Abstract
Epimedium polysaccharide (EPS) and isatis root polysaccharide (IRPS) were extracted, purified, and selenizingly modified by nitric acid-sodium selenite method to obtain nine selenizing EPSs (sEPSs), sEPS1–sEPS9 and nine selenizing IRPSs (sIRPSs), sIRPS1–sIRPS9, respectively. Their effects on chicken peripheral lymphocyte proliferation in vitro were compared by MTT assay. The results showed that selenium polysaccharides at appropriate concentration could promote lymphocyte proliferation more significantly than unmodified polysaccharides, sEPS5 and sIRPS5 with stronger actions were picked out and injected into the chickens vaccinated with Newcastle disease vaccine in vivo tests. The peripheral lymphocyte proliferation and serum antibody titer were determined. The results showed that sEPS5 and sIRPS5 could elevate serum antibody titer and promote lymphocyte proliferation more significantly than unmodified polysaccharides, sEPS5 possessed the strongest efficacy. These results indicate that selenylation modification can significantly enhance the immune-enhancing activity of EPS and IRPS, and sEPS5 can be as a new-type immunopotentiator of chickens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polysaccharides that exist widely in nature are highly hydrated polymers composed of repeating single units (monosaccharides) joined by glycosidic linkages. They can be homo- or heteropolymers and may be substituted with both organic and inorganic molecules. Plant polysaccharides exhibit more complex branching structure and may either be neutral or have polyanions [1]. In recent decades, polysaccharides have attracted a great deal of attention because of their various biological activities, largely due to their immunostimulatory, antiviral, antioxidant, and antiaging effects. A large number of immune tests proved that polysaccharides could not only activate complements and immune cells, such as T, B lymphocytes, macrophages, natural killer cells (NK) and so on, but also improve the production of cytokines and even play a regulatory role in the immune system [2, 3]. Epimedium herb (Herba Epimedii) and isatis root (Radix isatidis) are commonly used as traditional Chinese medicines, in which polysaccharide is one of their most important active ingredients.
Many researches found that appropriate molecular modification or structure reform could make polysaccharides generate new activity or further enhance original activity [4–6]. At present, people pay more attention to selenylation modification of polysaccharides. The most commonly used method is nitric acid-sodium selenite (HNO3-Na2SeO3) method since it is characterized by simpler reaction conditions, shorter duration, the derivant with higher selenium content, and so on.
According to our previous researches, epimedium polysaccharide (EPS) and isatis root polysaccharide (IRPS) were extracted by water decoction and ethanol precipitation, selenizingly modified by HNO3-Na2SeO3 method according to L9(34) orthogonal design of three factors, the usage amount of sodium selenite, reaction temperature, and reaction time, each at three levels to obtain nine selenizing EPSs, sEPS1–sEPS9, and nine selenizing IRPSs, sIRPS1–sIRPS9. Their selenium contents were determined by hydride generation atomic fluorescence spectrometry (HG-AFS), and the structures were identified by Fourier transform infrared (FT-IR). In vitro and in vivo experiments, their immune-enhancing activities were measured. The object of this study is to verify the probability that selenylation modification improves the immune-enhancing activity of unmodified polysaccharides, select out the best selenizing polysaccharide and its optimal modification conditions, and offer theoretical evidence for the development of new-type immunopotentiator.
Materials and Methods
Herbs and Reagents
Epimedium and isatis root were purchased from Nanjing Jinling pharmacy of Jiangsu province, the former was the product of Anhui Jiren Pharmaceutical Co., Ltd., and the latter was the product of Xuzhou Pengzu Chinese traditional medicine company, China.
Nitric acid (HNO3) and sodium nitrite (Na2SeO3) were the products of Shanghai Lingfeng Chemical Reagent Co., Ltd. Na2SeO3 was dissolved into 0.05 g mL−1 with ultrapure water. An Se element standard solution (standard values 1000 μg mL−1) that was provided by the National Center of Analysis and Testing for Nonferrous Metals and Electronic Materials was accurately diluted into 1 μg mL−1. Perchloric acid (HClO4) was the product of Tianjin Xinyuan Chemical Co., Ltd.
Sodium heparin was dissolved into 5 mg mL−1 with calcium and magnesium-free (CMF) phosphate-buffered saline (PBS, pH 7.4) and filtered through a 0.22-μm syringe filter. Hanks’ solution, pH was adjusted to 7.4 with 5.6 % sodium bicarbonate solution, supplemented with benzylpenicillin 100 and 100 IU mL−1 streptomycin. RPMI-1640 (Gibco) supplemented with 100 IU mL−1 benzylpenicillin, 100 IU mL−1 streptomycin and 10 % fetal bovine serum was used for washing and resuspending cells, diluting mitogen, and culturing the cells. The 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Biosharp) was dissolved into 5 mg mL−1 with CMF PBS and filtered through a 0.22-μm syringe filter. The sodium heparin solution was stored at −20 °C, the others were at 4 °C, and MTT solution was in a dark bottle. Lymphocyte Separation Medium was manufactured by Shanghai Hengxin Chemicals Ltd. Dimethylsulfoxide (DMSO) was the product of Shanghai Lingfeng Chemical Reagent Co., Ltd. Newcastle disease vaccine (ND vaccine, La Sota strain) was purchased from Nanjing Tianbang Biotechnology Co., Ltd.
Extraction and Purification of EPS and IRPS
Epimedium pieces and isatidis root pieces (1 kg of each) were respectively refluxed with 95 % alcohol to remove colored ingredients and small molecular impurities. After drying, the residues were extracted twice, every 2 h in hot water. Extraction solutions were concentrated into about 1000 mL and centrifuged at 3500 rpm for 15 min. The supernatant was precipitated with a fourfold volume of 90 % ethanol solution for 12 h at 4 °C. Precipitates were solubilized in deionized water, deproteinized by Sevage assay, freeze-dried to obtain EPS, and IRPS.
Selenylation Modification of EPS and IRPS
Three factors respectively at three levels, the usage amount of sodium selenite at 200, 300, and 400 mg for 500 mg of polysaccharide (A), the reaction temperature at 50, 70, and 90 °C (B), and the reaction time for 6, 8, and 10 h (C) were selected. Nine modification conditions were designed according to L9 (34) orthogonal test (Table 1).
Two kinds of polysaccharides were respectively divided into nine portions, each portion was 500 mg, respectively, added into the three-necked flask filled with 50 mL of 0.5 % HNO3 solution and stirred to make polysaccharide completely dissolve. Then, the sodium selenite solution was added, and the reaction was performed at a definitive temperature and duration designed in Table 1. After the reaction finished, the mixture was cooled to room temperature, adjusted pH to 5–6 with saturated sodium carbonate solution, centrifugated, and dialyzed in a dialysis sack with a 1-kDa ultrafiltration membrane against tap water until no free sodium selenium was detected by ascorbic acid method [7], and the polysaccharide solution was concentrated and freeze-dried. Nine selenizing EPSs named sEPS1–sEPS9 and nine selenizing IRPSs named sIRPS1–sIRPS9 were obtained.
Identification of sEPSs and sIRPSs
The contents of selenium and carbohydrate and the FT-IR spectra of sEPSs and sIRPSs were tested. The carbohydrate contents were determined by phenol-sulfuric acid method [8].
Selenium Content Assay of sEPSs and sIRPSs
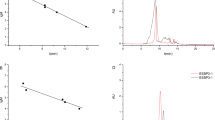
HG-AFS was used to determine selenium contents by SA-10 atomic fluorescence morphological analyzer (Beijing Jitian Instrument Co., Ltd.) [9]. The working conditions were as follows: the negative high voltage was 270 V, the height of atomizer was 8 mm, the atomization temperature was 200 °C, the discharges of carrier gas and shield gas flow were 400 mV min−1 and 1000 mL min−1 respectively, the injection volume was 1 mL, the reading mode was peak area, reading time was 10 s, and the delay time was 10 s. One hundred milliliters of standard selenium solution and 5 % HCl solution as diluent was linked to the HG-AFS. The concentrations of standard curve were set at 0, 2.5, 5, 10, 20, 40 ng mL−1, the corresponding absorbances were determined. The standard curve was drawn taking the selenium concentration as abscissa and fluorescence intensity as vertical axis.
Twenty milligrams of each selenium polysaccharide (nine sEPSs and nine sIRPSs) weighed accurately was dissolved in 10 mL of ultrapure water, 0.5 mL of selenium polysaccharide solution was accurately measured and added into a triangular flask with a cork, 10 mL of HClO4-HNO3 (v/v, 1:1) mixed acid solution was added to digest for 12 h at 4 °C then heated under 180 °C, replenishing the mixed acid solution timely. When the solution became clear, colorless, and accompanied by white smoke, it was concentrated to about 2 mL. After being cooled to room temperature, 8 mL of 6 mol L−1 HCl was added, shaken well, heated again under 180 °C until the solution was concentrated to about 2 mL, again cooled to room temperature, and diluted accurately to 25 mL with 5 % HCl solution, from which 1 mL of the solution was accurately shifted and diluted into 100 mL with 5 % HCl solution then the sample solution was obtained. The blank sample solution was prepared by the same method. The fluorescence intensities of the sample solution and the blank sample solution were detected by the spectrometer. The selenium contents were calculated according to the standard curve.
Infrared Spectroscopy Analysis of sEPSs and sIRPSs
After drying in a drying oven for 2 h, 1 mg of EPS, IRPS, sEPSs, or sIRPSs was mixed with 100–200 mg of dried potassium bromide (KBr), ground in the agate mortar and crushed into slice by the KBr pellets method. The FT-IR spectra of in a wavenumber range of 4000–400 cm−1 were recorded with a Nicolet 200 Magna-IR spectrometer (Nicolet instrument Corp.).
Comparison of Immune-enhancing Activity In Vitro of sEPSs and sIRPSs
The effect of sEPS1–sEPS9, sIRPS1–sIRPS9, EPS, and IRPS on chicken peripheral lymphocyte proliferation in vitro was determined by MTT assay [10]. Nine sEPSs and nine sIRPSs were respectively diluted with RPMI-1640 twofold serially from 1.563 to 0.098 μg mL−1, a total of five concentrations, based on the results of the safety concentration determination. Blood samples were collected from 50-day-old non-vaccinated chickens and transferred immediately into aseptic capped tubes with sodium heparin then diluted with an equal volume of Hanks’ solution and carefully layered on the surface of Lymphocyte Separation Medium. After 20 min of centrifugation at 2000 rpm, a white cloud-like lymphocytes’ band was collected and washed twice with RPMI-1640 media without fetal bovine serum. The resulting pellet was resuspended to 2.5 × 106 mL−1 with RPMI-1640 media added with fetal bovine serum and inoculated into 96-well culture plates, 100 μL per well. Then, in polysaccharide groups, the 20 polysaccharides at series of concentrations were respectively added, 100 μL per well, four wells each concentration, in cell control group (CC), RPMI-1640 media of 100 μL. The plates were incubated at 38.5 °C in a humid atmosphere of 5 % CO2. After incubation for 44 h, 20 μL of MTT (5 μg mL−1) was added into each well and continued to incubate for 4 h. The supernatant was removed carefully and 100 μL of DMSO were added into each well. The plates were shaken for 5 min to dissolve the crystals completely. The absorbance of cells in each well was measured by a microliter enzyme-linked immunosorbent assay reader (Model DG-3022, East China Vacuum Tube Manufacturer) at a wavelength of 570 nm (A 570 value) [11]. Meanwhile, the cellular proliferation rate was calculated to compare the strength of lymphocyte proliferation according to the equation [12, 13]: lymphocyte proliferation rate (%) = (A polysaccharide group − A control group) / A control group × 100 % (A was an average value of five concentration groups of polysaccharide or four wells of cell control group).
Comparison of Immune-enhancing Activity In Vivo of sEPS5 and sIRPS5
sEPS5 and sIRPS5 were selected to further compare their immune-enhancing activity in vivo based on the results of in vitro test. Four polysaccharides were respectively diluted into 2 mg mL−1 with deionized water according to the net contents of polysaccharides. The diluted preparations were sterilized by pasteurization and detected for endotoxin by pyrogen tests. When the endotoxin amount was up to the standard of the Chinese Veterinary Pharmacopoeia (less than 0.5 EU mL−1) (Veterinary Pharmacopoeia Commission of the People’s Republic of China, 2010), they were stored at 4 °C for the test.
Animals and Experimental Design
One-day-old White Roman chickens (male) were purchased from Tangquan Poultry Farm and housed in wire cages (100 × 60 × 40 cm) in air-conditioned rooms at 37 °C with 24 h light at the beginning of the pretrial period. The temperature was gradually decreased to room temperature and the light to 12 h per day. These parameters were maintained during the following days. The chickens were fed with commercial diet provided by the feed factory of Jiangsu Academy of Agricultural Science. All animal experiments were performed in accordance with the guideline approved by the Institutional Animal Care and Use Committee of the Laboratory Animal Research Center in Nanjing Agricultural University.
At 14 days old, their average maternal ND-HI antibody titer was 3.2 log2, 180 chickens were randomly assigned into six groups. The chickens except in blank control (BC) group were vaccinated with ND vaccine, repeated vaccination at 28 days old. At the same time of each vaccination, the chickens in four polysaccharide groups were intramuscularly injected respectively with 0.5 mL of sEPS5, sIRPS5, EPS, and IRPS, in vaccination control (VC) and BC groups, with the equal volume of physiological saline.
Serum HI Antibody Assay
On days 7 (D7), 14 (D14), 21 (D21), and 28 (D28) after the first vaccination, the blood samples of eight chickens randomly from each group were collected for examination of serum hemagglutination inhibition (HI) antibody titer by micro-method [14].
Peripheral Lymphocytes Proliferation Assay
On days 7 (D7), 14 (D14), 21 (D21), and 28 (D28) after the first vaccination, the blood samples of four chickens randomly from each group were collected for the determination of peripheral lymphocyte proliferation by MTT assay mentioned above. The cellular A 570 values were determined as the index of lymphocyte proliferation. Meanwhile, the average lymphocyte proliferation rates were calculated to compare the strength of lymphocyte proliferation according to the equation: Average lymphocytes proliferation rate (%) = (‾A polysaccharide group − ‾A BC group) / ‾A BC group × 100 % (‾A was an average value of each group).
Statistical Analysis
Data were expressed as means ± SD and the Duncan’s multiple range test was used to determine the difference among groups with the software SPSS 17.0. Differences between means were considered significant at P < 0.05.
Results
The Modification Conditions, Yields, and Contents of Selenium and Carbohydrate of sEPSs and sIRPSs
The modification conditions, yields, and contents of selenium and carbohydrate of sEPSs and sIRPSs are listed in Table 1. In nine sEPSs, the yield of sEPS1 was the highest, up to 36.54 %, and the next were sEPS7, sEPS4, and sEPS5. The highest selenium content was sEPS9 (21.89 mg g−1), and the next were sEPS6, sEPS3, and sEPS8. The highest carbohydrate content was sEPS5 (47.92 %), and the next were sEPS2, sEPS4, and sEPS8. Moreover, in nine sIRPSs, the yield of sIRPS5 was the highest, up to 27.34 %, and the next were sIRPS1, sIRPS2, and sIRPS8. The highest selenium content was sIRPS9 (15.21 mg g−1), and the next were sIRPS6, sIRPS8, and sIRPS3. The highest carbohydrate content was sIRPS3 (86.56 %), and the next were sIRPS6, sIRPS9, and sIRPS5.
The Infrared Spectroscopy Characteristic of sEPS and sIRPS
The FT-IR spectra of sEPS, sIRPS, EPS, and IRPS in the ranges of 4000–400 cm−1 are illustrated in Figs. 1 and 2. The spectra of EPS (Fig. 1b) and IRPS (Fig. 2b) exhibited the characteristic vibration bands of polysaccharides. The broad O–H stretching absorption band appeared at 3363.16 cm−1 in EPS and 3387.74 cm−1 in IRPS, and a weak C–H stretching vibration band at 2935.53 cm−1 in EPS and 2933.16 cm−1 in IRPS could be observed. The peak at 1637.89 cm−1 in EPS or at 1642.52 cm−1 in IRPS was characteristic of the C=O stretching vibration in COOH. The bands attributed to C–O–C stretching vibrations appeared at about 1400–1000 cm−1.
As compared with the spectrogram of unmodified polysaccharides, the FT-IR spectroscopy of sEPS (Fig. 1a) and sIRPS (Fig. 2a) presented one characteristic absorption band at 662.82 and 634.65 cm−1, respectively, describing an asymmetrical Se–O–C stretching vibration mode, which signified that EPS and IRPS were successfully modified in selenylation.
The Peripheral Lymphocyte Proliferation Changes of Polysaccharide In Vitro
The cellular A 570 values of every group are listed in Table 2. The A 570 values in sEPS1 and sEPS6 at 0.391–0.098 μg mL−1; sEPS2, sEPS4, and sEPS5 at 1.563–0.098 μg mL−1; sEPS3, sEPS7, and sEPS8 at 0.782–0.098 μg mL−1; sEPS9 at 0.196–0.098 μg mL−1; and EPS at 0.782–0.391 μg mL−1 groups were significantly larger than that of the corresponding cell control group (P < 0.05). The A 570 values in these ten polysaccharide groups from the rest of the concentration groups were larger than that of the corresponding cell control group (P > 0.05).
The A 570 values in sIRPS1, sIRPS3, and sIRPS5 at 0.782–0.098 μg mL−1, sIRPS2 at 0.391 and 0.098 μg mL−1, sIRPS4 at 0.391–0.196 μg mL−1, sIRPS6 at 1.563–0.098 μg mL−1, sIRPS7 at 0.782 μg mL−1, sIRPS8 at 0.782–0.196 μg mL−1, sIRPS9 at 0.391–0.098 μg mL−1, and IRPS at 1.563 μg mL−1 groups were significantly larger than that of the corresponding cell control group, respectively (P < 0.05). The A 570 values in these ten polysaccharide groups at the rest concentration groups were larger than that of the corresponding cell control group (P > 0.05).
The lymphocyte proliferation rates of every group are illustrated in Fig. 3. In nine sEPSs, the proliferation rate in sEPS5 group was the highest (25.50 %), the next were sEPS8 (20.56 %), sEPS4 (20.29 %), and sEPS2 (18.68 %) groups, these four groups were significantly higher than that of EPS group (P < 0.05). The proliferation rates of the rest of the polysaccharide groups were higher than that of EPS group (P > 0.05). In nine sIRPSs, the proliferation rate in sIRPS5 group was the highest (19.01 %), the next were sIRPS9 (16.41 %) and sIRPS8 (15.68 %), these three groups were significantly higher than that of IRPS group (P < 0.05). The proliferation rates of the rest of the polysaccharide groups were higher than that of IRPS group (P > 0.05). Among 18 selenium polysaccharides, the proliferation rate of sEPS5 group was the highest.
The Changes of Serum Antibody Titer In Vivo
The antibody titers of every group are illustrated in Table 3. On D7, the antibody titer in sEPS5 group was significantly higher than those in VC and BC groups (P < 0.05). On D14, the serum antibody titers in all polysaccharides except IRPS groups were significantly higher than that in VC group; in sEPS5 and sIRPS5 groups, the serum antibody titers in all polysaccharides except IRPS groups were significantly higher than those in EPS and IRPS groups, respectively. On D21, the serum antibody titers in all polysaccharide groups were significantly higher than those in VC and BC groups; in sEPS5 group, the serum antibody titers in all polysaccharide groups were significantly higher than that in EPS group. On D28, the serum antibody titers in all polysaccharide groups were significantly higher than those in VC and BC groups. On D7–D28, sEPS5 group was the highest.
The Changes of Lymphocyte Proliferation In Vivo
The cellular A 570 values of every group are illustrated in Table 4. On D7–D28, the A 570 values in sEPS5 and sIRPS5 groups were significantly higher than those in VC and BC groups at the same time points (P < 0.05). On D7–D21, the A 570 values in sEPS5 and sIRPS5 groups were significantly higher than those in EPS and IRPS groups at the same time points, respectively.
The average lymphocyte proliferation rates of every group are illustrated in Fig. 3. The lymphocyte proliferation rate in sEPS5 group was the highest on D21 (50.12 %), the following were sEPS5 group on D28 (48.36 %) and sIRPS5 group on D21 (41.71 %), they were significantly higher than that of the corresponding unmodified polysaccharide at the same time points (P < 0.05) (Fig. 4).
Discussion
It has been reported that alpha glycosidic bond may connect Se to monosaccharide residues in ganoderma lucidum polysaccharides. Se element might take the form of C–O–SeO3 or Se=O in selenium polysaccharide so that tertiary and quaternary structures of the polysaccharide chain were changed [15–17]. The results of our studies showed that the selenium contents of nine sEPSs were 3.32–21.89 mg g−1, and those of nine sRIPSs were 2.65–15.21 mg g−1. Infrared spectrum analysis showed that sEPS and sIRPS respectively presented one characteristic absorption band at 662.82 and 634.65 cm−1 as compared with the spectrogram of unmodified polysaccharides, describing an asymmetrical Se–O–C stretching vibration mode. This confirmed that there were selenite groups in the structures of sEPS and sIRPS, and EPS and IRPS were successfully modified in selenylation.
By stimulation of mitogens or antigens in vitro, lymphocytes can be converted into lymphoblasts then split and proliferated. Usually lymphocyte proliferation is an effective index to evaluate cellular immunity [11].The results of experiments in vitro displayed that the A 570 values of sEPS2, sEPS4, and sEPS5 at five; sEPS3, sEPS7, and sEPS8 at four; sEPS1 and sEPS6 at three; and sEPS9 and EPS at two concentration groups were significantly larger than that of the corresponding cell control group, respectively; sIRPS6 at five; sIRPS1, sIRPS3, and sIRPS5 at four; sIRPS2, sIRPS4, sIRPS8, and sIRPS9 at three; and sIRPS7 and IRPS at one concentration groups were significantly larger than that of the corresponding cell control group, respectively. This indicated that these polysaccharides at these concentrations could significantly enhance cellular immunity. Lymphocyte proliferation rate is an indicator to compare the strength of immune-enhancing activity of polysaccharide. In nine sEPSs, the lymphocyte proliferation rate of sEPS5 group was the highest, the following were sEPS8, sEPS4, and sEPS2 groups, these four groups were significantly larger than that of unmodified EPS group; in nine sIRPSs, the lymphocyte proliferation rate of sIRPS5 group was the highest, the following were sIRPS9 and sIRPS2 groups, these three groups were significantly larger than that of unmodified IRPS group. This indicated that selenylation modification of EPS and IRPS could significantly enhance the cellular immune activity. According to the above test results, sEPS5 and sIRPS5 have been selected out for vivo tests.
It was reported that selenium polysaccharides could significantly improve the cellular and humoral immune responses in mice [18]. An antibody is an important molecule to mediate humoral immunity. The antibody titer reflects humoral immune status of animals [19]. The experimental results in vivo showed that the serum antibody titers in all polysaccharide groups except EPS group on D7 and IRPS group on D7–D14 were significantly higher than that of the corresponding VC group at all time points. This indicated that these four polysaccharides could enhance the humoral immune response of ND vaccine. Furthermore, making a comparison between selenizing and non-selenizing polysaccharide groups, the serum antibody titers of sEPS5 group on D14 and D21 were significantly higher than that of the corresponding EPS group and the serum antibody titers of sIRPS5 group on D14 were significantly higher than that of the corresponding IRPS group. This indicated that selenylation modification of EPS and IRPS could significantly enhance a humoral immune response.
In in vivo test, the results of lymphocyte proliferation showed that the A 570 values in sEPS5 and sIRPS5 groups at all time points, EPS except on D7 and IRPS except on D14 at rest three time points were all significantly larger than those of the corresponding VC and BC groups, this indicated that four kinds of polysaccharides could all significantly enhance a cellular immune response. Making a comparison, the A 570 values in sEPS5 group on D7–D21 were significantly higher than that of EPS group, the A 570 values in sIRPS5 group at all time points were significantly higher than that of IRPS group, which indicated that the cellular immune-enhancing activities of sEPS5 and sIRPS5 were all significantly stronger than that of non-selenizing polysaccharides. The lymphocyte proliferation rates in four polysaccharide groups almost at all time points were significantly higher than that in VC group, in selenizing polysaccharide groups were all significantly higher than that in the corresponding non-selenizing polysaccharide group, and sEPS5 group on D14–D28 were the highest and on D21–D28, significantly higher than those of other polysaccharide groups at the same time point. This further confirmed that selenylation modification could significantly improve cellular immune response, and sEPS5 was the strongest.
As organic selenium compounds, selenium polysaccharides have the activities of both selenium and polysaccharides. Studies have shown that biological activities of selenium polysaccharides were generally higher than that of selenium and the corresponding polysaccharide. As compared with inorganic selenium, organic forms of selenium reduces the toxicity of selenium, it is easily absorbed and utilized by organism [20, 21]. As for the mechanisms of selenizing polysaccharides to enhance immunity, selenium plays an important role [22], it can promote the proliferation of T lymphocytes, improve the function of B lymphocytes, and increase the number of neutrophils [23, 24].
Our previous studies have shown that selenylation modification could significantly stimulate the immune-enhancing activity of garlic polysaccharide and angelica polysaccharide [25, 26]. This study demonstrated that selenylation modification could significantly improve the immune-enhancing activity of EPS and IRPS, sEPS5 presented the best efficacy and could be expected as a new-type immunologic adjuvant, its optimal modification conditions were 300 mg of sodium selenite for 500 mg of EPS, the reaction temperature of 70 °C, and the reaction time of 6 h.
References
Vandamme E, Baets S, Steinbuchel A (2002) Polysaccharides I: polysaccharides and prokaryotes (biopolymers series)
Jiang X, Xu M, Yin YJ (2009) The bioactivity of polysaccharide and its application in animal production. Chin J Vet Med 36:31–34
Rioux S, Girard C, Dubreuil JD, Jacques M (1998) Evaluation of the protective efficacy of Actinobacillus pleuropneumoniae serotype 1 detoxified lipopolysaccharides or O-polysaccharide-protein conjugate in pigs. Res Vet Sci 65:165–167
Melo MRS, Feitosa JPA, Freitas ALP, Paula RCM (2002) Isolation and characterization of soluble sulfated polysaccharide from the red seaweed Gracilaria cornea. Carbohydr Polym 49:491–498
Zhang M, Cheung PCK, Ooi VEC, Zhang LN (2004) Evaluation of sulfated fungal b-glucans from the sclerotium of Pleurotus tuber-regium as a potential watersoluble anti-viral agent. Carbohydr Res 339:2297–2301
Wang XH, Zhang LN (2009) Physicochemical properties and antitumor activities for sulfated derivatives of lentinan. Carbohydr Res 344:2209–2216
Li G, Miu J, Liu F (2001) Selenium polysaccharide compounds and their preparation methods. Chinese Patent NO: CNn21414C
Yu W, Yang XM, Liu WM, Liu F, Ma HL (2009) Assay study on content of polysaccharides in Ficus carica by phenol-sulfuric acid method. Food Sci Technol 10:256–258
Gao J, Qin S, Huang K (2006) Assay of organic selenium and inorganic selenium of enriched yeast by hydride generation atomic fluorescence spectrometry method. J Anal Chem 22:157–159
Wang DY, Hu YL, Sun JL, Kong XF, Zhang BK, Liu JG (2005) Comparative study on adjuvanticity of compound Chinese herbal medicinal ingredients. Vaccine 23:3704–3708
Thekisoe MMO, Mbati PA, Bisschop SPR (2004) Different approaches to the vaccination of free ranging village chickens against Newcastle disease in Qwa-Qwa, South Africa. Vet Microbiol 101:23–30
Yu J, Jiang Z, Yan H, Zhu L (2005) Effect of pachyman on cell-mediated immunity activity and anti-tumor function in chicken infected with vMDV. Chin J Vet Sci Technol 34:70–73
Lu Y, Wang DY, Hu YL, Huang XY, Wang JM (2008) Sulfated modification of epimedium polysaccharide and effects of the modifiers on cellular infectivity of IBDV. Carbohydr Polym 71:180–186
Abula SFD, Wang JM, Hu YL, Wang DY, Sheng X, Zhang J, Zhao XN, Nguyen TL, Zhang YQ (2011) Screening on the immune-enhancing active site of Siberian solomonseal rhizome polysaccharide. Carbohydr Polym 85:687–691
Shang DJ, Wang GJ, Wang XM, Tian X, An Y (2001) Isolation, purification and property identification of two kinds of polysaccharide containing selenium in Ganoderma lucidum. J Dalian Univ Technol 41:165–168
Shang DJ, Cui Q, Tian X (2002) Separation, purification and molecular composition analysis of Ganoderma lucidum selenium polysaccharide SeGLP-2A. Chin J Edible Fungi 21:35–37
Shang DJ, Wang XM, Xu YT, Tian X, An Y (2002) Separation, purification and analysis of selenium activity structure of SeGLP-1. Acta Edulis Fungi 9:22–27
Xu CL, Wang YZ, Jin ML, Yang XQ (2009) Preparation, characterization and immunomodulatory activity of selenium-enriched exopolysaccharide produced by bacterium Enterobacter cloacae Z0206. Bioresour Technol 100:2095–2097
Thekisoe MMQ, Mbati PA, Bisschop SPR (2003) Diseases of free-ranging chickens in the Qwa-Qwa District of the northeastern Free State province of South Africa. J S Afr Vet Assoc 74:11–14
Staaf M, Yang Z, Widmalm G (2000) Structural elucidation of the viscous exopolysaccharide produced by Lactobacillus helveticus Lb161. Carbohydr Res 326:113–119
Behne D, Kyriakopoulos A, Scheid S, Gessner H (1991) Effects of chemical form and dosage on the incorporation of selenium into tissue proteins in rats. J Nutr 121:806–814
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 521:273–1280
McKenzie RC, Rafferty TS, Beckett GJ (1998) Selenium: an essential element for immune function. Immunol Today 19:342–345
Hawkes WC, Kelley DS, Taylor PC (2001) The effects of dietary selenium on the immune system in healthy men. Biol Trace Elem Res 81:189–213
Qin T, Chen J, Wang DY, Hu YL, et al (2013) Selenylation modification can enhance immune-enhancing activity of Chinese angelica polysaccharide. Carbohydr Polym 95:183–187
Qiu SL, Chen J, Qin T, et al (2014) Effects of selenylation modification on immune-enhancing activity of garlic polysaccharide. PLoS One 9:e86377
Acknowledgments
The project was supported by National Natural Science Foundation of China (31272596), Special Fund for Agro-scientific Research in the Public Interest (201403051), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. We are grateful to all other staff in the Institute of Traditional Chinese Veterinary Medicine of Nanjing Agricultural University for their assistance in the experiments.
Compliance with Ethical Standards
ᅟ
Conflict of Interest
The authors declare that they have no conflict of interests.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Institutional Animal Care and Use Committee of the Laboratory Animal Research Center in Nanjing Agricultural University.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Hou, R., Yue, C. et al. The Selenylation Modification of Epimedium Polysaccharide and Isatis Root Polysaccharide and the Immune-enhancing Activity Comparison of Their Modifiers. Biol Trace Elem Res 171, 224–234 (2016). https://doi.org/10.1007/s12011-015-0511-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0511-4