Abstract
Selenizing astragalus polysaccharides-3 (sAPS3) was prepared by nitric acid–sodium selenite method. The effects of sAPS3 on carbon tetrachloride (CCl4) induced hepatocellular necrosis, and its underlying mechanisms were studied in male Wistar rats. Hepatic damage was induced by intraperitoneal injection of CCl4 twice a week, for 3 weeks. Meanwhile, the rats in addition to CCl4 were also exposed to sodium selenite (SS), astragalus polysaccharides (APS), SS + APS or sAPS3, in parallel by oral gavage once a day for 3 weeks. At the end of 3 weeks, blood and liver tissue were taken. Serum was collected to test the levels of alanine aminotransferase, aspartate aminotransferase and antioxidant status parameters. Liver tissue was collected for histopathological examination and determination of messenger RNA (mRNA) expression levels of CD68, TNF-α, IL-1β and ATG7 followed by the measurements of CD68, IL-1β and LC3II by immunohistochemistry assay (IHC), or TNF-α by immunofluorescence assay (IFA). The results showed that sAPS3 effectively ameliorated CCl4 induced hepatocellular necrosis and inflammation and significantly decreased the levels of aspartate aminotransferase, alanine aminotransferase, malondialdehyde and the expression levels of Kupffer cells (KCs)-specific biomarker CD68 and proinflammatory cytokines produced by activated KCs such as IL-1β and TNF-α (P < 0.01). While increasing the levels of total antioxidant capacity, glutathione, glutathione peroxidase and superoxide dismutase (P < 0.05) and reduced the expression levels of a key regulator of autophagy in KCs ATG7 or LC3II (P < 0.05). These findings indicate that sAPS3 could ameliorate CCl4-induced hepatocellular necrosis by inactivation of Kupffer cells and its activity may be superior to the application of selenium, APS or combination of selenium with APS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver is considered to be the main organ responsible for the metabolism of all toxic chemicals and drugs; therefore, it represents the primary target organ for nearly all toxic chemicals [1]. Kupffer cells (KCs) are considered as a population of liver resident macrophages, including nearly 90% of the tissue macrophages. KCs remain in the lumen of the liver sinusoids, for phagocytosis of larger particulates and foreign materials [2]. Multiple studies indicate that KCs contribute to the pathogenesis of different liver injuries and diseases including its pathogenetic role in carbon tetrachloride-induced hepatocellular necrosis and fibrosis [3, 4].They also play critical roles in establishing, maintenance and outcome of inflammation by releasing a large amount of inflammatory cytokines and chemokines after its activation. Carbon tetrachloride (CCl4) intoxication is a common animal model for detecting the mechanism of liver damage as well as the hepatoprotective activity of synthetic and natural compounds [5]. The administration of CCl4 causes a serious degree of necrosis and inflammation which was owing to more KCs infiltration prominently [6].
Selenium (Se) is a vital trace element that plays a major role in various physiologic processes including antioxidant system regulation in the body and amelioration of liver injuries [7, 8]. Herbal Chinese medicine was reported to be able to enhance the body immune function, protect the liver, improve blood circulation in the liver, regulate liver function and repair hepatocyte damages effectively [9]. The astragalus polysaccharides, derived from the roots of the Astragalus membranaceous, which is demonstrated to have many pharmacological applications, including anti-inflammatory, immunomodulatory, anticancer and antioxidant effects in addition to exhibiting hepatoprotective and antioxidant properties acting as a synergistic effect in CCl4-induced liver injury in mice [9–11].
Selenium–polysaccharide combination possesses higher and duple biological activities as compared with selenium or polysaccharide, and it is easily absorbed by the body [12, 13]. Selenium polysaccharides were used widely in immunomodulation, antitumor, antioxidation, antiageing and so on [14].
In our laboratory, we prepared selenizing astragalus polysaccharide (sAPS) that has combined properties of astragalus polysaccharides and selenium to ameliorate CCl4-induced liver injury in rats. The present study aimed to verify if administration of sAPS3 reduced the hepatocellular damage in the rats to a lesser extent, and also to underline the possible mechanisms.
Materials and Methods
Chemicals
Astragalus polysaccharides powder extract with purity 95% was obtained from Pharmagenesis Inc. Sodium selenite and nitric acid (HNO3) were provided from Shanghai Lingfeng Chemical Reagent Ltd. (China). Kits for glutathione (GSH), total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-Px), SOD, MDA, total protein and CCl4 were provided from the Institute of Jiancheng Biotechnology (Nanjing, China). TNF-α, CD68, IL1-β and microtubule-associated protein I1 light chain 3 (LC3II) monoclonal antibody were purchased from Abcam (Cambridge, UK).
Preparation of sAPS
Selenizing astragalus polysaccharides (sAPS) were prepared by nitric acid–sodium selenite method [14, 15].We prepared three selenizing molecules (sAPS1, sAPS2 and sAPS3) according to amount of sodium selenite (200, 300, 400 mg sodium selenite) for the whole reaction condition 500 mg of APS in 70 °C for 10 h [16]. The selenium contents of these selenizing molecules were 1.76, 1.89 and 2.21 mg/g, respectively, detected by atomic fluorescence spectrometry method [17, 18] and carbohydrate contents were 47.92, 50.9 and 62.21%, respectively, determined by the phenol–sulphuric acid method. APS and sAPS3 were diluted into 40 mg/mL, and the endotoxins were detected by pyrogen tests (less than 0.5 EU mL−1) according to (Veterinary Pharmacopoeia Commission of the People’s Republic of China) [14].
Dose-Response Experiment for Selenizing Astragalus Polysaccharides
The dose of 0.4 mg/kg for sodium selenite and 200 mg/kg for astragalus polysaccharides were used previously [19, 20] as an effective dose of both sodium selenite and astragalus polysaccharides. In the current study, a preliminary experiment with serum markers enzymes, AST and ALT as liver damage markers and GSH was carried out with three selenizing molecules (sAPS1, sAPS2 and sAPS3) for the selection of the maximum effective dose of sAPS in CCl4-treated rats. A total of 42 male Wistar rats weighing 190–210 g were obtained from the Center for Laboratory Animals, Yangzhou University (Yangzhou, China). Rats were divided into seven groups (six animals each) as follows: group 1 (control), group 2 (CCl4), group 3 (CCl4 + APS), group 4 (CCl4 + sodium selenite (SS)), group 5 (CCl4 + APS1), group 6 (CCl4 + sAPS2) and group 7 (CCl4 + sAPS3). Group 1 received an only intraperitoneal injection of olive oil 2 mL/kg BW twice a week for 3 weeks. Groups 2–5 received an intraperitoneal injection with CCl4 2 mL/kg BW, 1:1 in olive oil twice a week for 3 weeks [7]. Each rat in treated groups was administered orally with 1 mL containing 40 mg/day of APS [20], 80 μg/day of SS [19], 40 mg/day of APS1, 40 mg/day of sAPS2 and 40 mg/day of sAPS3 along with CCl4. Blood samples and liver tissues were collected by the end of the experiment and immediately stored at −70 °C.
Induction of Hepatocellular Necrosis and Study Design with the Selected Dose of sAPS
This experiment was approved by the Animal Care and Use Committee of Nanjing Agricultural University, (Certification No.: SYXK (Su) 2011-0036). A total of 48 male Wistar rats weighing 190–210 g were obtained from the Center for Laboratory Animals, Yangzhou University (Yangzhou, China). Rats were maintained under a controlled environmental condition at 25 ± 2 °C, with a normal day/night cycle and fed with normal basal diet and water ad libitum. Animals were divided into six groups (eight animals each) as follows: group 1 (control), group 2 (CCl4), group 3 (CCl4 + APS), group 4 (CCl4 + SS), group 5 (CCl4 + SS + APS) and group 6 (CCl4 + sAPS3). Group 1 received an only intraperitoneal injection of olive oil 2 mL/kg BW twice a week for 3 weeks. Groups 2–5 received an intraperitoneal injection with CCl4 2 mL/kg BW, 1:1 in Olive oil twice a week for 3 weeks. Each rat in treated groups was administered orally with 1 mL containing 40 mg/day of APS, 80 μg/day of SS, 40 mg/day of APS + 80 μg/day of SS or 40 mg/day of sAPS3 along with CCl4 (The most effective dose from the preliminary study).. Blood and liver samples were collected by the end of the experiment and immediately stored at −70 °C.
Determination of Serum Enzymes
ALT and AST were assayed in serum using an automated chemistry analyser. (BS-300, Mindray Medical International Limited).
Determination of Serum T-AOC, GSH-Px, SOD and MDA
T-AOC, GSH-Px, SOD and MDA were assayed in serum using an AF-610A atomic fluorescence spectrometer, according to the instructions of the commercial assay kits.
GSH Activity Assay of Liver Homogenate
A hundred milligrammes of frozen liver tissue in 1 mL of homogenised ice-cold buffer (1 mmol/L EDTA, 0.32 mol/L sucrose and 10 nmol/L Tris-HCl, pH = 7.4) was homogenised on ice with a Polytron-aggregate homogeniser at 12000 rpm. The homogenate was centrifuged at 2500 rpm for 10 min at 4 °C. The activity of GSH was measured from the supernatant using a commercial assay kit, according to the instructions.
Histopathology Examination
Liver tissues were immediately fixed in 10% neutral buffered formalin, processed for paraffin embedding. Haematoxylin–eosin (H&E) was performed using standard procedures [21].
Immunohistochemistry Assay
Liver sections were fixed in 10% neutral-buffered formalin, processed for paraffin embedding and were incubated with mouse monoclonal antibody against CD68, IL-1β and LC3II then incubated with the second antibody peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) then incubated with the streptavidin–peroxidase complex, then subsequently visualised using diaminobenzidine (DAB) solution. Finally, hepatic sections were counterstained with haematoxylin and then mounted on a coverslip.
Immunofluorescence Assay
Liver tissues were immediately fixed in 10% neutral-buffered formalin and processed for paraffin embedding. Liver tissues were then incubated with a specific mouse monoclonal antibody against rat TNF-α diluted at 1:50 with 1% BSA/PBS, followed by incubation with FITC-conjugated rabbit anti-mouse IgG (1:50 dilution) in 1% BSA/PBS. A fluorescent microscope was used to take images.
RNA Extraction and Quantitative Real-Time PCR Assay
The relative messenger RNA (mRNA) expression of TNF-α, IL-β1, CD68, and ATG7 were determined by quantitative real-time PCR assay (qRT-PCR). The primers were designed by online Primer-Blast of NCBI as shown in Table 1. The extraction of RNA and synthesis of complementary DNA (cDNA) were performed as previously reported [22]. Briefly, RNA was extracted from the liver tissue with TRIzol (Invitrogen), according to the instructions. Reverse transcription (RT) was performed to synthesise cDNA with PrimeScript RT Master Mix Perfect Real Time (Takara Co., Otsu, Japan), according to the instructions. The qRT-PCR was carried out on an ABI Prism 7300 Detection System (Applied Biosystems, USA). All cDNA samples were amplified using SYBR Green (Takara Co., Otsu, Japan). The relative levels of gene expression were determined by the ΔΔCT method; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. All reactions were performed in triplicates.
Statistical Analysis
The experimental data were analysed statistically using SPSS 18.0 for Windows. Experimental results were presented as mean ± S.E.M. One-way ANOVA was used to examine the statistical significance. The differences between groups were determined by Duncan’s contrasts; P < 0.01 was considered to be highly significant difference between the groups, while P < 0.05 was considered to be significant between the groups.
Results
Preliminary Study for Selection of the Maximum Effective Dose of sAPS
As indicated by serum ALT, AST and activity of GSH from the liver homogenate (Table 2). All treated groups had significant (P < 0.05) decrease in the serum activities of ALT and AST or GSH activity compared with the CCl4-treated group. sAPS3 showed the best reduction of ALT, AST or GSH compared with other treatments. Accordingly, we selected sAPS3 as the most appropriate dose to complete the main study.
Determination of Serum Enzymes
Assays of ALT and AST in animal serum were given in (Fig. 1a, b). The CCl4-treated group significantly (P < 0.01) had elevated serum activities of ALT compared with the control group. The serum activities of ALT in rats treated with APS, SS, SS + APS or sAPS3 significantly decreased (P < 0.05) compared with the CCl4-treated group. The SS + APS and sAPS3 groups showed a significant decrease (P < 0.05) in the serum activities of ALT compared with the APS or SS group. There is no significant difference between SS and APS groups.
Effects of APS, SS, SS + APS, and sAPS3 on liver marker enzymes in the serum and glutathione (GSH) levels in liver tissues a serum alanine aminotransferase; b serum aspartate aminotransferase and c the levels of GSH. Data are represented as mean ± SEM (n = 8). Columns with different letters differ significantly (P < 0.05)
The CCl4-treated group significantly (P < 0.01) had elevated the serum activities of AST compared with control group. The serum activities of AST in rats treated with APS, SS, SS + APS or sAPS3 significantly decreased (P < 0.05) compared with the CCl4-treated group. sAPS3 was more effective than APS, SS or SS + APS group (P < 0.05). While SS and SS + APS showed a better result compared to APS.
T-AOC, GSH-Px, SOD and MDA Levels
The T-AOC, SOD and GSH-Px activities, decreased significantly in the CCl4-treated group compared with that of the control group (P < 0.05). The groups treated with APS, SS, SS + APS or sAPS3 improved the T-AOC, SOD and GSH-Px activities (Fig. 2a–c) compared to the CCl4-treated group (P < 0.05). There was no significant difference in the T-AOC activities between the SS + APS and sAPS3 groups. However, the sAPS3 group showed further increase in the SOD and GSH-Px activities, compared with the APS, SS and SS + APS supplementation (P < 0.05). MDA levels significantly increased in serum of the CCl4-treated group compared to the control group (P < 0.05; Fig. 2d). However, APS, SS, SS + APS or sAPS3 supplementation significantly lowered the MDA levels compared with the CCl4-treated group. The SS + APS and sAPS3 groups showed a better result compared to APS or SS (P < 0.05).
Effects of APS, SS, SS + APS or sAPS3 on GSH Levels in Liver Tissues
The level of hepatic GSH was an important indicator of liver oxidative damage. The activities of GSH were showed in (Fig. 1c). CCl4-treated group significantly had reduced (P < 0.05) antioxidant activities of GSH compared with the control group. While the levels of GSH were highly increased in the APS, SS, SS + APS or sAPS3 group compared with CCl4-treated group (P < 0.05).
Histopathological Examination
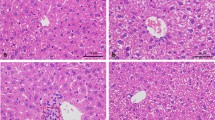
The histopathological sections of the liver of control and treated rats were showed in (Fig. 3).The liver histopathological appearance of the CCl4-treated group showed massive necrosis, hepatocyte ballooning, and inflammation associated with hepatotoxicity (Fig. 3b). In the control group, no significant inflammation and necrosis are observed (Fig. 3a). APS and SS groups revealed moderate necrosis, inflammation and hepatocyte ballooning (Fig. 3c, d). SS + APS group revealed mild necrosis and inflammation (Fig. 3e). While sAPS3 administration ameliorated the hepatic necrosis and inflammation induced by CCl4 (Fig. 3f).
Effects of sAPS3 on the Expression of KCs Specific Biomarker CD68
To clarify the effect of sAPS3 on the activation degree of KCs, we measured specific biomarker of KCs CD68 (Fig. 4a).The mRNA expression levels of CD68 in CCl4-treated group was highly increased (P < 0.01), compared with the control group. While the mRNA expression levels of CD68 in the APS, SS, SS + APS or sAPS3 group were significantly decreased compared with the CCl4-treated group (P < 0.01).sAPS3 was more effective than APS, SS or SS + APS group in the mRNA expression levels of this gene (P < 0.05). SS + APS showed a better result than APS or SS. However, there was no significant difference in the mRNA expression levels of CD68 between APS and SS groups. This result was confirmed by staining CD68 in liver tissue sections by immunohistochemistry (IHC) assay. As shown in (Fig. 5). The CD68 expression did not show in the liver tissue of the control rats (Fig. 5a), while CCl4-treated group showed extensive expression of CD68 mainly around portal tracts and liver sinusoid (Fig. 5b). SS and APS groups showed moderate expressions of hepatic CD68 (Fig. 5c, d). SS + APS showed mild expressions of hepatic CD68 (Fig. 5e). Hepatic expression of CD68 has been completely reduced by sAPS3 administration (Fig. 5f).
Effects of APS, SS, SS + APS and sAPS3 on the genes expressions. Quantitative real-time PCR (qRT-PCR) analysis detected the mRNA expression levels of CD68, TNF-α, IL-1β and ATG7 in the liver. GAPDH served as an internal control. Data are represented as mean ± SEM (n = 8). Columns with different letters differ significantly (P < 0.05)
Effects of sAPS3 on the Expression of Proinflammatory Cytokines Produced by Activated KCs
To assess the effect on the activation degree of KCs, we measured the mRNA expression levels of TNF-α and IL-β1 in the liver tissues (Fig. 4b, c). CCl4-treated group had highly increased mRNA expression levels of TNF-α and IL-β1 (P < 0.01) compared with the control group. The groups treated with APS, SS, SS + APS or sAPS3 showed reduced mRNA expression levels of TNF-α and IL-β1 compared with the CCl4-treated group (P < 0.05). The sAPS3 group showed a further reduction in the mRNA expression levels of IL-β1 (P < 0.05) compared with APS, SS or SS + APS group. While SS + APS and sAPS3 more effective than APS and SS in reducing mRNA expression levels of TNF-α. However, there was no significant difference in the mRNA expression levels of these genes between the SS and APS groups. This result was confirmed by staining IL-β1 in liver tissue sections by immunohistochemistry (IHC) assay. As shown in (Fig. 6), the ccl4-treated group showed massive expressions of IL-β1 especially around degenerated hepatocytes of the liver (Fig. 6b), compared with the control group (Fig. 6a). SS, APS, and SS + APS groups showed sporadically expressions of IL-β1 (Fig. 6c–e) compared with the CCl4-treated group, while administration of sAPS3 markedly reduced the degree of IL-β1 in the liver tissue (Fig. 6f). We also investigate the expressions of TNF-α in liver tissue sections by immunofluorescence (IFA) assay. As shown in (Fig. 7), immunofluorescence analysis showed that the green signals representing TNF-α were highly localised in the cytoplasm of hepatocytes of the CCl4-treated group (Fig. 7b) compared with the control group (Fig. 7a). The moderate density of TNF-α was shown in the SS and APS groups (Fig. 7c, d), while the density of TNF-α in the cytoplasm of hepatocytes was reduced in the SS + APS or sAPS3 group (Fig. 7e, f).
Effects of sAPS3 on the Autophagy Impairment in KCs after CCl4 Treatment
Autophagy is considered to be a key regulator of inflammation and its reduction can trigger various inflammatory conditions [6]. To detect the autophagy activation in KCs, we measured the mRNA expression levels of ATG7, a key regulator of autophagy activation (Fig. 4d). The mRNA expression levels of ATG7 in CCl4-treated group was significantly increased (P < 0.05), compared with the control group. However, the mRNA expression levels of ATG7 in the APS, SS, SS + APS or sAPS3 group were highly decreased compared with the CCl4-treated group (P < 0.05). SS + APS and sAPS3 were more effective in the reduction of ATG7 mRNA expression levels (P < 0.05) compared with the APS or SS group. While there was no significant difference in the mRNA expression levels of ATG7 between the APS and SS groups. This result was confirmed by staining microtubule-associated protein I1 light chain 3 (LC3II) in liver tissue sections by immunohistochemistry (IHC) assay. As shown in (Fig. 8), the CCl4-treated group showed strong expressions of LC3II all around the hepatic lobules (Fig. 8b) compared with the control group (Fig. 8a). SS and APS groups showed relatively low expressions of LC3II (Fig. 8c, d). No expression was observed in the administration of SS + APS or sAPS3-treated group (Fig. 8e, f).
Discussion
Liver damage is one of the major health problems worldwide, frequently occurring as the result of oxidative damage and intoxication [23]. KCs are resident macrophages of the liver and play a major role in its homoeostasis and involved in the acute and chronic responses of the liver to various toxic agents including CCl4. Activation of KCs directly or indirectly by toxic compounds results in the release of a number of inflammatory cytokines, growth factors, and reactive oxygen species. This activation is commonly associated with acute hepatocellular damage as well as chronic liver conditions including hepatic cancer [24]. Therefore, inactivation of KCs may results in amelioration of hepatocellular necrosis to a lesser extent. The studying of hepatoprotective activity of medicinal plants and foods and their bioactive ingredients s is one of the main goals of medical research today [23]. Selenium (Se) is a fundamental nutrient element that can ameliorate the progress of liver injury and reduce the hepatic fibrosis [25]. However, some investigations showed that APS had hepatoprotective effects on liver damage induced by CCl4 in mice [26]. The combination of selenium with polysaccharides into organic selenium polysaccharides as selenizing polysaccharides may improve the activity compared to polysaccharide and selenium. Therefore, this study was conducted to prepare a sAPS product that has the combined effects of selenium and APS. Our results showed that administration of sAPS3 was effective in reducing CCl4-induced hepatocellular necrosis via inactivation of KCs. This study is the first to determine the effect of sAPS3 on CCl4-induced hepatocellular necrosis in rats and elucidate the underlying mechanisms.
Specific serum marker enzymes, such as ALT and AST, are commonly recognised as indicators of liver damage and they are increased in hepatocellular necrosis and liver dysfunction [7, 27, 28]. The present findings showed that the levels of ALT and AST were highly elevated in the serum of CCl4-treated rats, suggesting severe damage of hepatocytes. Administration of sAPS3 significantly reduced the levels of the of the above-mentioned serum enzymes. These results were confirmed by the finding of histopathological examination that revealed the damage of hepatic lobule with severe hepatocytes necrosis, ballooning, and inflammation in the CCl4-treated rats. There is some evidence indicating that CCl4 causes massive hepatocellular necrosis and inflammation [7, 25] while sAPS3 could effectively reduce the histopathological alterations induced by CCl4. These finding indicated that sAPS3 could prevent the liver from hepatocellular necrosis.
Oxidative stress was a major contributor in the pathophysiologic process of liver injury [29]. Free radicals accumulation in oxidative stress process can damage the hepatocytes membrane mainly through lipid peroxidation [30]. Nevertheless, antioxidants have protective effects against induced liver injury by lessening oxidative stress in cells [31]. Glutathione (GSH) is one of the important non-enzymatic antioxidants involved in the protection of cells from oxidative stress. However, activation of KCs results in the release of reactive oxygen species and driving more oxidative stress in the injured liver by CCl4 [24, 29]. The levels of T-AOC, GSH, GSH-Px, and SOD significantly decreased in CCl4-treated rats and were elevated toward normal values by sAPS3. The peroxidative status showed inhibited by sAPS3 according to the lower lipid peroxide (MDA). These findings indicate that sAPS3 was able to ameliorate the deleterious effect of oxidative stress.
CD68 is the major specific marker of KCs; it has been used to monitor KCs. Activation when increased may indicate hyperfunction of KCs in the injured liver tissue [6, 32, 33]. The expression levels of CD68 were significantly increased in CCl4-treated rats, while administration of sAPS3 reduced the expression of this marker. These results indicate that sAPS3 was able to ameliorate the hepatocellular necrosis by inactivation of KCs.
KCs are the primary source of inflammatory cytokines when activated by toxic compounds including CCl4 secreted variety of these cytokines including IL-β1 and TNF-α. These cytokines can act on hepatocytes to cause cell death [24, 34]. The expression levels of IL-β1 and TNF-α were significantly increased in CCl4-treated rats, while administration of sAPS3 reduced the expression of these inflammatory cytokines. These findings indicate that sAPS3 was able to ameliorate the liver damage by inhibition of KCs and their cytokines production.
Autophagy is a major lysosomal catabolic pathway of eliminating intracellular components and also recycling damaged macromolecules, including dysfunctional organelles, proteins and lipids. Many studies indicate that autophagy deficiency increases inflammation as a key regulator of inflammation. Under toxic stimuli, excess autophagy can eliminate essential cellular components and may lead to cellular death. [6, 35]. Recent studies have indicated that impaired autophagy induces necrosis and thus stimulates the inflammatory response [36]. Others indicated that CCl4 could stimulate autophagy activation significantly in young KCs [6]. Our study indicated that CCl4 stimulates autophagy in KCs by increasing the expression of a key regulator of autophagy activation ATG7 in addition to a biological marker of autophagy microtubule-associated protein I1 light chain 3 (LC3II). Meanwhile, administration of sAPS3 decreased the expression of ATG7 and LC3II in the liver tissue. These findings indicated that autophagy in KCs might increase due to strong stimulation of CCl4 and hyperactivation of KCs compared with KCs of sAPS3 rats, but these results need a further study.
In conclusion, sAPS3 shows strong protective effects against CCl4-induced hepatocellular necrosis by inactivation of KCs. In addition, the hepatoprotective activities of sAPS3 may be superior to that of selenium, APS or selenium +APS and the combination of selenium with APS was more effective than APS or selenium alone. Further study is required to investigate the role of CCl4 in the autophagy in KCs. Further analysis is also needed to determine the quality and standard of the materials and about clinical application of sAPS3 in animals.
References
Bissell DM, Gores GJ, Laskin DL, Hoofnagle JH (2001) Drug-induced liver injury: mechanisms and test systems. Hepatology 33(4):1009–1013
Bilzer M, Roggel F, Gerbes AL (2006) Role of Kupffer cells in host defense and liver disease. Liver Int 26(10):1175–1186
Tsukamoto H, LU SC (2001) Current concepts in the pathogenesis of alcoholic liver injury. FASEB J 15(8):1335–1349
Rivera C, Bradford B, Hunt K, Adachi Y, Schrum L, Koop D, Burchardt E-R, Rippe R, Thurman R (2001) Attenuation of CCl4-induced hepatic fibrosis by GdCl3 treatment or dietary glycine. American Journal of Physiology-Gastrointestinal and Liver Physiology 281(1):G200–G207
Cheshchevik V, Lapshina E, Dremza I, Zabrodskaya S, Reiter R, Prokopchik N, Zavodnik I (2012) Rat liver mitochondrial damage under acute or chronic carbon tetrachloride-induced intoxication: protection by melatonin and cranberry flavonoids. Toxicol Appl Pharmacol 261(3):271–279
Yang X, Liang L, Zong C, Lai F, Zhu P, Liu Y, Jiang J, Yang Y, Gao L, Ye F (2016) Kupffer cells-dependent inflammation in the injured liver increases recruitment of mesenchymal stem cells in aging mice. Oncotarget 7(2):1084
Liu Y, Liu Q, Ye G, Khan A, Liu J, Gan F, Zhang X, Kumbhar S, Huang K (2014) Protective effects of selenium-enriched probiotics on carbon tetrachloride-induced liver fibrosis in rats. J Agric Food Chem 63(1):242–249
Behne D, Alber D, Kyriakopoulos A (2010) Long-term selenium supplementation of humans: selenium status and relationships between selenium concentrations in skeletal muscle and indicator materials. J Trace Elem Med Biol 24(2):99–105
Lu A, Qi M, Li Z, Lv H (2010) Callus cultivation and determination of flavonoids from Tetrastigma hemsleyanum. Zhong yao cai= Zhongyaocai= Journal of Chinese medicinal materials 33(7):1042–1045
Zhao L-H, Ma Z-X, Zhu J, Yu X-H, Weng D-P (2011) Characterization of polysaccharide from Astragalus radix as the macrophage stimulator. Cell Immunol 271(2):329–334
Jia R, Cao L, Xu P, Jeney G, Yin G (2012) In vitro and in vivo hepatoprotective and antioxidant effects of Astragalus polysaccharides against carbon tetrachloride-induced hepatocyte damage in common carp (Cyprinus carpio). Fish Physiol Biochem 38(3):871–881
Liu J, Chen X, Yue C, Hou R, Chen J, Lu Y, Li X, Li R, Liu C, Gao Z (2015) Effect of selenylation modification on immune-enhancing activity of Atractylodes macrocephala polysaccharide. Int J Biol Macromol 72:1435–1440
Li X, Hou R, Yue C, Liu J, Gao Z, Chen J, Lu Y, Wang D, Liu C, Hu Y (2016) The selenylation modification of epimedium polysaccharide and isatis root polysaccharide and the immune-enhancing activity comparison of their modifiers. Biol Trace Elem Res 171(1):224–234
Qiu S, Chen J, Chen X, Fan Q, Zhang C, Wang D, Li X, Chen X, Chen X, Liu C (2014) Optimization of selenylation conditions for Lycium barbarum polysaccharide based on antioxidant activity. Carbohydr Polym 103:148–153
Qin T, Chen J, Wang D, Hu Y, Wang M, Zhang J, Nguyen TL, Liu C, Liu X (2013) Optimization of selenylation conditions for Chinese angelica polysaccharide based on immune-enhancing activity. Carbohydr Polym 92(1):645–650
Li G, Miu J, Liu F (2001) Selenium polysaccharide compounds and their preparation methods. Chinese Patent NO: CNn21414C
Gao J-z, S-y Q, K-h H (2006) Assay of organic selenium and inorganic selenium of enriched yeast by hydride generation atomic fluorescence spectrometry method. Journal of Analytical Science 22(2):157–159
Tyson J, Palmer C (2009) Simultaneous detection of selenium by atomic fluorescence and sulfur by molecular emission by flow-injection hydride generation with on-line reduction for the determination of selenate, sulfate and sulfite. Anal Chim Acta 652(1):251–258
Abdo KM (1994) NTP technical report on toxicity studies of sodium selenate and sodium selenite (CAS Nos. 13410-01-0 and 10102-18-8) administered in drinking water to F344/N rats and B6C3F1 mice. US Department of Health and Human Services, Public Health Service, National Institutes of Health,
Dang SS, Zhang X, Jia XL, Cheng YA, Song P, Liu EQ, He Q, Li ZF (2008) Protective effects of emodin and astragalus polysaccharides on chronic hepatic injury in rats. Chin Med J 121(11):1010–1014
Nido SA, Shituleni SA, Mengistu BM, Liu Y, Khan AZ, Gan F, Kumbhar S, Huang K (2016) Effects of selenium-enriched probiotics on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in mice fed a high-fat diet. Biol Trace Elem Res 171(2):399–409
Gan F, Ren F, Chen X, Lv C, Pan C, Ye G, Shi J, Shi X, Zhou H, Shituleni SA (2013) Effects of selenium-enriched probiotics on heat shock protein mRNA levels in piglet under heat stress conditions. J Agric Food Chem 61(10):2385–2391
Sánchez-Valle V, Chavez-Tapia N, Uribe M, Méndez-Sánchez N (2012) Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem 19(28):4850–4860
Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE (2007) Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci 96(1):2–15
Ianăş O, Olinescu R, Bădescu I, Simionescu L, Popovici D (1994) The influence of "selenium organicum" upon the hepatic function of carbon tetrachloride poisoned rats. Romanian journal of internal medicine= Revue roumaine de medecine interne 33(1–2):113–120
Yan F, Zhang Q-Y, Jiao L, Han T, Zhang H, Qin L-P, Khalid R (2009) Synergistic hepatoprotective effect of Schisandrae lignans with astragalus polysaccharides on chronic liver injury in rats. Phytomedicine 16(9):805–813
Cao G, Li Q, Chen X, Cai H, Tu S (2014) Hepatoprotective effect of superfine particles of herbal medicine against CCl4-induced acute liver damage in rats. BioMed research international 2014
Al-Rasheed NM, Attia HA, Mohamad RA, Al-Rasheed NM, Al-Amin MA, AL-Onazi A (2015) Aqueous date flesh or pits extract attenuates liver fibrosis via suppression of hepatic stellate cell activation and reduction of inflammatory cytokines, transforming growth factor-β1 and angiogenic markers in carbon tetrachloride-intoxicated rats. Evidence-based complementary and alternative medicine 2015
Jayakumar T, Ramesh E, Geraldine P (2006) Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl4-induced liver injury in rats. Food Chem Toxicol 44(12):1989–1996
Liu C, Chen J, Li E, Fan Q, Wang D, Li P, Li X, Chen X, Qiu S, Gao Z (2015) The comparison of antioxidative and hepatoprotective activities of Codonopsis pilosula polysaccharide (CP) and sulfated CP. Int Immunopharmacol 24(2):299–305
Cederbaum AI, Lu Y, Wu D (2009) Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol 83(6):519–548
Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, Duffield JS, Iredale JP (2007) Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol 178(8):5288–5295
Boltjes A, Movita D, Boonstra A, Woltman AM (2014) The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J Hepatol 61(3):660–671
Nieto N (2006) Oxidative-stress and IL-6 mediate the fibrogenic effects of rodent Kupffer cells on stellate cells. Hepatology 44(6):1487–1501
Gonzalez Y, Aryal B, Chehab L, Rao VA (2014) Atg7-and Keap1-dependent autophagy protects breast cancer cell lines against mitoquinone-induced oxidative stress. Oncotarget 5(6):1526
Kroemer G, Mariño G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40(2):280–293
Acknowledgements
This work was funded by the Natural Science Foundation of China (Grant numbers 31602123, 31472253); Jiangsu Agricultural science and technology independent innovation foundation of China (CX (15)1067) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Hamid, M., Liu, D., Abdulrahim, Y. et al. Inactivation of Kupffer Cells by Selenizing Astragalus Polysaccharides Prevents CCl4-Induced Hepatocellular Necrosis in the Male Wistar Rat. Biol Trace Elem Res 179, 226–236 (2017). https://doi.org/10.1007/s12011-017-0970-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-0970-x