Abstract
Nutritional immunity describes mechanisms for withholding essential transition metals as well as directing the toxicity of these metals against infectious agents. Zinc is one of these transition elements that are essential for both humans and microbial pathogens. At the same time, Zn can be toxic both for man and microbes if its concentration is higher than the tolerance limit. Therefore a “delicate” balance of Zn must be maintained to keep the immune cells surveilling while making the level of Zn either to starve or to intoxicate the pathogens. On the other hand, the invading pathogens will exploit the host Zn pool for its survival and replication. Apparently, different sets of protein in human and bacteria are involved to maintain their Zn need. Metallothionein (MT)—a group of low molecular weight proteins, is well known for its Zn-binding ability and is expected to play an important role in that Zn balance at the time of active infection. However, the differences in structural, functional, and molecular control of biosynthesis between human and bacterial MT might play an important role to determine the proper use of Zn and the winning side. The current review explains the possible involvement of human and bacterial MT at the time of infection to control and exploit Zn for their need.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To prevent pathogenesis of infectious microorganisms, humans restrict access to essential metals in a process known as nutritional immunity. Broadly, nutritional immunity describes mechanisms for withholding essential transition metals as well as directing the toxicity of these metals against infectious agents. Scope of nutritional immunity has broaden from its original concept of referring to iron (Fe) to include other transition metals such as zinc (Zn), copper (Cu), and manganese (Mn) [1]. While Fe and Cu are known to have redox potential and are involved in large number of oxidoreductases or other electron transfer proteins, Zn plays critical role in structural as well as catalytic proteins both in eukaryotes and prokaryotes [2]. Zn is frequently incorporated into metalloenzymes, storage proteins, and transcription factors and become the second most abundant transition metal in most living systems after Fe. For example, ∼80% enzymes in archaea and bacteria are Zn-containing proteins while those in eukaryotes are ∼50%. However, Zn-binding proteins, including Zn-dependent transcription factors, make up a larger proportion of the total proteome in eukaryotes as compared to bacteria and archaea [3]. Thus, Zn is essential for both humans and microbial pathogens to survive. At the same time, Zn can be toxic if its concentration is higher than the tolerance limit both for man and microbes.
Cells of the human body use a number of sophisticated mechanisms to maintain intracellular and extracellular Zn homeostasis. Role of Zn in life processes has been thoroughly reviewed [4,5,6]. Dietary Zn deficiency results in loss of immune function and resistance to infection suppressing thymic function, T lymphocyte development, lymphocyte proliferation, and T cell-dependent B cell functions [7]. At the same time, to acquire the required amount of Zn in Zn-deficient conditions and to prevent lethal effects of Zn in Zn excess conditions, pathogenic bacteria also use a number of mechanisms to maintain key cellular processes including growth and replication [8,9,10].
Therefore, it is expected that a “delicate” balance must be maintained at the time of infection that in one hand limit Zn availability to the pathogens; at the same time, the Zn level should be good enough to cause toxicity to the pathogens as well activation to the immune cells (Fig. 1). The site of infection might govern the strategy to be adopted by the invading pathogens since Zn availability may vary in different tissues. For example, group A Streptococcus is suggested to face Zn toxicity during colonization of the nasopharynx, but Zn deprivation on the skin [9]. Apparently, different sets of proteins in human and bacteria are involved in maintaining the balance. A family of low molecular weight proteins, namely, metallothionein (MT), a bonafide Zn-binding protein that is ubiquitously present in both prokaryotic and eukaryotic organisms, is also expected to play an important role in that balance, i.e., in nutritional immunity.
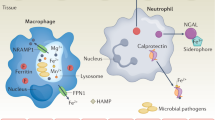
Using zinc at the time of infection. Immune cells of the host require enough Zn supply to maintain immune response against the pathogenic insult. While the Zn availability must be limited to abate survival and proliferation of the pathogens, and at the same time, free Zn must incur toxic insult to the pathogens to kill them
MTs in human are primarily involved in homeostasis of essential metals such as Zn and Cu, and detoxifying of toxic metals, such as Cd and mercury (Hg) [11,12,13,14]. In the last few decades, MT expression in humans was linked with a number of inducers (or initiators) such as heavy metals, endotoxins, cytokines, glucocorticoids (GCs), reactive oxygen species (ROS), and toxic organic compounds [15,16,17,18,19,20]. Expression of MT in human tissues is also induced during different pathological condition [15, 21]. In bacteria, MTs are mainly involved in metal resistance, for example, Cd resistance by Escherichia coli [22] and Salmonella enterica [23], lead resistance by Providencia vermicola [24], and Cu resistance by Mycobacterium tuberculosis [25].
However, the role of MT in the cross talk between human MT and bacterial MT in nutritional immunity more particularly at the time of active infection is largely unknown. The role of bacterial MT in Zn speciation and homeostasis is also largely unknown [10]. The current review will attempt to propose possible involvement of human and bacterial MT at the time of infection to control and exploit Zn with special reference to nutritional immunity. The focus of the current review will be on the synthesis or degradation of MT in response to infectious diseases, the human-MT mediated Zn homeostasis in response to infectious insult, role of MT in directing Zn to activate immune response against the infection, mechanism of exploitation of as well as resistance against host Zn pool by the infectious agents, any possible competition between host (human) MT and bacterial MT for host Zn pool, and host-MT-mediated changes at the site of infection (Fig. 2).
Zn Distribution and Homeostasis in Human
Zinc is widely distributed in various tissues in human with a total amount of 1.4–2.3 g in adults, 85% of which are localized in the muscles and bones, 11% in the skin and liver, and the remaining 4% in other tissues [26]. Highest concentration of Zn is present in the retina and choroid of the eye, followed by the prostate, bones, liver, and kidneys [27,28,29]. Virtually, all Zn is intracellular: 30–40% in the nucleus; 50% in the cytosol, organelles, and specialized vesicles; and the remainder is associated with cell membranes [30]. In human, plasma Zn maintains a homeostatic level of approximately 10–18 mol/L that represents only 0.1% of total body Zn [31]. In human, the global Zn storage is mediated by hormones such as glucagon and epinephrine that in turn can increase MT expression and Zn storage in liver [32].
The number of in vivo Zn-binding proteins in humans was estimated to be 2800, corresponding to 10% of the human proteome. Among these, the most abundant class of Zn-binding proteins is that of Zn fingers, with Cys4 and Cys2-His2 binding coordination [3, 33].
The intracellular homeostasis and distribution of Zn is controlled by specialized sets of proteins: Zn2+ importer family (14 ZIPs, solute-linked transporter (SLC) 39A) and Zn2+ transporter family (10 ZnTs, SLC 30A) transporters [34]. ZnTs generally transport Zn2+ out of the cytosol, whereas ZIPs import them from cellular compartments or the extracellular space into the cytosol [35]. Most ZnTs are present in intracellular compartments, such as endosomes, Golgi, or endoplasmic reticulum, while only ZnT1 appears to be located at the plasma membrane as it is the primary regulator of cellular Zn efflux [36]. Most ZIPs are observed at the plasma membrane; however, Zip7 is located at the Golgi apparatus [37]. The localization of some ZIPs changes according to Zn availability or physiologic conditions. Zip5 has a basolateral plasma membrane orientation in polarized cells during dietary zinc sufficiency [38, 39]. Similarly, ZIP14 is mobilized to the sinusoidal membrane of the mouse hepatocyte during acute inflammation and, therefore, increases zinc uptake as a component of the acute phase response [35, 40].
Zn in Host Immunity Against Infectious Diseases
Zinc regulates an array of developmental and functional aspects of cell-mediated immunity involving neutrophils, NK cells, and macrophages; cytokine production by immune cells; and the growth and function of T and B cells. Zn also mediates protection from the adverse effect of ROS that are produced not only during metabolism but also during inflammatory processes. Free intracellular Zn2+ is essential in extravasation to the site of the infection and uptake and killing of microorganisms by neutrophils [41]. Role of Zn in immunity has been thoroughly reviewed [1, 42,43,44]. A number of important roles of Zn in immunity that are relevant to the focus of the current review are highlighted below.
Zinc modulates the nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) signaling pathway. NF-κB influences the expression of pro-inflammatory cytokines (e.g., interleukin (IL)-1b, IL-6, IL-8, tumor necrosis factor alpha (TNF-α), and MCP-1), chemokines, acute phase proteins (CRP and fibrinogen), matrix metalloproteinases (MMPs), adhesion molecules, growth factors, and other factors involved in inflammatory response, such as COX-2 and iNOS [45, 46]. Zinc importer ZIP8 (SLC39A8) is the most significantly upregulated transporter in response to cytokines, bacteria, and sepsis. ZIP8 increases cytosolic Zn content by promoting extracellular uptake or release from subcellular organelles. The thiol-reactive cytosolic Zn induces NF-κB inhibition downstream from MAPKs; hence, ZIP8-mediated Zn influx works as a negative feedback regulator of NF-κB in response to infection [47, 48].
In human, a number of proteins that exert their antimicrobial effects are Zn dependent. Such as, the cathelicidin LL-37, secretion of which by intestinal epithelial cells is Zn dependent [49], shows antimicrobial activity against Pseudomonas aeruginosa, Staphylococcal species and E. coli, and Candida albicans [50]. Zinc-dependent secretory proteins, namely, human peptidoglycan recognition proteins (PGLYRPs), inhibit many Gram-positive and Gram-negative bacteria [51]. Biological functions of thymulin, a serum Zn-dependent thymus-specific hormone, binds receptors on T cells, induces T cell markers, and promotes allogenic cytotoxicity, suppressor T cell functions, and IL-2 production [52]. Furthermore, Zinc is also crucial for the balance between the different T cell subsets [31, 43, 44]. Paradoxically, the activity of NADPH oxidase, involved in the destruction of pathogens after phagocytosis, may be inhibited by both Zn-deficient and Zn excess conditions.
Inflammatory processes during active infectious stage are associated with remarkable changes in Zn homeostasis. During the active infectious stage, a rapid decrease of the serum Zn level takes place due to its redistribution from plasma into organs, predominantly the liver. An upregulated expression of ZIP14 in liver in response to the pro-inflammatory cytokine IL-6 has been shown to mediate the redistribution [40], thus limit Zn availability for the invading pathogens. Furthermore, the Zn chelation by calprotectin is mostly released by the leukocytes and has been shown to suppress the reproduction of bacteria and C. albicans [53]. At the same time, an increased Zn concentration in macrophages can intoxicate phagocytosed microorganisms [7, 44]. Again, increased intracellular hepatocyte Zn promotes energy metabolism, neutralizes ROS, and guarantees the synthesis of acute phase proteins in the liver [54, 55] that are needed to fight the pathogens (Fig. 2).
Potential roles of MT in nutritional immunity in controlling Zn availability for invading pathogens. Most bacterial pathogens use metalloproteinases to invade host tissue. The invaded tissues are degraded due to apoptosis or necrosis and release Zn. Increased Zn at the site of infection results in upregulated expression of ZIP by the circulating leukocytes or infiltrated inflammatory cells, resulting in upregulated expression of MT through the activation of metal-responsive elements (MREs). These intracellular MTs protect leukocytes from the increased influx of Zn at the site of infections. Inflammatory cytokines released at the site of infection may eventually induce adrenal cortex to release glucocorticoid (GC) hormones. Once GC reached the circulation, MT synthesis in leukocytes can be induced through GRE. Kidney also responds to the inflammatory cytokines and secretes more MT in the circulation. Pathogenic invasion also redistributes Zn from serum to liver. With the increased amount of Zn in the liver, MT biosynthesis in hepatocytes is upregulated. Both hepatic and renal MT may induce bone marrow hematopoietic stem cells to produce more circulatory leukocytes. At the site of infection, macrophages also face increased Zn and reactive oxygen species (ROS) during phagocytosis. Both the Zn and ROS can induce MT biosynthesis. The resulting upregulated MT protects the phagocytes from Zn toxicity as well as minimize the Zn level for the invading pathogens. Depending on the Zn starvation (downward arrow) or Zn excess (upward arrow) condition, invading bacteria may upregulate either Zn-influx or Zn-efflux mechanism, respectively (inset). Some of eh cytoplasmic Zn induce bacterial MT biosynthesis that in turn help the bacteria to maintain required Zn content
Zn reduces the incidence and severity of diarrhea and acute lower respiratory tract infections in infants and children [56, 57] as well as the incidence of Staphylococcus aureus pneumonia, Streptococcus pneumonia tonsillitis, and E. coli urinary tract infections in sickle cell anemia patients [58]. Zn supplementation significantly decreases the incidence of infections in elderly subjects [59]. Moreover, Zn augments monocyte adhesion to endothelial cells in vitro and affects production of pro-inflammatory cytokines, such as IL-1b, IL-6, and TNF-α. Zn aids NK cells to recognize major histocompatibility complex (MHC) class I, and the lytic activity. In vitro, moderate Zn supplementation increases the differentiation of CD34+ cells toward NK cells and their cytotoxic activity.
Immune suppression in Zn-deficient conditions is well documented with an increased susceptibility to various infectious agents, including F. tularensis [60], Listeria monocytogenes, Salmonella enteritidis, M. tuberculosis, and many viruses, protozoan parasites, and eukaryotes [7, 61,62,63]. A delayed production of protective antibodies in Zn-deficient condition has been reiterated.
Zn in Pathogenesis and Virulence
Zinc is essential to the survival of a pathogen in the host. Bacteria are predicted to incorporate Zn into 5–6% of all proteins [3]. A number of Zn-dependent virulence factors contribute to the survival and pathogenesis of the invading bacteria (Table 1).
Zinc-dependent microbial metalloproteases are a group of well-documented virulence factors and one of the four major groups of extracellular proteases. The other three groups of microbial proteases include serine proteases (EC 3.4.21), cysteine (or thiol) proteases (EC 3.4.22), and aspartate proteases (EC 3.4.23). Metalloprotease typically exhibits broad proteolytic specificity that facilitates the pathogen to disrupt physiological barriers to invade host, degrade key signaling intermediates, and release metals from host metalloproteins. Cytokines or interleukins that are important for the neutrophils and macrophage recruitment at the site of infection can be disrupted by bacterial metalloproteases to avoid immune clearance. A list of well-known virulence factors that belong to the Zn-dependent bacterial metalloproteases are presented in Table 1. These virulence factors augment pathogenesis of the respective pathogens in various ways.
Zn as a Regulator of MT Biosynthesis and Induction
In human cells, MT biosynthesis is regulated by metal (MRE), antioxidant (ARE), and glucocorticoid (GRE) response elements. Thus, the divalent trace elements such as Zn, ROS, and stress hormones such as GC are potent MT inducers in human cells [88,89,90,91]. Zn has a direct impact on the MT biosynthesis and induction. Zn binds MRE-binding transcription factors (MTFs) and activates MRE. After Zn occupancy, MTF-1 binds specifically to the MRE sequence to initiate transcription of MT genes. The requirement of additional Zn for the binding of the MTF-1 with its promoter in cell-free system attests the definitive role of Zn in MT biosynthesis [90, 92,93,94]. Induction of MT by other elements such as Cd and Cu [90, 95,96,97] is also Zn dependent as these metals displace Zn from the Zn-containing protein which in turn allows the free Zn to induce MT expression [98, 99].
A number of steps might be involved in Cu- and Cd-induced expressions of MT genes. Firstly, Cd and Cu may displace Zn from the binding sites of Zn-containing metalloproteins including MT. Subsequently, free Zn may bind to the Zn finger of MTF-1 and regulate the expression of MT gene [98, 99]. GRE within the promoter region of the MT gene can act independently to induce MT transcription in the presence of GC, a stress hormone [100,101,102]. ARE also plays an important role in the induced expression of MT in response to ROS, such as hydrogen peroxide [100, 103]. Notably, both GC and ROS are increased in an active infectious state. Furthermore, maintaining the physiological concentrations of Zn is necessary to avoid oxidative stress, since both Zn deficiency and Zn overload are pro-oxidant conditions [104].
Differences Between Human and Bacterial MT
To date, four major isoforms, namely, MT-1, MT-2, MT-3, and MT-4, have been identified in human. In human, MT-1 and MT-2 were detected in all organs [105, 106]; MT-3 in the brain, lung, kidney, and reproductive organs [107,108,109,110,111]; and MT-4 in differentiating stratified squamous epithelial cells [112]. In human, eight functional MT-1 isogenes have been identified, namely, MT-1A, MT-1B, MT-1E, MT-1F, MT-1G, MT-1H, MT-1M, and MT-1X [113, 114].
The very first MT-like proteins in bacteria were identified in the marine cyanobacterium Synechococcus sp. (strain RRIMP N1) and later in the freshwater strain Synechococcus TX-20 [91, 115]. These bacterial MTs were considerably different from human MTs, as they contain aromatic amino acid His [116]. Bacterial MTs do not have any significant sequence homology with human MTs, except for a high Cys content. In 1990s, the gene for MT from Synechococcus PCC7942) was sequenced (SmtA), along with the gene for the metal-responsive transcription factor SmtB, and the operator-promoter region between the two genes [117, 118]. A glutathione S-transferase (GST)-fusion protein of SmtA expressed in E. coli revealed that SmtA is capable of binding Zn2+, Cd2+, Cu2+, and Hg2+ [118].
The determination of the pH values for half-displacement of bound metal suggested for SmtA in comparison with mammalian MTs a relatively higher affinity for Zn2+ and a relatively lower affinity for Cd2+. Later, it was confirmed that the purified SmtA binds four Zn2+ or Cd2+, where nine Cys residues participate in the metal binding [119]. In 2008, Gold et al. (2008) reported a copper-binding MT (MymT), of ∼5 kDa, in M. tuberculosis expression of which can be induced by other metals such as Zn, Cd, Co, and Ni [120]. The MymT can also be induced by nitrosative and oxidative stress, as well as mildly acidic conditions and cell wall perturbation.
The inorganic core of SmtA strongly resembles the Zn4Cys11 cluster of mammalian MT, despite different amino acid sequences. In SmtA, four Zn2+ binds in a Zn4Cys9His2 cluster. InSmtA, the two ZnCys3His sites and one of the ZnCys4 sites readily exchange Zn2+ for exogenous Cd2+, while the remaining ZnCys4 remains inert [121]. This metal binding behavior of SmtA is different from most of the MTs where metal binding is generally kinetically labile [122, 123]. Moreover, “all-cysteine” MTs bind Cd2+ 10 times more strongly than Zn2+, and stoichiometric amounts of Cd2+ are usually sufficient to displace all of the Zn2+ from MT under similar conditions to those used here [122].
Fighting for Zn: Man vs. Microbes
The human body is a rich reservoir of Zn and needs to maintain Zn homeostasis within the physiological range (as discussed earlier). A number of microbial pathogens have evolved to exploit the Zn reserve in different organs of the human body. As a countermeasure, human body uses a number of defense mechanism to limit the availability of free Zn but also to maintain Zn homeostasis. Zn-binding proteins in eukaryotes, namely, psoriasin [124], calgranulin C [125, 126] and calprotectin [127,128,129], exert their antimicrobial potential through Zn2+ chelation. The same families of proteins also have pro-inflammatory properties causing inflammation-mediated pathologies [130]. Ironically, a number of pathogens such as S. enterica [131, 132], Campylobacter jejuni [133], Haemophilus influenzae [134], L. monocytogenes [8], and Streptococcus pneumoniae [114] can counteract the antimicrobial potential of those proteins in different ways.
In an active infectious state, neutrophils that are recruited at the site of infection secrete calprotectin, which mainly binds Zn and Mn [135, 136], thus make the Zn unavailable for the microbial invaders. Interestingly, Neisseria meningitidis was shown to scavenge calprotectin-chelated Zn, thus evade neutrophil-mediated killing [137]. Again, detection of the bacterial invaders via lipopolysaccharide can induce IL-6 expression, which in turn increases MT expression and reduces free Zn2+ [138, 139]. Paradoxically, GC signaling can either induce MT biosynthesis, hence reducing the free Zn2+ or induce Zn2+ secretion from pancreatic cells aiding microbial Zn2+ feast [94]. MT controls human matrix metalloproteinases (MMPs) by regulating Zn [140]. Host-derived MMP controls influx of effector cells, killing of pathogens, resolution of inflammation, and remodeling of extracellular matrix [141].
Bacterial pathogen uses three strategies to combat host-imposed Zn starvation or poisoning: (i) transcriptional regulation by metal-sensing metalloregulatory proteins; (ii) Zn efflux and acquisition across cell membranes; and (iii) Zn sparing (increase the expression of non-Zn-requiring proteins to replace essential Zn-dependent enzymes and proteins), and allocation of Zn to Zn-requiring enzymes, processes that are governed by Zn speciation in the cytoplasm [10]. An invading pathogen acquires host Zn mostly (90%) from skeletal muscle and bone and for the rest from the liver and kidneys [142, 143]. Intracellular Zn in these tissues is present at 100–500 μM, a large portion of which is bound to MTs [144, 145]. Only a small part of the total body Zn, i.e., 0.1%, is present in blood serum (1.25 μg/mL serum) that are bound to albumin (73–91%), macroglobin (9–27%), or various serum proteins and amino acids (2–8%) [146,147,148].
In bacterial cells, the total cell-associated Zn is in the millimolar range; however, the bioavailable Zn in the bacterial cytoplasm is predicted to be in the picomolar to nanomolar range [10, 149]. In one hand, Zn buffering between this 106-fold concentration difference tells the overcapacity of bacterial cell to chelate Zn, while the mechanism of which remains unknown. Many bacterial pathogens such as L. monocytogenes, S. enterica, Brucella abortus, and Yersinia pestis depend on the ATP-binding cassette (ABC) transporters to acquire Zn from human host [8, 131, 150]. These ABC transporters, common across Gram-positive and Gram-negative bacteria [151], contains three components: the periplasmic binding protein ZnuA binds a single Zn2+ with high affinity, the ZnuB permease that actively transports Zn through the inner membrane, and the ZnuC ATPase provides energy by ATP hydrolysis [152, 153]. In contrast to Zn starvation condition, bacteria such as M. tuberculosis uses Zn-efflux pumps to survive in macrophages [154]. Using liver homogenate, Choudhuri et al. (1992) showed that at a lysosomal pH of around 4.7, about 60% of Zn can be displaced from MT, thereby making it susceptible to degradation [155]. Hence, an increasing Zn excess condition due to its release from MT and the subsequent degradation of the apo-MT might overthrow the Zn-efflux pumps of the invading pathogen. In a severely Zn starvation condition, the Zn-free (apo) form of Zn uptake repressor (Zur) of most bacteria shows low affinity for the operator and overlapping promoter regions of high-affinity Zn uptake system(s) [10]. In addition, the Zn efflux systems are repressed by the apo form of the Zn efflux repressor, ZntR in Zn-limited conditions. With an increased bioavailable Zn, the Zn-bound form of Zur binds to the operator site, thus preventing transcription of the Zn uptake systems [156]. Likewise, the efflux regulator, ZntR, binds Zn (in Zn-excess condition) and allosterically activates transcription of Zn-specific P-type ATPase efflux transporter (zntA) [157].
Metal-specific outer membrane also functions in Zn uptake in Gram-negative bacteria [158, 159]. For example, Neisseria ZnuD might be capable of transporting free, hydrated Zn2+, as suggested by the structural and computational studies [159]. In an escalation in the Zn acquisition “arms race” between microbe and host, an outer-membrane porin-designated CbpA, a candidate bacterial receptor for CP-Zn complexes, is thought to capture this CP-bound Zn, consistent with a direct role in Zn piracy [137].
Competition Between Host MT and Bacterial MT for Host-Zn Pool
While Zn in human MTs is bound to Cys residues, the same in bacterial MTs can be bound to Cys and aromatic amino acid, His [119]. MT binds Zn exceptionally strongly owing to the exclusive coordination of the metal with cysteine sulfur ligands (stability constant of Zn7MT-2 = 3.2 × 1013 M−1 at pH 7.4). Again, the Zn-binding constants of most of the enzymes studied are at least 1000 times lower than that of MT [160]. Commonly, Zn2+ forms tetrahedral complex involving His, Glu or Asp, and Cys, in metalloproteins. The side chains of residues are capable of binding one or two Zn2+ [161, 162]. Notably, up to 20% of intracellular Zn are complexed by MTs [163, 164]. In mammalian MT, Zn2+ are bound tetrahedrally to Cys in both domains. Zn-S cluster with in MT is very sensitive to changes of cellular redox state. Therefore, a shift to more oxidizing environment releases Zn from MT, whereas a shift to more reducing environment leads Zn binding to apo-MT [165, 166]. Thus, Zn2+, only rapidly released by MTs, is able to play its relevant function against oxidative stress and participate in immune responses.
In healthy human serum, MT-1 plus MT-2 (MT-1/2) concentration (n = 200) could be as low as 10 ng/mL and as high as >90 ng/mL [167]. Earlier, it was reported that the MT-1/2 concentration in human serum could be in the range of 10–30 ng/mL [168] with an average of 23 ± 4.6 ng/mL [169]. However, an increased level of MT-1/2 was detected in various liver diseases such as chronic hepatitis [167].
Changes of Host-MT Expression in Response to Infectious Diseases
Bacterial Infection and MT Expression
A number of evidence has shown the link between the MT expression in different human organs in relation to bacterial infectious diseases. Given the fact that there is instant increase of hepatic MT expression in response to bacterial infection, an effect that is generally mediated by endotoxin (lipopolysaccharide (LPS)), leads to classify MTs as acute phase proteins [170, 171]. Bacterial lipopolysaccharide-induced MT overexpression in liver is often mediated by pro-inflammatory cytokines, including IL-1, IL-6, TNF-α, interferon (IFN)-γ [172], nitric oxide [173], and the stress hormone glucocorticoids [174].
MT expression in inflammatory bowel diseases (IBD) is somewhat inconclusive. In organ biopsies of the IBD patients, MT expression was generally lower, such as in ulcerative colitis and Crohn’s disease, compared to the control specimens [175,176,177,178]. However, MT overexpression was observed in fibroblasts and intestinal epithelial cells of ulcerative and fissural lesions in ulcerative colitis and Crohn’s disease [179]. Since MT expression depends on the time and degree of inflammation as well as on the tissue of origin, hence the inconsistencies could be explained by different sampling [170].
Viral Infection and MT Expression
O’Connor et al. (2014) reported a significant upregulation of MT genes when compared to the IFN-stimulated genes in hepatitis-C virus-infected liver biopsies of IFNL-3 rs8099917 responders [180]. Fibrosis scores were also inversely correlated with MT levels in the liver biopsies. The higher MT expression in the responders was seen as reason for the improved HCV clearance, hence was linked with clinical relevance. In a murine experimental model of coxsackievirus infection, MT expression was increased by fivefold (P < 0.01) in liver and kidneys, and in spleen by 34% (P < 0.05) [181].
Role of Host MT in Directing Zn to Activate Immune Response Against the Infection
Proliferation of lymphocytes in the presence of concanavalin A or lipopolysaccharides [182,183,184], and proliferation of cytotoxic T lymphocytes (CTLs) in mixed lymphocyte reactions, can be augmented by MT [185]. The exo-MT was suggested to facilitate the proliferation of immature T cells, but suppress their terminal differentiation [185].
Macrophages treated with the in vitro exo-MT produce superoxide through respiratory burst to destroy antigen [183]. In PBL, ROS is produced during respiratory burst as a self-defense mechanism [186,187,188]. In PBL, pre-synthesized MTs from their precursors or freshly synthesized MT induced by the dietary Zn [95, 189] provide protection against apoptosis, necrosis, or DNA breakdown caused by ROS. Zn supplementation may also help to prevent oxidative damage of DNA due to arsenic exposure by induction of MT expression [190, 191]. Furthermore, transportation of MT to the cell membrane is necessary for their immunoregulatory properties, where Zn is involved in transporting MT to the cell membrane and regulating T cell [192].
Summary: Host Winning Factor in Zn Regulation Using MT
Fighting for the Zn in nutritional immunity using MT offers a number of advantages for the human host. For example, (i) the number of Zn atoms bound per MT is higher in human MT (seven Zn) compared to that of bacterial MT (four Zn); (ii) exchange of Zn between free Zn2+ and MT-bound Zn is thermodynamically favorable for human MT, as at least one MT-bound Zn in bacterial MT is unlikely to be released; (iii) at the time of infection, human MT synthesis can be upregulated by a number of infection related responses such as ROS, and GC; (iv) bioavailable Zn2+ in bacterial cells remains in picomolar to nanomolar range, while in immune cells such as lymphocytes and macrophages, that amount may range in micromolar level; and (v) in response to infection resulting in the redistribution of Zn, upregulated MT biosynthesis is not limited to the site of infection but can be observed by number of organs such as kidney and liver. Thus, it is expected that at the time of active infection, upregulated biosynthesis of human MT might play a major role in nutritional immunity.
References
Hood MI, Skaar EP (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi:10.1038/nrmicro2836
Andreini C, Bertini I, Cavallaro G et al (2008) Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem 13:1205–1218. doi:10.1007/s00775-008-0404-5
Andreini C, Banci L, Bertini I, Rosato A (2006) Zinc through the three domains of life. J Proteome Res 5:3173–3178. doi:10.1021/pr0603699
Fischer Walker C, Black RE (2004) Zinc and the risk for infectious disease. Annu Rev Nutr 24:255–275. doi:10.1146/annurev.nutr.23.011702.073054
Kulkarni H, Mamtani M, Patel A (2012) Roles of zinc in the pathophysiology of acute diarrhea. Curr Infect Dis Rep 14:24–32. doi:10.1007/s11908-011-0222-8
Jarosz M, Olbert M, Wyszogrodzka G et al (2017) Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling Inflammopharmacology 25:11–24. doi:10.1007/s10787-017-0309-4
Shankar AH, Prasad AS (1998) Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 68:447S–463S
Corbett D, Wang J, Schuler S et al (2012) Two zinc uptake systems contribute to the full virulence of Listeria monocytogenes during growth in vitro and in vivo. Infect Immun 80:14–21. doi:10.1128/IAI.05904-11
Ong CY, Gillen CM, Barnett TC et al (2014) An antimicrobial role for zinc in innate immune defense against group A streptococcus. J Infect Dis 209:1500–1508. doi:10.1093/infdis/jiu053
Capdevila DA, Wang J, Giedroc DP (2016) Bacterial strategies to maintain zinc metallostasis at the host-pathogen interface. J Biol Chem 291:20858–20868. doi:10.1074/jbc.R116.742023
Carpene E, Andreani G, Isani G (2007) Metallothionein functions and structural characteristics. J Trace Elem Med Biol 21(Suppl 1):35–39. doi:10.1016/j.jtemb.2007.09.011
Nordberg M, Nordberg GF (2000) Toxicological aspects of metallothionein. Cell Mol Biol (Noisy-le-grand) 46:451–463
Rigby Duncan KE, Stillman MJ (2006) Metal-dependent protein folding: metallation of metallothionein. J Inorg Biochem 100:2101–2107. doi:10.1016/j.jinorgbio.2006.09.005
Vasak M (2005) Advances in metallothionein structure and functions. J Trace Elem Med Biol 19:13–17. doi:10.1016/j.jtemb.2005.03.003
Chang X, Jin T, Chen L et al (2009) Metallothionein I isoform mRNA expression in peripheral lymphocytes as a biomarker for occupational cadmium exposure. Exp Biol Med (Maywood) 234:666–672. doi:10.3181/0811-RM-336
Karin M, Herschman HR (1980) Glucocorticoid hormone receptor mediated induction of metallothionein synthesis in HeLa cells. J Cell Physiol 103:35–40. doi:10.1002/jcp.1041030106
Karin M, Imbra RJ, Heguy A, Wong G (1985) Interleukin 1 regulates human metallothionein gene expression. Mol Cell Biol 5:2866–2869
Nourani MR, Ebrahimi M, Roudkenar MH et al (2011) Sulfur mustard induces expression of metallothionein-1A in human airway epithelial cells. Int J Gen Med 4:413–419. doi:10.2147/IJGM.S17916
Phillippi JA, Klyachko EA, Kenny JP 4th et al (2009) Basal and oxidative stress-induced expression of metallothionein is decreased in ascending aortic aneurysms of bicuspid aortic valve patients. Circulation 119:2498–2506. doi:10.1161/CIRCULATIONAHA.108.770776
Yamada H, Koizumi S (1991) Metallothionein induction in human peripheral blood lymphocytes by heavy metals. Chem Biol Interact 78:347–354
Boonprasert K, Ruengweerayut R, Aunpad R et al (2012) Expression of metallothionein isoforms in peripheral blood leukocytes from Thai population residing in cadmium-contaminated areas. Environ Toxicol Pharmacol 34:935–940. doi:10.1016/j.etap.2012.08.002
Khan Z, Nisar MA, Hussain SZ et al (2015) Cadmium resistance mechanism in Escherichia coli P4 and its potential use to bioremediate environmental cadmium. Appl Microbiol Biotechnol 99:10745–10757. doi:10.1007/s00253-015-6901-x
Khan Z, Rehman A, Hussain SZ et al (2016) Cadmium resistance and uptake by bacterium, Salmonella enterica 43C, isolated from industrial effluent. AMB Express 6:54. doi:10.1186/s13568-016-0225-9
Sharma J, Shamim K, Dubey SK, Meena RM (2017) Metallothionein assisted periplasmic lead sequestration as lead sulfite by Providencia vermicola strain SJ2A. Sci Total Environ 579:359–365. doi:10.1016/j.scitotenv.2016.11.089
Rowland JL, Niederweis M (2012) Resistance mechanisms of Mycobacterium tuberculosis against phagosomal copper overload. Tuberculosis (Edinb) 92:202–210. doi:10.1016/j.tube.2011.12.006
Calesnick B, Dinan AM (1988) Zinc deficiency and zinc toxicity. Am Fam Physician 37:267–270
Tipton IH, Schroeder HA, Perry HMJ, Cook MJ (1965) Trace elements in human tissue. 3. Subjects from Africa, the Near and Far East and Europe. Health Phys 11:403–451
Karcioglu ZA (1982) Zinc in the eye. Surv Ophthalmol 27:114–122
Karcioglu ZA, Stout R, Hahn HJ (1984) Serum zinc levels in retinitis pigmentosa. Curr Eye Res 3:1043–1048
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
Foster M, Samman S (2012) Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients 4:676–694. doi:10.3390/nu4070676
Cousins RJ, Dunn MA, Leinart AS et al (1986) Coordinate regulation of zinc metabolism and metallothionein gene expression in rats. Am J Phys 251:E688–E694
Andreini C, Banci L, Bertini I, Rosato A (2006) Counting the zinc-proteins encoded in the human genome. J Proteome Res 5:196–201. doi:10.1021/pr050361j
Lichten LA, Cousins RJ (2009) Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29:153–176. doi:10.1146/annurev-nutr-033009-083312
Cousins RJ, Liuzzi JP, Lichten LA (2006) Mammalian zinc transport, trafficking, and signals. J Biol Chem 281:24085–24089. doi:10.1074/jbc.R600011200
Palmiter RD, Findley SD (1995) Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J 14:639–649
Huang L, Kirschke CP, Zhang Y, Yu YY (2005) The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J Biol Chem 280:15456–15463. doi:10.1074/jbc.M412188200
Wang F, Kim B-E, Petris MJ, Eide DJ (2004) The mammalian Zip5 protein is a zinc transporter that localizes to the basolateral surface of polarized cells. J Biol Chem 279:51433–51441. doi:10.1074/jbc.M408361200
Dufner-Beattie J, Kuo Y-M, Gitschier J, Andrews GK (2004) The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem 279:49082–49090. doi:10.1074/jbc.M409962200
Liuzzi JP, Lichten LA, Rivera S et al (2005) Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A 102:6843–6848. doi:10.1073/pnas.0502257102
Hasan R, Rink L, Haase H (2016) Chelation of free Zn(2)(+) impairs chemotaxis, phagocytosis, oxidative burst, degranulation, and cytokine production by neutrophil granulocytes. Biol Trace Elem Res 171:79–88. doi:10.1007/s12011-015-0515-0
Prasad AS (2008) Zinc in human health: effect of zinc on immune cells. Mol Med 14:353–357. doi:10.2119/2008-00033.Prasad
Bonaventura P, Benedetti G, Albarede F, Miossec P (2015) Zinc and its role in immunity and inflammation. Autoimmun Rev 14:277–285. doi:10.1016/j.autrev.2014.11.008
Haase H, Rink L (2014) Zinc signals and immune function. Biofactors 40:27–40. doi:10.1002/biof.1114
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1:a001651. doi:10.1101/cshperspect.a001651
Hayden MS, Ghosh S (2014) Regulation of NF-kappaB by TNF family cytokines. Semin Immunol 26:253–266. doi:10.1016/j.smim.2014.05.004
Liu M-J, Bao S, Galvez-Peralta M et al (2013) ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB. Cell Rep 3:386–400. doi:10.1016/j.celrep.2013.01.009
Galvez-Peralta M, Wang Z, Bao S et al (2014) Tissue-specific induction of mouse ZIP8 and ZIP14 divalent cation/bicarbonate symporters by, and cytokine response to, inflammatory signals. Int J Toxicol 33:246–258. doi:10.1177/1091581814529310
Talukder P, Satho T, Irie K et al (2011) Trace metal zinc stimulates secretion of antimicrobial peptide LL-37 from Caco-2 cells through ERK and p38 MAP kinase. Int Immunopharmacol 11:141–144. doi:10.1016/j.intimp.2010.10.010
Gordon YJ, Huang LC, Romanowski EG et al (2005) Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res 30:385–394. doi:10.1080/02713680590934111
Wang M, Liu L-H, Wang S et al (2007) Human peptidoglycan recognition proteins require zinc to kill both gram-positive and gram-negative bacteria and are synergistic with antibacterial peptides. J Immunol 178:3116–3125
Beck FW, Kaplan J, Fine N et al (1997) Decreased expression of CD73 (ecto-5′-nucleotidase) in the CD8+ subset is associated with zinc deficiency in human patients. J Lab Clin Med 130:147–156
Sohnle PG, Hunter MJ, Hahn B, Chazin WJ (2000) Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14). J Infect Dis 182:1272–1275. doi:10.1086/315810
Powanda MC, Cockerell GL, Pekarek RS (1973) Amino acid and zinc movement in relation to protein synthesis early in inflammation. Am J Phys 225:399–401
Haase H, Rink L (2009) The immune system and the impact of zinc during aging. Immun Ageing 6:9. doi:10.1186/1742-4933-6-9
Sazawal S, Black RE, Bhan MK et al (1995) Zinc supplementation in young children with acute diarrhea in India. N Engl J Med 333:839–844. doi:10.1056/NEJM199509283331304
Sazawal S, Black RE, Jalla S et al (1998) Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and preschool children: a double-blind, controlled trial. Pediatrics 102:1–5
Prasad AS, Beck FW, Kaplan J et al (1999) Effect of zinc supplementation on incidence of infections and hospital admissions in sickle cell disease (SCD). Am J Hematol 61:194–202
Prasad AS, Beck FWJ, Bao B et al (2007) Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr 85:837–844
Celli J, Zahrt TC (2013) Mechanisms of Francisella tularensis intracellular pathogenesis. Cold Spring Harb Perspect Med 3:1–14. doi:10.1101/cshperspect.a010314
Coghlan LG, Carlomagno MA, McMurray DN (1988) Effect of protein and zinc deficiencies on vaccine efficacy in guinea pigs following pulmonary infection with Listeria. Med Microbiol Immunol 177:255–263
Kidd MT, Qureshi MA, Ferket PR, Thomas LN (1994) Dietary zinc-methionine enhances mononuclear-phagocytic function in young turkeys. Zinc-methionine, immunity, and Salmonella. Biol Trace Elem Res 42:217–229
McMurray DN, Bartow RA, Mintzer CL, Hernandez-Frontera E (1990) Micronutrient status and immune function in tuberculosis. Ann N Y Acad Sci 587:59–69
Chang AK, Kim HY, Park JE et al (2005) Vibrio vulnificus secretes a broad-specificity metalloprotease capable of interfering with blood homeostasis through prothrombin activation and fibrinolysis. J Bacteriol 187:6909–6916. doi:10.1128/JB.187.20.6909-6916.2005
Kooi C, Subsin B, Chen R et al (2006) Burkholderia cenocepacia ZmpB is a broad-specificity zinc metalloprotease involved in virulence. Infect Immun 74:4083–4093. doi:10.1128/IAI.00297-06
Grimwood BG, Plummer THJ, Tarentino AL (1994) Purification and characterization of a neutral zinc endopeptidase secreted by Flavobacterium meningosepticum. Arch Biochem Biophys 311:127–132. doi:10.1006/abbi.1994.1217
Tarentino AL, Quinones G, Grimwood BG et al (1995) Molecular cloning and sequence analysis of flavastacin: an O-glycosylated prokaryotic zinc metalloendopeptidase. Arch Biochem Biophys 319:281–285. doi:10.1006/abbi.1995.1293
Miyoshi N, Shimizu C, Miyoshi S, Shinoda S (1987) Purification and characterization of Vibrio vulnificus protease. Microbiol Immunol 31:13–25
Elgaml A, Miyoshi S-I (2017) Regulation systems of protease and hemolysin production in Vibrio vulnificus. Microbiol Immunol 61:1–11. doi:10.1111/1348-0421.12465
Jin F, Matsushita O, Katayama S et al (1996) Purification, characterization, and primary structure of Clostridium perfringens lambda-toxin, a thermolysin-like metalloprotease. Infect Immun 64:230–237
Morihara K (1964) Production of elastase and proteinase by Pseudomonas aeruginosa. J Bacteriol 88:745–757
Heck LW, Alarcon PG, Kulhavy RM et al (1990) Degradation of IgA proteins by Pseudomonas aeruginosa elastase. J Immunol 144:2253–2257
Myers LL, Firehammer BD, Shoop DS, Border MM (1984) Bacteroides fragilis: a possible cause of acute diarrheal disease in newborn lambs. Infect Immun 44:241–244
Moncrief JS, Obiso RJ, Barroso LA et al (1995) The enterotoxin of Bacteroides fragilis is a metalloprotease. Infect Immun 63:175–181
Wu S, Lim KC, Huang J et al (1998) Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci U S A 95:14979–14984
Plaut AG, Genco RJ, Tomasi TBJ (1974) Isolation of an enzyme from Streptococcus sanguis which specifically cleaves IgA. J Immunol 113:589–591
Molla A, Matsumoto K, Oyamada I et al (1986) Degradation of protease inhibitors, immunoglobulins, and other serum proteins by Serratia protease and its toxicity to fibroblast in culture. Infect Immun 53:522–529
Kerr MA, Loomes LM, Senior BW (1995) Cleavage of IgG and IgA in vitro and in vivo by the urinary tract pathogen Proteus mirabilis. Adv Exp Med Biol 371A:609–611
Loomes LM, Kerr MA, Senior BW (1993) The cleavage of immunoglobulin G in vitro and in vivo by a proteinase secreted by the urinary tract pathogen Proteus mirabilis. J Med Microbiol 39:225–232. doi:10.1099/00222615-39-3-225
Brezski RJ, Jordan RE (2010) Cleavage of IgGs by proteases associated with invasive diseases: an evasion tactic against host immunity? MAbs 2:212–220
Warfel JM, Steele AD, D’Agnillo F (2005) Anthrax lethal toxin induces endothelial barrier dysfunction. Am J Pathol 166:1871–1881. doi:10.1016/S0002-9440(10)62496-0
Smith H, Stanley JL (1962) Purification of the third factor of anthrax toxin. J Gen Microbiol 29:517–521. doi:10.1099/00221287-29-3-517
Leppla SH, Arora N, Varughese M (1999) Anthrax toxin fusion proteins for intracellular delivery of macromolecules. J Appl Microbiol 87:284
Smith H (2002) Discovery of the anthrax toxin: the beginning of studies of virulence determinants regulated in vivo. Int J Med Microbiol 291:411–417
Kastrup CJ, Boedicker JQ, Pomerantsev AP et al (2008) Spatial localization of bacteria controls coagulation of human blood by “quorum acting”. Nat Chem Biol 4:742–750
Mintz CS, Miller RD, Gutgsell NS, Malek T (1993) Legionella pneumophila protease inactivates interleukin-2 and cleaves CD4 on human T cells. Infect Immun 61:3416–3421
Chung M-C, Popova TG, Millis BA et al (2006) Secreted neutral metalloproteases of Bacillus anthracis as candidate pathogenic factors. J Biol Chem 281:31408–31418. doi:10.1074/jbc.M605526200
Davis SR, Cousins RJ (2000) Metallothionein expression in animals: a physiological perspective on function. J Nutr 130:1085–1088
Ruttkay-Nedecky B, Nejdl L, Gumulec J et al (2013) The role of metallothionein in oxidative stress. Int J Mol Sci 14:6044–6066. doi:10.3390/ijms14036044
Selvaraj A, Balamurugan K, Yepiskoposyan H et al (2005) Metal-responsive transcription factor (MTF-1) handles both extremes, copper load and copper starvation, by activating different genes. Genes Dev 19:891–896. doi:10.1101/gad.1301805
Olafson RW, Abel K, Sim RG (1979) Prokaryotic metallothionein: preliminary characterization of a blue-green alga heavy metal-binding protein. Biochem Biophys Res Commun 89:36–43
Westin G, Schaffner W (1988) A zinc-responsive factor interacts with a metal-regulated enhancer element (MRE) of the mouse metallothionein-I gene. EMBO J 7:3763–3770
Palmiter RD (1994) Regulation of metallothionein genes by heavy metals appears to be mediated by a zinc-sensitive inhibitor that interacts with a constitutively active transcription factor, MTF-1. Proc Natl Acad Sci U S A 91:1219–1223. doi:10.1073/pnas.91.4.1219
Guo L, Lichten LA, Ryu M-S et al (2010) STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells. Proc Natl Acad Sci U S A 107:2818–2823. doi:10.1073/pnas.0914941107
Chang X-L, Jin T-Y, Zhou Y-F (2006) Metallothionein 1 isoform gene expression induced by cadmium in human peripheral blood lymphocytes. Biomed Environ Sci 19:104–109
Jonai H, Yamada H, Suzuki K et al (1992) Estimation of metallothionein synthesis in cadmium-exposed human lymphocytes by gel electrophoresis and silver staining. Ind Health 30:129–137
Smirnova IV, Bittel DC, Ravindra R et al (2000) Zinc and cadmium can promote rapid nuclear translocation of metal response element-binding transcription factor-1. J Biol Chem 275:9377–9384
Waldron KJ, Rutherford JC, Ford D, Robinson NJ (2009) Metalloproteins and metal sensing. Nature 460:823–830. doi:10.1038/nature08300
Zhang B, Georgiev O, Hagmann M et al (2003) Activity of metal-responsive transcription factor 1 by toxic heavy metals and H2O2 in vitro is modulated by metallothionein. Mol Cell Biol 23:8471–8485
Haq F, Mahoney M, Koropatnick J (2003) Signaling events for metallothionein induction. Mutat Res 533:211–226
Kelly EJ, Sandgren EP, Brinster RL, Palmiter RD (1997) A pair of adjacent glucocorticoid response elements regulate expression of two mouse metallothionein genes. Proc Natl Acad Sci U S A 94:10045–10050
Quaife C, Hammer RE, Mottet NK, Palmiter RD (1986) Glucocorticoid regulation of metallothionein during murine development. Dev Biol 118:549–555
Dalton T, Palmiter RD, Andrews GK (1994) Transcriptional induction of the mouse metallothionein-I gene in hydrogen peroxide-treated Hepa cells involves a composite major late transcription factor/antioxidant response element and metal response promoter elements. Nucleic Acids Res 22:5016–5023
Maret W (2000) The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr 130:1455S–1458S
Coyle P, Philcox JC, Carey LC, Rofe AM (2002) Metallothionein: the multipurpose protein. Cell Mol Life Sci 59:627–647
Moffatt P, Denizeau F (1997) Metallothionein in physiological and physiopathological processes. Drug Metab Rev 29:261–307
Garrett SH, Sens MA, Todd JH et al (1999) Expression of MT-3 protein in the human kidney. Toxicol Lett 105:207–214
Moffatt P, Seguin C (1998) Expression of the gene encoding metallothionein-3 in organs of the reproductive system. DNA Cell Biol 17:501–510. doi:10.1089/dna.1998.17.501
Neal JW, Singhrao SK, Jasani B, Newman GR (1996) Immunocytochemically detectable metallothionein is expressed by astrocytes in the ischaemic human brain. Neuropathol Appl Neurobiol 22:243–247
Suzuki K, Nakajima K, Otaki N, Kimura M (1994) Metallothionein in developing human brain. Biol Signals 3:188–192
Werynska B, Pula B, Muszczynska-Bernhard B et al (2013) Expression of metallothionein-III in patients with non-small cell lung cancer. Anticancer Res 33:965–974
Quaife CJ, Findley SD, Erickson JC et al (1994) Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry 33:7250–7259
Mao J, Yu H, Wang C et al (2012) Metallothionein MT1M is a tumor suppressor of human hepatocellular carcinomas. Carcinogenesis 33:2568–2577. doi:10.1093/carcin/bgs287
Moleirinho A, Carneiro J, Matthiesen R et al (2011) Gains, losses and changes of function after gene duplication: study of the metallothionein family. PLoS One 6:e18487. doi:10.1371/journal.pone.0018487
Olafson RW, McCubbin WD, Kay CM (1988) Primary- and secondary-structural analysis of a unique prokaryotic metallothionein from a Synechococcus sp. cyanobacterium. Biochem J 251:691–699
Higham DP, Sadler PJ, Scawen MD (1986) Cadmium-binding proteins in Pseudomonas putida: pseudothioneins. Environ Health Perspect 65:5–11
Huckle JW, Morby AP, Turner JS, Robinson NJ (1993) Isolation of a prokaryotic metallothionein locus and analysis of transcriptional control by trace metal ions. Mol Microbiol 7:177–187
Shi J, Lindsay WP, Huckle JW et al (1992) Cyanobacterial metallothionein gene expressed in Escherichia coli. Metal-binding properties of the expressed protein. FEBS Lett 303:159–163
Blindauer CA (2011) Bacterial metallothioneins: past, present, and questions for the future. J Biol Inorg Chem 16:1011–1024. doi:10.1007/s00775-011-0790-y
Gold B, Deng H, Bryk R et al (2008) Identification of a copper-binding metallothionein in pathogenic mycobacteria. Nat Chem Biol 4:609–616. doi:10.1038/nchembio.109
Blindauer CA, Harrison MD, Parkinson JA et al (2001) A metallothionein containing a zinc finger within a four-metal cluster protects a bacterium from zinc toxicity. Proc Natl Acad Sci U S A 98:9593–9598. doi:10.1073/pnas.171120098
Oz G, Pountney DL, Armitage IM (1998) NMR spectroscopic studies of I = 1/2 metal ions in biological systems. Biochem Cell Biol 76:223–234
Robinson NJ, Whitehall SK, Cavet JS (2001) Microbial metallothioneins. Adv Microb Physiol 44:183–213
Glaser R, Harder J, Lange H et al (2005) Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol 6:57–64. doi:10.1038/ni1142
Moroz OV, Antson AA, Grist SJ et al (2003) Structure of the human S100A12-copper complex: implications for host-parasite defence. Acta Crystallogr D Biol Crystallogr 59:859–867
Moroz OV, Burkitt W, Wittkowski H et al (2009) Both Ca2+ and Zn2+ are essential for S100A12 protein oligomerization and function. BMC Biochem 10:11. doi:10.1186/1471-2091-10-11
Corbin BD, Seeley EH, Raab A et al (2008) Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962–965. doi:10.1126/science.1152449
McCormick A, Heesemann L, Wagener J et al (2010) NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect 12:928–936. doi:10.1016/j.micinf.2010.06.009
Urban CF, Ermert D, Schmid M et al (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5:e1000639. doi:10.1371/journal.ppat.1000639
Hsu K, Champaiboon C, Guenther BD et al (2009) Anti-infective protective properties of S100 calgranulins. Antiinflamm Antiallergy Agents Med Chem 8:290–305
Ammendola S, Pasquali P, Pistoia C et al (2007) High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect Immun 75:5867–5876. doi:10.1128/IAI.00559-07
Campoy S, Jara M, Busquets N et al (2002) Role of the high-affinity zinc uptake znuABC system in Salmonella enterica serovar typhimurium virulence. Infect Immun 70:4721–4725
Davis LM, Kakuda T, DiRita VJ (2009) A Campylobacter jejuni znuA orthologue is essential for growth in low-zinc environments and chick colonization. J Bacteriol 191:1631–1640. doi:10.1128/JB.01394-08
Rosadini CV, Gawronski JD, Raimunda D et al (2011) A novel zinc binding system, ZevAB, is critical for survival of nontypeable Haemophilus influenzae in a murine lung infection model. Infect Immun 79:3366–3376. doi:10.1128/IAI.05135-11
Kehl-Fie TE, Chitayat S, Hood MI et al (2011) Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 10:158–164. doi:10.1016/j.chom.2011.07.004
Nisapakultorn K, Ross KF, Herzberg MC (2001) Calprotectin expression in vitro by oral epithelial cells confers resistance to infection by Porphyromonas gingivalis. Infect Immun 69:4242–4247. doi:10.1128/IAI.69.7.4242-4247.2001
Stork M, Grijpstra J, Bos MP et al (2013) Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLoS Pathog 9:e1003733. doi:10.1371/journal.ppat.1003733
Gaetke LM, McClain CJ, Talwalkar RT, Shedlofsky SI (1997) Effects of endotoxin on zinc metabolism in human volunteers. Am J Phys 272:E952–E956
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454. doi:10.1056/NEJM199902113400607
Zitka O, Kukacka J, Krizkova S et al (2010) Matrix metalloproteinases. Curr Med Chem 17:3751–3768
Elkington PTG, O’Kane CM, Friedland JS (2005) The paradox of matrix metalloproteinases in infectious disease. Clin Exp Immunol 142:12–20. doi:10.1111/j.1365-2249.2005.02840.x
Wastney ME, Aamodt RL, Rumble WF, Henkin RI (1986) Kinetic analysis of zinc metabolism and its regulation in normal humans. Am J Phys 251:R398–R408
Wang Y, Tang JW, Ma WQ et al (2010) Dietary zinc glycine chelate on growth performance, tissue mineral concentrations, and serum enzyme activity in weanling piglets. Biol Trace Elem Res 133:325–334. doi:10.1007/s12011-009-8437-3
Plum LM, Rink L, Haase H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7:1342–1365. doi:10.3390/ijerph7041342
Fukada T, Yamasaki S, Nishida K et al (2011) Zinc homeostasis and signaling in health and diseases: zinc signaling. J Biol Inorg Chem 16:1123–1134. doi:10.1007/s00775-011-0797-4
Folin M, Contiero E, Vaselli GM (1994) Zinc content of normal human serum and its correlation with some hematic parameters. Biometals 7:75–79
Foote JW, Delves HT (1984) Albumin bound and alpha 2-macroglobulin bound zinc concentrations in the sera of healthy adults. J Clin Pathol 37:1050–1054
Prasad AS, Oberleas D (1974) Thymidine kinase activity and incorporation of thymidine into DNA in zinc-deficient tissue. J Lab Clin Med 83:634–639
Osman D, Cavet JS (2011) Metal sensing in Salmonella: implications for pathogenesis. Adv Microb Physiol 58:175–232. doi:10.1016/B978-0-12-381043-4.00005-2
Desrosiers DC, Bearden SW, Mier IJ et al (2010) Znu is the predominant zinc importer in Yersinia pestis during in vitro growth but is not essential for virulence. Infect Immun 78:5163–5177. doi:10.1128/IAI.00732-10
Ma Z, Jacobsen FE, Giedroc DP (2009) Coordination chemistry of bacterial metal transport and sensing. Chem Rev 109:4644–4681. doi:10.1021/cr900077w
Patzer SI, Hantke K (1998) The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol Microbiol 28:1199–1210
Hantke K (2001) Bacterial zinc transporters and regulators. Biometals 14:239–249
Botella H, Peyron P, Levillain F et al (2011) Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10:248–259. doi:10.1016/j.chom.2011.08.006
Choudhuri S, McKim JMJ, Klaassen CD (1992) Role of hepatic lysosomes in the degradation of metallothionein. Toxicol Appl Pharmacol 115:64–71
Gilston BA, Wang S, Marcus MD et al (2014) Structural and mechanistic basis of zinc regulation across the E. coli Zur regulon. PLoS Biol 12:e1001987. doi:10.1371/journal.pbio.1001987
Philips SJ, Canalizo-Hernandez M, Yildirim I et al (2015) Transcription. Allosteric transcriptional regulation via changes in the overall topology of the core promoter Science 349:877–881. doi:10.1126/science.aaa9809
Pederick VG, Eijkelkamp BA, Begg SL et al (2015) ZnuA and zinc homeostasis in Pseudomonas aeruginosa. Sci Rep 5:13139. doi:10.1038/srep13139
Calmettes C, Ing C, Buckwalter CM et al (2015) The molecular mechanism of zinc acquisition by the neisserial outer-membrane transporter ZnuD. Nat Commun 6:7996. doi:10.1038/ncomms8996
Petering DH, Krezoski S, Villalobos J et al (1987) Cadmium-zinc interactions in the Ehrlich cell: metallothionein and other sites. Experientia Suppl 52:573–580
Maret W (2012) New perspectives of zinc coordination environments in proteins. J Inorg Biochem 111:110–116. doi:10.1016/j.jinorgbio.2011.11.018
Patel K, Kumar A, Durani S (2007) Analysis of the structural consensus of the zinc coordination centers of metalloprotein structures. Biochim Biophys Acta 1774:1247–1253. doi:10.1016/j.bbapap.2007.07.010
Cousins RJ (1985) Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev 65:238–309
Vasak M, Hasler DW (2000) Metallothioneins: new functional and structural insights. Curr Opin Chem Biol 4:177–183
Jacob C, Maret W, Vallee BL (1998) Control of zinc transfer between thionein, metallothionein, and zinc proteins. Proc Natl Acad Sci U S A 95:3489–3494. doi:10.1073/pnas.95.7.3489
Maret W, Vallee BL (1998) Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc Natl Acad Sci U S A 95:3478–3482
Nakazato K, Tomioka S, Nakajima K et al (2014) Determination of the serum metallothionein (MT)1/2 concentration in patients with Wilson’s disease and Menkes disease. J Trace Elem Med Biol 28:441–447. doi:10.1016/j.jtemb.2014.07.013
Nagamine T, Nakajima K (2013) Development of a high sensitivity ELISA for the assay of metallothionein. Curr Pharm Biotechnol 14:427–431
Nakajima K, Kodaira T, Kato M et al (2010) Development of an enzyme-linked immunosorbent assay for metallothionein-I and -II in plasma of humans and experimental animals. Clin Chim Acta 411:758–761. doi:10.1016/j.cca.2010.02.058
Waeytens A, De Vos M, Laukens D (2009) Evidence for a potential role of metallothioneins in inflammatory bowel diseases. Mediat Inflamm. doi:10.1155/2009/729172
Everhardt Queen A, Moerdyk-Schauwecker M, McKee LM et al (2016) Differential expression of inflammatory cytokines and stress genes in male and female mice in response to a lipopolysaccharide challenge. PLoS One 11:e0152289. doi:10.1371/journal.pone.0152289
De SK, McMaster MT, Andrews GK (1990) Endotoxin induction of murine metallothionein gene expression. J Biol Chem 265:15267–15274
Arizono K, Kagawa S, Hamada H, Ariyoshi T (1995) Nitric oxide mediated metallothionein induction by lipopolysaccharide. Res Commun Mol Pathol Pharmacol 90:49–58
Itoh N, Kasutani K, Muto N et al (1996) Blocking effect of anti-mouse interleukin-6 monoclonal antibody and glucocorticoid receptor antagonist, RU38486, on metallothionein-inducing activity of serum from lipopolysaccharide-treated mice. Toxicology 112:29–36
Clarkson JP, Elmes ME, Jasani B, Webb M (1985) Histological demonstration of immunoreactive zinc metallothionein in liver and ileum of rat and man. Histochem J 17:343–352
Mulder TP, Verspaget HW, Janssens AR et al (1991) Decrease in two intestinal copper/zinc containing proteins with antioxidant function in inflammatory bowel disease. Gut 32:1146–1150
Sturniolo GC, Mestriner C, Lecis PE et al (1998) Altered plasma and mucosal concentrations of trace elements and antioxidants in active ulcerative colitis. Scand J Gastroenterol 33:644–649
Kruidenier L, Kuiper I, Van Duijn W et al (2003) Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J Pathol 201:17–27. doi:10.1002/path.1408
Bruwer M, Schmid KW, Metz KA et al (2001) Increased expression of metallothionein in inflammatory bowel disease. Inflamm Res 50:289–293. doi:10.1007/PL00000246
O’Connor KS, Parnell G, Patrick E et al (2014) Hepatic metallothionein expression in chronic hepatitis C virus infection is IFNL3 genotype-dependent. Genes Immun 15:88–94. doi:10.1038/gene.2013.66
Ilbäck NG, Frisk P, Mohamed N et al (2007) Virus induces metal-binding proteins and changed trace element balance in the brain during the course of a common human infection (coxsackievirus B3) in mice. Sci Total Environ 381:88–98. doi:10.1016/j.scitotenv.2007.03.025
Lynes MA, Garvey JS, Lawrence DA (1990) Extracellular metallothionein effects on lymphocyte activities. Mol Immunol 27:211–219
Lynes MA, Borghesi LA, Youn J, Olson EA (1993) Immunomodulatory activities of extracellular metallothionein. I Metallothionein effects on antibody production Toxicology 85:161–177
Youn J, Borghesi LA, Olson EA, Lynes MA (1995) Immunomodulatory activities of extracellular metallothionein. II Effects on macrophage functions J Toxicol Environ Health 45:397–413. doi:10.1080/15287399509532004
Youn J, Lynes MA (1999) Metallothionein-induced suppression of cytotoxic T lymphocyte function: an important immunoregulatory control. Toxicol Sci 52:199–208
Bulua AC, Simon A, Maddipati R et al (2011) Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 208:519–533. doi:10.1084/jem.20102049
Robinson JM (2008) Reactive oxygen species in phagocytic leukocytes. Histochem Cell Biol 130:281–297. doi:10.1007/s00418-008-0461-4
Santos SS, Brunialti MKC, Rigato O et al (2012) Generation of nitric oxide and reactive oxygen species by neutrophils and monocytes from septic patients and association with outcomes. Shock 38:18–23. doi:10.1097/SHK.0b013e318257114e
Pauwels M, van Weyenbergh J, Soumillion A et al (1994) Induction by zinc of specific metallothionein isoforms in human monocytes. Eur J Biochem 220:105–110
Rahman MT, De Ley M (2017) Arsenic induction of metallothionein and metallothionein induction against arsenic cytotoxicity. Rev Environ Contam Toxicol. doi:10.1007/398_2016_2
Qu W, Waalkes MP (2015) Metallothionein blocks oxidative DNA damage induced by acute inorganic arsenic exposure. Toxicol Appl Pharmacol 282:267–274. doi:10.1016/j.taap.2014.11.014
Spiering R, Wagenaar-Hilbers J, Huijgen V et al (2014) Membrane-bound metallothionein 1 of murine dendritic cells promotes the expansion of regulatory T cells in vitro. Toxicol Sci 138:69–75. doi:10.1093/toxsci/kft268
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahman, M.T., Karim, M.M. Metallothionein: a Potential Link in the Regulation of Zinc in Nutritional Immunity. Biol Trace Elem Res 182, 1–13 (2018). https://doi.org/10.1007/s12011-017-1061-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1061-8