective than other forms of Se at scavenging free radicals; it also has additional benefits such as low toxicity and acceptable bioavailability [13,14,15,16], in that NS exhibits high specific surface area, small particle size, and good intestinal absorption [17, 18]. Se bioavailability increased egg deposition when organic sources were compared with inorganic sources, and organic sources led to a higher deposition of Se in egg contents [19]. Diet supplemented with 0.30 mg/kg of nano-Se could effectively increase the growth performance of chickens and the Se concentrations of the organ [18]. And about 0.3 mg Se/kg diet was suggested to be the suitable level [20].
In the past years, the role of trace elements in animal health has been well established, and their presence in feed in adequate quantities has been taken for granted [1]. However, a mineral intake with a high level has interaction effects on other minerals [21], and previous studies demonstrate that a high level of calcium supplementation may cause or accentuate poor utilization of zinc (Zn) from soy products [22, 23]. Specially, it was reported that Se deficiency would cause an overload of iron (Fe) and unbalance in vivo distributions of other elements, such as copper (Cu) and Zn, and the interactions in vivo might occur by a mechanism other than a simple mutual interaction [24]. Egg is an important international food commodity, and China accounts for approximately a quarter of the world’s egg production at roughly 39.68 billion pounds; female birds may deposit minerals into the egg [25, 26].
The main objective of the present study was to investigate the effects of supplemental Se (0.3 mg/kg) from different Se compounds (NS, SY, and SS) in laying hens on antioxidant enzyme activity and the interactions between Se and other essential trace elements involved in the antioxidant system.
Materials and Methods
Ethics Statement
Experiments were carried out in accordance with the ethical guidelines of Hunan Agricultural University for the care and use of laboratory animals.
Animals and Management
A total of 288 21-week-old Hyline Sophie laying hens were selected from a commercial flock (Huangpu District, Guangzhou City, China) and randomly divided into four groups with 72 hens each group in eight replicates. And four treatments were as follows: (1) The CON group received basal diet without selenium supplementation; (2) the SS group received diet supplementation with 0.3 mg kg−1 Se of SS; (3) the SY group received diet supplementation with 0.3 mg kg−1 Se of SY; (4) and the NS group received diet supplementation with 0.3 mg kg−1 Se of NS. The trial lasted for 35 days. Three birds were housed in a (38 × 35 × 35 cm) wire cage with three ladders, and then three wire cages formed an experimental unit that was randomly distributed in the shed. The light regimen was 16 h light: 8 h darkness and the birds were fed twice (05:30 AM and 13:30 PM) ad libitum and had free access to water during the experiment period. Feed intake was recorded daily, and the feed conversion rate and daily average feed intake per bird were calculated for a 5-week period. The number of eggs per replicate was counted, and the eggs were weighed daily; the incidences of soft-shelled and cracked eggs were also recorded.
Diets
All hens were fed corn-soybean-based diets in mash form. The corn-soybean basal diet was formulated to contain adequate nutrient concentrations as suggested in the NRC (1994) for laying hens except for Se (Table 1). All four groups of hens received the basal layer diet during the 1-week adaptation period. After the adaptation period, three treatments of birds received basal diet supplemented with 0.3 mg kg−1 Se from SS, NS, and SY (provided by Tanke Bio-tech Co., Ltd., 510890, Guangzhou), respectively.
Sample Collection and Preparation
On the 35th day of the trial, a total 64 birds, 16 birds per treatment (2 per replicate), were randomly selected, and blood samples were collected from the main wing vein. Blood was centrifuged for 15 min at 3000×g under room temperature; then, serum was obtained and stored at − 20 °C for subsequent analysis. In addition, a total of 128 normal eggs were randomly selected (n = 32 eggs/treatment) to evaluate the egg quality traits and receive yolk. Yolk and albumen were separated manually by a plastic egg separator, and trace element analysis and antioxidant enzyme activity of yolk were analyzed.
Egg Quality Traits
Egg quality traits including egg weight, eggshell strength, albumen height, Haugh unit, and shell thickness were evaluated. The egg weight, albumen height, and Haugh unit were determined by an egg quality analyzer (EA-01, Orka Food Technology Ltd., Ramat HaSharon, Israel). The eggshell strength was determined by an egg force reader (Orka Food Technology Ltd., Ramat HaSharon, Israel). The shell thickness was determined by a shell thickness gauge (Orka Food Technology Ltd., Ramat HaSharon, Israel) at three different locations (bottom, middle, and top of the egg) [27].
Trace Element Concentration in Feed, Yolk, and Serum
Total Se concentration of the feed, yolk, and serum samples was determined by an atomic fluorescence spectrophotometer (AFS-2000, Beijing, China). 0.5 g of feed or yolk samples and 1 ml of serum samples were mineralized in 5 ml of HNO3 in closed vessels in a microwave oven (Topex, Shanghai, China). The vessels were heated at 140 °C for 10 min, and this temperature was maintained for 8 min. After cooling, the solution was diluted with 2 ml of HCLO4 in a 100-ml Erlenmeyer flask, and heated at 140 °C until the appearance of white fumes. After cooling, the digested sample was transferred to a cuvette and made up to a final volume of 25 ml with 15 ml HCL and ultrapure water; the supernatant was measured directly on the machine [11].
For serum and yolk sample dilution and preparation of standards, ultrapure water and ultrapure acids (HNO3:HClO4 = 4:1) were used. Samples were analyzed with preliminary treatment electric oven digestion; then, Fe, Zn, Mn, and Cu content in ICP-OES (iCAP 6000 series, Shanghai, China) was determined [28].
Measurement of Antioxidant Enzyme Activity in Yolk
Total superoxide dismutase (T-SOD) activity, glutathione peroxidase (GSH-Px) activity, total antioxidant capability (T-AOC), and malondialdehyde (MDA) content in the serum and yolk were determined by assay kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions [29].
Statistical Analysis
This experiment was performed at a completely randomized design. All data statistical analyses were performed using SPSS 17.0 software for Windows (Chicago, IL, USA). A multivariate linear model was used to analyze the data where Se, Zn, Cu, manganese (Mn), and Fe were the concentration variables, while treatments and tissues (blood and yolk) were the fixed factors, and the model [30] was
where Y denotes Se, Zn, Cu, Mn, and Fe concentrations.
The statements of significance presented in this study were based on p < 0.05. In the table, the data are showed as the mean ± standard error (SE) of each of the main effects.
Other data in this experiment was shown as mean ± standard error of mean (SEM). Significant differences among treatment means were determined by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (SPSS 17.0). The differences were considered significant when p < 0.05. The data of laying performance and laying ratio is the average value of 5 weeks.
Results
Effects of Supplemental Se on Laying Performance and Egg Quality Traits
The laying performances of hens are presented in Table 2. Se supplementation significantly decreased the average egg production and increased feed conversion ratios (p < 0.05) in the NS group when compared with the CON group. The results of egg quality traits are shown in Table 3. Se supplementation significantly increased the eggshell thickness (p < 0.05) in the NS group compared with the CON group. When compared with the SS group, Se supplementation decreased significantly the egg shape index in the SY group (p < 0.05).
Effects of Supplemental Se on Interaction Between Se and Other Trace Elements
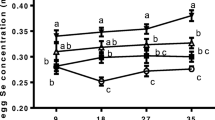
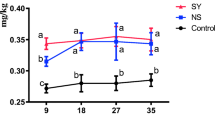
Supplementation of diet with 0.3 mg/kg of Se from different Se compounds significantly (p < 0.001) increased Se concentration (data pooled for deposition in serum and yolk effects), compared with the Se concentration of laying hens fed the diet without Se supplementation (CON group) (Table 4). Se concentration was affected significantly (p < 0.001, data pooled for treatment to exacerbate deposition effects; Table 4). Se concentration in descending order was egg > blood, and this pattern was marked for all treatments as indicated by the significant treatment × tissue interactions (p < 0.001).
Zn concentration was not affected by different Se compound addition to laying hens’ diets (Table 4). However, Zn concentration was 4.48 times greater in yolk than its concentration in serum (p < 0.001). Furthermore, the treatment × tissue interactions (p = 0.040) indicated that different Se compound supplementation had significant effects on Zn concentration. In detail, Zn concentration in serum of laying hens fed diets SY (5.78 ± 0.31 mg kg−1) and NS (6.39 ± 0.46 mg kg−1) was lower than that of laying hens fed the diet without Se supplementation (11.58 ± 0.86 mg kg−1).
Different Se compound supplementation had not affected the concentration of Fe (Table 4). Fe concentration of the SS, SY, and NS groups was increased by 8.7, 11.1, and 15.1%, respectively, compared with the concentration of the CON group (p = 0.125). However, Fe concentration was significantly affected (p < 0.001), and Fe concentration in yolk was 4.1 times greater than that in serum (p < 0.001). In detail, Fe concentration in yolk of laying hens fed diet NS (61.94 ± 2.11 mg kg−1) was higher than that of laying hens fed the diet without Se supplementation (52.99 ± 3.04 mg kg−1).
Mn concentration was not affected by different Se compound addition (Table 4). Yolk contained higher (p < 0.001) concentration of Mn compared with serum. Furthermore, the treatment × tissue interactions (p = 0.011) indicated that different Se compound supplementation had significant effects on Mn concentration. The present study revealed significant interactions between treatment and tissue regarding Mn accumulation. In detail, Mn concentration in yolk of laying hens fed diet SY (0.82 ± 0.16 mg kg−1) was lower than that of laying hens fed the diet without Se supplementation (1.16 ± 0.11 mg kg−1).
Copper concentration was not affected by different Se compound addition (Table 4). Yolk contained higher (p < 0.001) concentration of Cu compared with serum. In detail, Cu concentration in yolk of laying hens fed diet NS (1.70 ± 0.05 mg kg−1) was higher than that of laying hens fed the diet without Se supplementation (1.53 ± 0.06 mg kg−1).
Effects of Supplemental Se on Antioxidant Capacity in Serum and Yolk
The effects of dietary supplementation with different Se compounds on antioxidant enzyme activity in serum were shown in Table 5. The serum T-SOD activity in the NS and SY groups was significantly higher than that in the CON group (p < 0.05). Meanwhile, SS, SY, and NS supplementation all improved the GSH-Px activity in serum of the hens compared with the CON group (p < 0.05).
The effects of dietary supplementation with different Se compounds on antioxidant enzyme activity in yolk were shown in Table 6. After 3 days in the storage at room temperature, the yolk T-AOC values decreased significantly in the SY and NS group in comparison with CON group, as well as the yolk MDA value in NS group significantly (p < 0.05). After 10 days in the storage at room temperature, the yolk T-SOD activity and MDA values decreased significantly in the SY group in comparison with the CON group (p < 0.05).
Discussion
Some previous studies show that dietary supplementation with different levels and sources of Se (from 0.18 to 0.51 mg/kg of SY, Se-enriched kale sprouts, or other organic Se source) has no effect on egg production or feed intake [2, 31, 32,32,33,34,36], but in our present study, NS supplementation decreased the EP and increased FCR; a potential reason for such result may be due to higher bioavailability but low metabolism requirements for Se in poultry. Meanwhile, NS supplementation increased the eggshell thickness, because during the process of eggshell formation, sources of mineral nutrients play an important role in maintaining eggshell quality [37]; it was speculated that NS supplementation may help improve eggshell quality via interactions with other mineral nutrients.
An interaction exists among different ions, and changes in the ion concentration may play some roles in the regulation of physiological processes [38]. Optimal nutrition guarantees proper functions of the organism, among which the most important are structural, physiological, catalytic, and regulatory [39]. In chicken muscles, Mn and Cu are significantly influenced by Se deficiency, and an imbalance in the level of Cu and Mn influences the activity of enzymes [40]. It is reported that Se in the body is first to supply the tissues and organs that need it and is distributed in various tissues and organs through blood circulation [41]. This study revealed that Se compound supplementation in laying hens’ diet increased the Se concentration of serum and egg yolk. Eggs have been very useful in studying the absorption of various Se compounds, and egg Se concentration is significantly affected by the dosage level and source of Se [10, 11, 34, 35]. In the present study, Se supplementation with SY or NS or SS increased Se concentrations in egg yolk which is consistent with the result in the published literature [10, 19, 33,33,35]. Furthermore, different Se compounds affected other trace elements’ retention in egg yolk, whereas in the present work, supplemental SY decreased Mn concentration and NS increased Fe and Cu concentration, which was speculated that trace elements might interact with each other at the levels of absorption, distribution, and retention [30]. It is reported that there may be an antagonistic effect between Se (sodium selenite) and Mn, Cu, and other elements in chicken muscles [40], and Se (sodium selenite) supplementation increases some essential microelements, such as Zn in the chicken liver [39]. Se (sodium selenite) also reduces cadmium (Cd) accumulation and antagonizes Cd-triggered apoptosis in the chicken pancreas, and Se has interactions with heavy metal, which exerts its toxicity via changing the ion homeostasis in animal organisms [42]. It was suggested that the complex interactions might occur between Se and other elements in laying hens’ blood and egg, not only synergistic and antagonistic effects but also some complicated modulating effects among these ions. And different Se compounds may be related to these changing ion profiles in laying hens.
In addition, Mn, as an essential trace element, is a crucial component of the metalloenzyme, Mn superoxide dismutase (MnSOD), which is an antioxidant enzyme for detoxification of reactive oxygen species (ROS) [43]. Cu and Zn are also involved in the metabolism of oxygen and the biochemistry of redox reactions included in enzyme CuZn superoxide dismutase (SOD), which catalyzes the dismutation of superoxide, and Se was positively correlated with Zn, Cu, and Fe [24]. And Fe is an essential micronutrient for various physiological functions including energy metabolism, electron transfer and oxygen transport, and redox reactions, but excessive storage and intake of Fe cause various diseases [44,45,46]. Subsequently, the trace elements’ retention in egg yolk in the present study may affect the egg yolk antioxidant status, which may be related to the egg quality.
Se has antioxidant effects because it forms selenocysteine, a part of the active center of glutathione peroxidase (GSH-Px), which has an antioxidative action by catalyzing the reduction of hydrogen peroxide and lipid peroxides [9, 47, 48]. It has been reported that inorganic, organic, or nano-Se affects the GSH-Px activity previously. Broilers supplemented with 0.3 mg/kg of NS increased the GSH-Px activity compared with the control group without supplementation [15]. And Jing et al. reported that the organic sources (SY and Se-Met) exhibited a greater ability to increase the GSH-Px activity than the SS that was added at 0.3 mg Se kg−1 [49]. Previous work also suggests that there was significantly higher serum GSH-Px activity in NS-supplemented groups of broilers [47], similarly in laying hens [50]. Consistent with this, the present study showed that serum GSH-Px activities were all increased in the Se compound supplementation treatments. However, in contrast to the previous result that total superoxide dismutase activity in serum of broilers supplemented with 0.3 mg/kg was not affected by nano-Se levels [15], in the present study, dismutase activity in the SY or NS group was increased. A potential reason for such discrepancies may be due to differences in the length and process control of the experiments. Many ions are involved in important biological processes, and normal biochemical function in animals requires the proper amount of minerals, and Se could alleviate ion metabolism to some extent [5]. In this study, the alteration of serum Zn balance both in the SY and NS groups may concomitantly influence the antioxidant defense systems due to the positive Se-Zn correlation attributed to metallothionein (MT) [30]. Therefore, SY or NS supplementation may improve the T-SOD activity of serum in laying hens to ensure laying performance.
In the present study, the SY or NS supplementation increased the total antioxidant capacity in yolk of stored eggs at room temperature for 3 days, and it was speculated that SY and NS might improve egg quality during storage. Egg yolk is a natural supramolecular assembly of proteins and lipids with different organization levels [51]. The increase of Se content in broiler feed increases linolenic acid in thigh muscle tissue of broilers [52]. And Se sources (SS or SY) decrease the MDA content of chicken breast meat [53]. The NS supplementation affected the reduction of MDA content in yolk of stored eggs at room temperature for 3 days, and SY supplementation affected MDA content in yolk of stored eggs at room temperature for 10 days. MDA is the product of lipid peroxidation, and an increasing MDA level is an index of enhanced lipid peroxidation [5, 54]. It was speculated that supplementation SY or NS might be beneficial for egg yolk lipid retention, and a higher concentration of organic selenium in eggs may be a factor that positively affected the indicators of egg freshness [55]. However, further studies should be carried out to investigate the underlying mechanism on how other nutrients such as Se, Cu, Zn, and Mn metabolize and how they are related to the layer’s oxidative status.
Conclusions
In conclusion, the supplementation of NS with 0.3 mg kg−1 decreased the EP and increased FCR and eggshell thickness. And serum Zn concentration was decreased in both the SY and NS groups; the egg yolk Mn retention was also decreased in the SY group, whereas Fe and Cu concentrations were increased in the yolk of the NS group. The supplementation of SY and NS trended to increase the serum GSH-Px and T-SOD activities. It demonstrated that egg production, egg quality, serum trace mineral concentration, antioxidative capacity, egg yolk trace mineral concentration, and antioxidant status were affected by different Se compounds. And there may be existing complex interactions between different Se compounds and other elements in laying hens, including synergistic, antagonistic, and modulating effects among these ions; thus, the trace elements’ retention in eggs affected the egg antioxidant status. Based on these findings, it was speculated that the source of Se compounds may play important and complex roles in the alteration of the balance and correlation to other trace elements, such as Zn, Mn, Fe, and Cu, and may then affect the antioxidant defense system in blood and egg of laying hens, which provides a reference for choosing a suitable Se source.
References
López-Alonso M (2012) Trace minerals and livestock: not too much not too little. ISRN Vet Sci 2012:1–18. https://doi.org/10.5402/2012/704825
Chantiratikul A, Chinrasri O, Chantiratikul P (2018) Effect of selenium from selenium-enriched kale sprout versus other selenium sources on productivity and selenium concentrations in egg and tissue of laying hens. Biol Trace Elem Res 182(1):1–6. https://doi.org/10.1007/s12011-017-1069-0
Yu J, Yao H, Gao X, Zhang Z, Wang JF, Xu SW (2015) The role of nitric oxide and oxidative stress in intestinal damage induced by selenium deficiency in chickens. Biol Trace Elem Res 163(1–2):144–153. https://doi.org/10.1007/s12011-014-0164-8
Placha I, Takacova J, Ryzner M, Cobanova K, Laukova A, Strompfova V, Venglovska K, Faix S (2014) Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br Poult Sci 55(1):105–114 https://www.tandfonline.com/doi/abs/10.1080/00071668.2013.873772
Li J, Xing L, Zhang R (2017) Effects of se and cd co-treatment on the morphology, oxidative stress, and ion concentrations in the ovaries of laying hens. Biol Trace Elem Res 183:156–163. https://doi.org/10.1007/s12011-017-1125-9
Li, S., Gao, F., Huang, J., Wu, Y., Wu, S., & Lei, X. G. (2018). Regulation and function of avian selenogenome. Biochim Biophys Acta Gen Subj, S0304416518300916. https://doi.org/10.1016/j.bbagen.2018.03.029
Xu D, Li W, Huang Y, He J, Tian Y (2014) The effect of selenium and polysaccharide of atractylodes macrocephala koidz. (pamk) on immune response in chicken spleen under heat stress. Biol Trace Elem Res 160(2):232–237. https://doi.org/10.1007/s12011-014-0056-y
Huang, J. Q., Zhou, J. C., Wu, Y. Y., Ren, F. Z., & Lei, X. G (2018) Role of glutathione peroxidase 1 in glucose and lipid metabolism-related diseases. Free Radic Biol Med, 127, S0891584918309109-. https://doi.org/10.1016/j.freeradbiomed.2018.05.077, 108, 115
Yang Z, Liu C, Zheng W, Teng X, Li S (2016) The functions of antioxidants and heat shock proteins are altered in the immune organs of selenium-deficient broiler chickens. Biol Trace Elem Res 169(2):341–351. https://doi.org/10.1007/s12011-015-0407-3
Pan CL, Huang KH, Zhao YX, Qin SY, Chen F, Hu QH (2007) Effect of selenium source and level in hen’s diet on tissue selenium deposition and egg selenium concentrations. J Agric Food Chem 55(3):1027–1032. https://doi.org/10.1021/jf062010a
Delezie E, Rovers M, Van der Aa A, Ruttens A, Wittocx S, Segers L (2014) Comparing responses to different selenium sources and dosages in laying hens. Poult Sci 93(12):3083–3090. https://doi.org/10.3382/ps.2014-04301
Boostani A, Sadeghi AA, Mousavi SN, Chamani M, Kashan N (2015) The effects of organic, inorganic, and nano-selenium on blood attributes in broiler chickens exposed to oxidative stress. Acta Sci Vet, 43(1). http://bepls.com/sep_2014/25.pdf
Peng D, Zhang J, Liu Q, Taylor EW (2007) Size effect of elemental selenium nanoparticles (nano-se) at supranutritional levels on selenium accumulation and glutathione s-transferase activity. J Inorg Biochem 101(10):1457–1463. https://doi.org/10.1016/j.jinorgbio.2007.06.021
Zhang J, Wang X, Xu T (2008) Elemental selenium at nano size (nano-se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol Sci 101(1):22–31. https://doi.org/10.1093/toxsci/kfm221
Cai SJ, Wu CX, Gong LM, Song T, Wu H, Zhang LY (2012) Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult Sci 91(10):2532–2539. https://doi.org/10.3382/ps.2012-02160
Kim J, Lee KY, Lee CM (2016) Selenium nanoparticles formed by modulation of carrageenan enhance osteogenic differentiation of mesenchymal stem cells. J Nanosci Nanotechnol 16(3):2482–2487. https://doi.org/10.1166/jnn.2016.10764
Hu CH, Li YL, Xiong L, Zhang HM, Song J, Xia MS (2012) Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim Feed Sci Technol 177(3–4):204–210. https://doi.org/10.1016/j.anifeedsci.2012.08.010
Liu S, Tan H, Wei S, Zhao J, Yang L, Li S, Zhong C, Yin Y, Chen Y, Peng Y (2015) Effect of selenium sources on growth performance and tissue selenium retention in yellow broiler chicks. J Appl Anim Res 43(4):487–490. https://doi.org/10.1080/09712119.2014.978780
Reis RN, Vieira SL, Nascimento PC, Pena JE, Barros R, Torres CA (2009) Selenium contents of eggs from broiler breeders supplemented with sodium selenite or zinc-l-selenium-methionine. J Appl Poult Res 18(2):151–157. https://doi.org/10.3382/japr.2008-00069
He JH, Ohtsuka A, Hayashi K (2000) Selenium influences growth via thyroid hormone status in broiler chickens. Br J Nutr 84(5):727–732. https://doi.org/10.1017/S0007114500002087
Sirirat N, Lu JJ, Hung Alex TY, Chen SY, Tu FL (2012) Effects different levels of nanoparticles chromium picolinate supplementation on growth performance, mineral retention, and immune responses in broiler chickens. J Agric Sci (1916-9752), 4(12). https://doi.org/10.5539/jas.v4n12p48
Forbes RM, Weingartner KE, Parker HM, Bell RR, Erdman JW (1979) Bioavailability to rats of zinc, magnesium and calcium in casein-, egg- and soy protein-containing diets. J Nutr 109(9):1652–1660. https://doi.org/10.1093/jn/109.9.1652
Lin X, Liu Y, Meng T, Xie C, Wu X, Yin Y (2018) Circadian calcium feeding regime in laying hens related to zinc concentration, gene expression of circadian clock, calcium transporters and oxidative status[J]. J Trace Elem Med Biol 50:518–526. https://doi.org/10.1016/j.jtemb.2018.03.002
Feroci G, Badiello R, Fini A (2005) Interactions between different selenium compounds and zinc, cadmium and mercury. J Trace Elem Med Biol 18(3):227–234. https://doi.org/10.1016/j.jtemb.2004.09.005
Jia R, Ma Q, Fan Y, Ji C, Zhang J, Liu T et al (2016) The toxic effects of combined aflatoxins and zearalenone in naturally contaminated diets on laying performance, egg quality and mycotoxins residues in eggs of layers and the protective effect of bacillus subtilis biodegradation product. Food Chem Toxicol 90:142–150. https://doi.org/10.1016/j.fct.2016.02.010
Hargitai R, Nagy G, Nyiri Z, Bervoets L, Eke Z, Eens M, Török J (2016) Effects of breeding habitat (woodland versus urban) and metal pollution on the egg characteristics of great tits (parus major). Sci Total Environ 544:31–38. https://doi.org/10.1016/j.scitotenv.2015.11.116
Liu Y, Lin X, Zhou X, Wan D, Wang Z, Wu X, Yin Y (2017) Effects of dynamic feeding low and high methionine diets on egg quality traits in laying hens. Poult Sci 96:1459–1465. https://doi.org/10.3382/ps/pew398
Ni Y, Wu Y, Kokot S (2002) Improved icp-oes analysis of trace calcium in rare-earth matrices with the use of iterative target transformation factor analysis and kalman filter. J Anal At Spectrom 17(6):596–602. https://doi.org/10.1039/b110325n
Chen F, Zhu L, Qiu H, Qin S (2017) Selenium-enriched Saccharomyces cerevisiae improves growth, antioxidant status and selenoprotein gene expression in arbor acres broilers. J Anim Physiol Anim Nutr (Berl) 101(2):259–266. https://doi.org/10.1111/jpn.12571
Pappas AC, Zoidis E, Georgiou CA, Demiris N, Surai PF, Fegeros K (2011) Influence of organic selenium supplementation on the accumulation of toxic and essential trace elements involved in the antioxidant system of chicken. Food Addit Contam Part A 28(4):446–454. https://doi.org/10.1080/19440049.2010.549152
Chantiratikul A, Chinrasri O, Chantiratikul P (2008) Effect of sodium selenite and zinc-l-selenomethionine on performance and selenium concentrations in eggs of laying hens. Asian Australas J Anim Sci 21(7):1048–1052. https://doi.org/10.5713/ajas.2008.70576
Jiakui L, Xiaolong W (2005) Effect of dietary organic versus inorganic selenium in laying hens on the productivity, selenium distribution in egg and selenium content in blood, liver and kidney. J Trace Elem Med Biol 18(1):65–68. https://doi.org/10.1016/j.jtemb.2004.04.002
Payne RL, Lavergne TK, Southern LL (2005) Effect of inorganic versus organic selenium on hen production and egg selenium concentration. Poult Sci 84(2):232–237. https://doi.org/10.1093/ps/84.2.232
Paton ND, Cantor AH, Pescatore AJ, Ford MJ, Smith CA (2002) The effect of dietary selenium source and level on the uptake of selenium by developing chick embryos. Poult Sci 81(10):1548–1554. https://doi.org/10.1093/ps/81.10.1548
Utterback PL, Parsons CM, Yoon I, Butler J (2005) Effect of supplementing selenium yeast in diets of laying hens on egg selenium content. Poult Sci 84:1900–1901. https://doi.org/10.1093/ps/84.12.1900
Bennett DC, Cheng KM (2010) Selenium enrichment of table eggs. Poult Sci 89(10):2166–2172. https://doi.org/10.3382/ps.2009-00571
Lichovnikova M (2007) The effect of dietary calcium source, concentration and particle size on calcium retention, eggshell quality and overall calcium requirement in laying hens. Br Poult Sci 48(1):71–75. https://doi.org/10.1080/00071660601148203
Xu T, Gao X, Liu G (2016) The antagonistic effect of selenium on lead toxicity is related to the ion profile in chicken liver. Biol Trace Elem Res 169(2):365–373. https://doi.org/10.1007/s12011-015-0422-4
Suttle N, Suttle N (2009) Mineral nutrition of livestock. Cabi Bookshop 215(6):1–8. https://doi.org/10.1079/9781845934729.0000
Yao H, Zhao X, Fan R, Sattar H, Zhao J, Zhao W et al (2017) Selenium deficiency-induced alterations in ion profiles in chicken muscle. Plos One 12(9):e0184186. https://doi.org/10.1371/journal.pone.0184186
Zheng SF, Xing HJ, Zhang QJ, Xue H, Zhu FT, Xu SW (2018) Pharmacokinetics of sodium selenite administered orally in blood and tissues of selenium-deficient ducklings. Biol Trace Elem Res. https://doi.org/10.1007/s12011-018-1567-8
Jin, X., Jia, T., Liu, R., & Xu, S. (2018). The antagonistic effect of selenium on cadmium-induced apoptosis via ppar-γ/pi3k/akt pathway in chicken pancreas. J Hazard Mater, S0304389418304412-. https://doi.org/10.1016/j.jhazmat.2018.06.003
Gao T, Wang F, Li S, Luo X, Zhang K (2011) Manganese regulates manganese-containing superoxide dismutase (mnsod) expression in the primary broiler myocardial cells. Biol Trace Elem Res 144(1–3):695–704. https://doi.org/10.1007/s12011-011-9093-y
Feng J, Ma WQ, Xu ZR, He JX, Wang YZ, Liu JX (2009) The effect of iron glycine chelate on tissue mineral levels, fecal mineral concentration, and liver antioxidant enzyme activity in weanling pigs. Anim Feed Sci Technol 150(1):106–113. https://doi.org/10.1016/j.anifeedsci.2008.07.004
Zhang Y, Wan D, Zhou X, Long C, Wu X, Li L, He L, Huang P, Chen S, Tan B, Yin Y (2017) Diurnal variations in iron concentrations and expression of genes involved in iron absorption and metabolism in pigs. Biochem Biophys Res Commun 490(4):1210–1214. https://doi.org/10.1016/j.bbrc.2017.06.187
Soares MP, Hamza I (2016) Macrophages and iron metabolism. Immunity 44(3):492–504. https://doi.org/10.1016/j.immuni.2016.02.016
Zhou X, Wang Y (2011) Influence of dietary nano elemental selenium on growth performance, tissue selenium distribution, meat quality, and glutathione peroxidase activity in Guangxi yellow chicken. Poult Sci 90(3):680–686. https://doi.org/10.3382/ps.2011-01921
Rao SVR, Prakash B, Panda AK, Poonam S, Murthy OK (2013) Effect of supplementing organic selenium on performance, carcass traits, oxidative parameters and immune responses in commercial broiler chickens. Asian Australas J Anim Sci 26(2):247–252. https://doi.org/10.5713/ajas.2012.12299
Jing CL, Dong XF, Wang ZM, Liu S, Tong JM (2015) Comparative study of dl-selenomethionine vs sodium selenite and seleno-yeast on antioxidant activity and selenium status in laying hens. Poult Sci 94(5):965–975. https://doi.org/10.3382/ps/pev045
Meng TT, Liu YL, Xie CY, Zhang B, Huang YQ, Zhang YW et al (2018) Effects of different selenium sources on laying performance, egg selenium concentration, and antioxidant capacity in laying hens. Biol Trace Elem Res. https://doi.org/10.1007/s12011-018-1490-z
Anton M (2013) Egg yolk: structures, functionalities and processes. J Sci Food Agric 93(12):2871–2880. https://doi.org/10.1002/jsfa.6247
Kralik Z, Kralik G, Biazik E, Straková E, Suchy P (2013) Effects of organic selenium in broiler feed on the content of selenium and fatty acid profile in lipids of thigh muscle tissue. Acta Vet Brno 82(3):277–282. https://doi.org/10.2754/avb201382030277
Ahmad H, Tian J, Wang J, Khan MA, Wang Y, Zhang L, Wang T (2012) Effects of dietary sodium selenite and selenium yeast on antioxidant enzyme activities and oxidative stability of chicken breast meat. J Agric Food Chem 60(29):7111–7120. https://doi.org/10.1021/jf3017207
Liu CP, Fu J, Xu FP, Wang XS, Li S (2015) The role of heat shock proteins in oxidative stress damage induced by se deficiency in chicken livers. Biometals 28(1):163–173. https://doi.org/10.1007/s10534-014-9812-x
Baylan M, Canogullari S, Ayasan T, Copur G (2011) Effects of dietary selenium source, storage time, and temperature on the quality of quail eggs. Biol Trace Elem Res 143(2):957–964. https://doi.org/10.1007/s12011-010-8912-x
Funding
This research received support from the Qingyuan Technology Program (2018B02) and Guangzhou Tanke Bio-tech Co., Ltd., Guangdong, China.
Author information
Authors and Affiliations
Contributions
Yurong Zhao and Jiahua He designed the experiment; Xue Lin analyzed the data; and Xue Lin, Ting Yang, and Yinli Ji performed the experiments. Xue Lin wrote the manuscript, and Yurong Zhao, Jianhua He, and Hua Li revised it. Xue Lin, Ting Yang, and Yinli Ji contributed to data collection and analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Experiments were carried out in accordance with the ethical guidelines of Hunan Agricultural University for the care and use of laboratory animals.
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, X., Yang, T., Li, H. et al. Interactions Between Different Selenium Compounds and Essential Trace Elements Involved in the Antioxidant System of Laying Hens. Biol Trace Elem Res 193, 252–260 (2020). https://doi.org/10.1007/s12011-019-01701-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01701-x