Abstract
A total of 80 female albino mice were randomly allotted into five groups (n = 16) as follows: (A) normal control, (B) high-fat diet (HFD),; (C) HFD + probiotics (P), (D) HFD + sodium selenite (SS), and (E) HFD + selenium-enriched probiotics (SP). The selenium content of diets in groups A, B, C, D, and E was 0.05, 0.05, 0.05, 0.3, and 0.3 μg/g, respectively. The amount of probiotics contained in groups C and E was similar (Lactobacillus acidophilus 0.25 × 1011/mL and Saccharomyces cerevisiae 0.25 × 109/mL colony-forming units (CFU)). The high-fat diet was composed of 15 % lard, 1 % cholesterol, 0.3 % cholic acid, and 83.7 % basal diet. At the end of the 4-week experiment, blood and liver samples were collected for the measurements of lipid metabolism, antioxidative status, histopathological lesions, and related gene expressions. The result shows that HFD significantly increased the body weights and liver damages compared to control, while P, SS, or SP supplementation attenuated the body weights and liver damages in mice. P, SS, or SP supplementation also significantly reversed the changes of alanine aminotransferase (AST), aspartate aminotransferase (ALT), total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), total protein (TP), high-density lipoprotein (HDL), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalasa (CAT), and malondialdehyde (MDA) levels induced by HFD. Generally, adding P, SS, or SP up-regulated mRNA expression of carnitine palmitoyltransferase-I (CPT1), carnitine palmitoyltransferase II (CPT2), acetyl-CoA acetyltransferase II (ACAT2), acyl-coenzyme A oxidase (ACOX2), and peroxisome proliferator-activated receptor alpha (PPARα) and down-regulated mRNA expression of fatty acid synthase (FAS), lipoprotein lipase (LPL), peroxisome proliferator-activated receptor gamma (PPARγ), and sterol regulatory element-binding protein-1 (SREBP1) involved in lipid metabolism. Among the group, adding SP has a maximum effect in improving lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in mice fed a HFD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a worldwide epidemic that is characterized not only by excessive fat deposition but also by systemic microinflammation, high oxidative stress, and increased cardiovascular risk factors [1, 2]. It is known that an oversupply of fat is associated with the development of obesity in mice [3]. Long-term feeding on a high-fat diet can induce obesity with hyperplasia, hypergluconemia, hyperlipidemia, and insulin resistance [4]. Furthermore, obesity, especially abdominal obesity, has an association with dyslipidemia characterized by increasing triglyceride (TG) and decreasing high-density lipoprotein cholesterol (HDL-C) concentrations [5].
Probiotics are live microbial food supplements that are beneficial to the health of the host when administered in adequate amounts [6]. Diet-induced obese mice treated with probiotics showed reduced body weight gain and fat accumulation as well as lowered plasma insulin, total-cholesterol, and liver toxicity biomarkers [7].
Selenium is an essential element in the human diet. Interestingly, there has been an increased consumption of dietary supplements containing this element in the form of either inorganic or organic compounds. The effect of using selenium as a dietary supplement in yogurt has been evaluated [8]. It seems likely that selenoproteins are central for antioxidant system regulation in the body [9].
Selenium-enriched probiotics (SP) is a new product developed by utilizing several strains of probiotics that can transform and enrich organic Se form from inorganic source. In our laboratory, a newly developed SP product was produced by culturing Lactobacillus acidophilus (L. acidophilus) and Saccharomyces cerevisiae (S. cerevisiae) with sodium selenite being added into the culture medium under suitable conditions of the microenvironment. Both strains have a strong ability to convert sodium selenite into organic Se. Previous studies of dietary SP supplementation used in our laboratory for livestock, poultry, rats, and mice showed that the SP has the combined effects of selenium and probiotics on lowering body weight, improving serum levels, antioxidative status, and gene expression. Based on the above criteria, we speculated that SP could manipulate the lipid profile, improve the antioxidative status, and mRNA lipid gene expression in induced obese mice by a high-fat diet. The aim of the present study was to evaluate the effects of SP on the growth performance, lipid metabolism, antioxidative status, histopathological lesions, biochemical indices, and related gene expression in mice fed a high-fat diet.
Materials and Methods
Animals, Diet, and Experimental Design
Eighty (80) imprinting control region (ICR) female mice at 4 weeks of age with an average weight of 23 g were purchased from the Center of Laboratory Animals, Yangzhou University (Yangzhou, China). The animals were housed according to the guidelines for laboratory animal experiments by the Nanjing Agricultural University Animal Care Committee, which was approved by the Jiangsu Science and Technology Department [approval ID: Syxk (Jiangsu) 2011-0036]. The mice were provided 12-h dark/12-h light, with a room temperature of 25 ± 2 °C according to the experimental conditions. The basal diets were formulated to meet the National Research Council (NRC, 1995) recommendations (with selenium content being 0.05 mg/kg) for mice. The animals were randomly allotted into five groups: (A) normal control, (B) high-fat diet (HFD) control, (C) probiotics (P), (D) HFD + sodium selenite (SS), and (E) HFD + SP. After 1 week of an acclimatization period on a normal chow diet, 64 mice were randomly subjected to HFD-induced obesity. The SS and selenium-enriched probiotics (SP) diets were made by grinding the HFD diet into powder and mixed with SS and SP. Sodium selenite (Na2SeO3) was purchased from the Beijing Chemical Reagent Company (Beijing, China) and the high-fat diet from the institute of Shoude (Nanjing, China).
P, SS, and SP Products
The SP and P products used for this study both contained the same level of two probiotic species 1011/mL colony-forming units (CFU) of L. acidophilus and 109/mL (CFU) of yeast (S. cerevisiae). However, both species in the SP product were selenium-enriched. The total content of Se in the SP was 10.0 mg/L, as detected by AF-610A atomic fluorescence spectrometer. Greater than 90 % Se was organic Se and >75 % in the form of Selenomethionine.
A method has been developed for the determination of selenomethionine in selenium-enriched yeast by gas chromatography-mass spectrometry (GC-MS). Three extraction methods were compared for extraction efficiency of selenomethionine from the samples. Selenomethionine in the samples was extracted for 24 h with proteinase in Tris buffer. The selenomethionine was derivatized with butanol and trifluoroacetic acid (TFA). The derivatization was accomplished in two steps, starting with the esterification of the carboxyl group of the selenoamino acid using butanol, followed by the acylation of the amino group with trifluoroacetic acid anhydride. The selected ion for monitoring selenomethionine was at m/z 349. The instrument operating conditions were optimized. The samples were analyzed by GC-MS with an external standard method. Standard GC-MS chromatograms and mass spectra for selenomethionine were also obtained. The method was proved to be accurate and reliable. The recoveries of 98.5–103.7 % with relative standard deviations (RSDs) of 0.9–2.4 % (n = 6) and the correlation coefficient of 0.9978 were obtained. The detection limit of selenomethionine for the method was 0.5 mg/L (S/N = 3), and the selenomethionine contents of real sample was given [10].
The total Se content in the SS stock solution was 100 mg/L. The SP product was developed by the Institute of Nutritional and Metabolic Disorders at the Nanjing Agricultural University (Nanjing, China), and the product was granted a patent in China in the year of 2006 (number ZL 2005 10040990.2). The high-fat diet was composed of 15 % lard, 1 % cholesterol, 0.3 % cholic acid, and 83.7 % basal diet. A practical diet contained corn, wheat, soybean meal, bran, fish meal, cod liver oil, eggs, barley,, amino acids, vitamins, and minerals; the main nutrient contents were water <10 %, crude protein >18 %, fat >4 %, crude fiber >5 %, crude ash <8 %, calcium 1 %, and phosphorus 0.2 %. These were all purchased from Nanjing city (Jiangsu, China).
Chemical
Total protein (TP), total cholesterol (TC), alanine aminotransferase (AST), and aspartate aminotransferase (ALT) were determined by Hitachi BS-300 Automated Chemistry Analyzer Machine using the corresponding commercials TP, TC, AST, and ALT purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). The low-density lipoprotein (LDL), HDL, TG, glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalasa (CAT), and malondialdehyde (MDA) (also purchased from the same Institute) were analyzed according to the method described in our lab by [11] using an AF-610A atomic fluorescence spectrometer Analysis Instrument (Beijing, China).
Histopathological Examination
The liver was removed, rinsed with physiological saline solution, blotted dry with filter paper, and weighed. Pathological lesions were observed, and the relative liver weight calculated. The liver samples were immersion fixed and stored in 10 % neutral buffered formalin and were processed routinely to paraffin. Five-micrometer sections were cut and stained with hematoxylin and eosin (H&E). Preparation of tissue for histopathology examination was done according to the methods described by [12].
mRNA Extraction and Real-Time PCR Assay
The mRNA expression levels of carnitine palmitoyltransferase-I (CPT1), carnitine palmitoyltransferase II (CPT2), acyl-coenzyme A oxidase ((ACOX2), acetyl-CoA acetyltransferase II (ACAT2), fatty acid synthase (FAS), lipoprotein lipase (LPL), peroxisome proliferator-activated receptor alpha (PPARα), peroxisome proliferator-activated receptor gamma (PPARγ), and sterol regulatory element-binding protein-1 (SREBP1) were determined by real-time PCR. The primer of the reference gene (β-actin) and target genes was designed by Primer 5.0 t1 online software and are shown in Table 1. Quantitative real-time PCR was performed on an ABI Prism 7300 Detection System (Applied Biosystems, USA). All reactions were performed in duplicate using a Kit provided by TaKaRa Biotechnology Company.
The relative gene mRNA levels were determined using the Δ cycle threshold (ΔCt) method with β-actin serving as a reference gene. For each of the target genes, the ΔΔCt values of all the samples were calculated by subtracting the average ΔCt of the control group from the average △Ct of the HFD, HFD + P, HFD + SS, or HFD + SP groups. The ΔΔCt values were converted to fold differences by raising 2 to the power of −ΔΔCt (i.e., 2−ΔΔCt) [13].
Statistical Analysis
All data were analyzed using the SPSS software computer program (version 19.0) for windows (IBM SPSS Statistics). All results are presented as the mean ± SEM. A one-way ANOVA was performed to statistical analysis, the differences among the five dietary groups were determined by Duncan’s contrasts, and a p value was considered to be significant at 0.05.
Results
Body Performance, Liver Weight, and Relative Liver Weight
After a 4-week feeding period, the HFD group showed significantly increased body weight compared with that of the control group (Table 2). There were no significant differences in the initial body weights among the five groups. P, SS, and SP supplementation ameliorated the increased body weight of HFD-fed mice, although the difference was not statistically significant (Table 2). The body weight gain of the HFD group was also increased compared to the control group, where P, SS, and SP attenuated this increase; however, the body weight gain of HFD + P group was still higher than that of the control group. These results revealed that the supplementation of P, SS, and SP moderately attenuated the increased body weight of HFD-fed mice.
The amount of food intake was significantly reduced in the HFD and HFD + P groups compared to the control group. In addition, the food efficiency ratio (FER), which is the total grams of body weight gained on a test food divided by the total grams of food consumed during an animal feeding study, was significantly increased compared with that of the control group.
The liver weight in the HFD, P, SS, or SP group was increase compared with the control group. However, there was no significant difference in the liver weight of the P, SS, and SP groups. As shown in Table 2, there was an increase in the relative liver weight of the HFD, P, SS, or SP groups compared with the control group (p < 0.05). However, the difference was not statistically significant in the relative liver weight between the HFD-fed, P, SS, or SP groups. P, SS, and SP supplementation did not affect the food intake in the HFD-fed mice.
Gross Examination of the Liver
The results showed that HFD feeding generated enlarged, pale, and greasy livers in groups B, C, D, and E (Fig. 1b–e) in comparison with the control group (Fig. 1a). Liver from Control A showed normal appearence (Fig. 1a). The groups C, D, and E showed moderated changes (Fig. 1c–e) than the group B where severe changes were shown (Fig. 1b). The group E fed with SP showed better color ((Fig. 1e) compared to groups B, C, and D (Fig. 1b–d) while the control group showed no changes. The group C fed with P and group D fed with SS showed a better color compared to B fed with HFD. The severity of lesiones is less in D and E (Fig. 1d–e) compared to B (Fig. 1b).
Histopathological Examination
Feeding the HFD caused remarkable fat accumulation in the liver. The hematoxylin-eosin (H&E)-stained sections showed the differences in liver tissue structures and lipids accumulation of the five groups. The mice livers of the control group had a well-organized structure. In the HFD group, the structures of the livers displayed large degrees of damages characterized by enlarged hepatocytes and an increasing degree of steatosis represented by the vacuolation in hepatocytes (Fig. 2b–e). Hepatocytes steatosis was obviously alleviated by P, SS, and SP supplementation compared with the HFD group. The study suggested that many massive lipid droplets were accumulated in the liver tissues in the HFD group, and the lipid droplets had obviously decreased in the P, SS, and SP groups (Fig. 2c–e).
Histopathological examination of liver tissue showing damage and hepatocytes arranged by penetrated fat vacuoles (×40 magnifications) (n = 16). a Control group showing normal liver with no lipid deposits. b HFD group showing liver tissue with the presence of steatosis. c HFD + P group appearing with several steatosis. d HFD + SS group cells affected with many large fat vacuoles. e HFD + SP group with moderate steatosis, where the number of hepatocytes with small lipid droplets tended to decrease
Serumal Lipid Profile
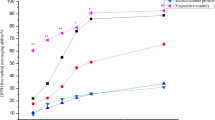
The effect of SP on serum levels of AST, ALT, TP, TC, HDL-C, LDL-C, and TG are summarized in Table 3 and Fig. 3. The AST, ALT, TC, LDL-C, and TG serum levels of mice fed a HFD greatly increased compared with the control group (p < 0.05). The groups treated with P, SS, and SP showed reduced biochemical levels compared to the HFD group. The elevated LDL-C and TG in the HFD group were indicative of progressive liver damage. P, SS, and SP could significantly reduce (p < 0.05) the serum levels of AST, ALT, TC, TG, and LDL-C compared with the HFD group while serum HDL-C and TP increased; SP was more effective than P and SS. However, SS showed a better result compared to P. The groups treated with P showed reduced biochemical levels compared to the HFD group.
Graph showing increased levels of HDL in groups which received supplementation with P, SS, and SP figures. The histograms are expressed as mean ± SD (n = 16). Bars with different letters differ significantly (p < 0.05). HDL high-density lipoprotein, LDL low-density lipoprotein. Groups which received P, SS and SP showing increased HDL-C (a) while the serum levels LDL-C significantly reduce (p< 0.05; b)
SOD, GSH-Px and CAT Activities and MDA Levels
The SOD, GSH-Px, and CAT levels decreased significantly in the HFD group compared with that of the control group (p < 0.05). The groups fed with P, SS, and SP supplementation improved the SOD, GSH-Px, and CAT activities (Fig. 4a–c) compared to the HFD group (p < 0.05). There was no significant difference in the SOD activities between the SS and SP groups. However, the SP group showed a further increase compared with the SS supplementation (p < 0.05; Fig. 4a). MDA levels significantly increased in liver of the HFD group compared to the control group (p < 0.05). However, P, SS, and SP supplementation significantly lowered the MDA levels compared with the HFD-treated group (p < 0.05; Fig. 4d). The groups which received P, SS, and SP showed lower levels of MDA. The SS and SP showed better results than the group which received P. The groups which received P showed lower levels of MDA compared to the HFD.
mRNA Expression Levels Associated with Lipid Metabolism
The mRNA expression levels associated with lipid metabolism are illustrated in Figs. 5 and 6. Feeding the mice with HFD significantly increased genes involved in the regulation of adipogenesis (PPARγ, SREBP1, FAS) and lipogenesis (LPL) and decreased genes involved in fatty acid βeta-oxidation (CPT1, CPT2, ACAT2, ACOX2, and PPARα) compared to the control group. We found that SP supplementation significantly reversed HFD gene expression changes including up-regulation of fatty acid βeta-oxidation (CPT1, CPT2, ACAT2, ACOX2, and PPARα) (Fig. 5) and down-regulation of adipogenesis (PPARγ, SREBP1, and FAS). SP supplementation showed better results up-regulating genes involved in fatty acid βeta-oxidation compared to control, P, and SS; however, groups which received SS showed a better result by up-regulating fatty acid βeta-oxidation (CPT1, CPT2, ACAT2, PPARα) compared to the control and P, but P supplementation showed better results compared to control in genes involved in fatty acid βeta-oxidation.
Genes involved in the regulation of adipogenesis showed increased levels of FAS, LPL, and SREBP1 (Fig. 6a–d) in the HFD fed groups compared to the control group. The groups which received P, SS, and SP showed decreased levels. The SP supplementation had better results in relation to the decline hepatic lipogenic genes. Its mRNA expression levels in the HFD, HFD + P HFD + SS, and HFD + SP groups were 3.0-, 2.7-, 2.5-, and 1.8-fold higher than the control group, respectively. SP decreased its levels compared with the HFD group; however, the groups which received SS showed a better result compared to P.
Discussion
Obesity represents a major risk factor for severe pathologies including non-alcoholic fatty liver disease (NAFLD), diabetes, coronary heart disease, hypertension, stroke, and cancer. This implies increased morbidity and mortality rates as well as high health-care costs.
After a 4-week feeding period, the HFD group showed significantly increased body weight (p < 0.01) compared with that of the control group (Table 2). There were no significant differences in the initial body weights among the five groups. In the present study, we found that SP supplementation ameliorated the increased body weight of the HFD-fed mice, although the difference was not statistically significant which corresponds with the results that were reported by [7] that diet-induced obese mice treated with probiotics (Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032) showed ameliorated body weight gain.
Also, the HFD generated an increase in the liver size and weight thus, making the liver large, yellowish, and greasy in color as well as increasing the percentage of lipid accumulation compared with the control. A similar result was reported earlier by [14, 15] that mice fed a HFD generated enlarged, yellowish, and greasy liver coloration. Liver weight was decreased by 35 % in the rats fed on a high-fat, high-cholesterol diet containing the probiotic [16].
The liver is a major site of glucose, fatty acid (FA), and triglyceride (TG) synthesis and serves as a major regulator of whole body nutrient homeostasis. An earlier report showing that chronic exposure of humans or rodents to high-calorie diets promotes non-alcoholic fatty liver disease (NAFLD), characterized by neutral lipid accumulation in lipid droplets (LD) of hepatocytes [17].
The HFD significantly increased the serums AST, ALT, TC, TG, and LDL-C resulting in lipid abnormalities in blood. The high-fat diet significantly (p < 0.01) elevates serumal total cholesterol, triglycerides, and low-density lipoprotein and decreases high-density lipoprotein [14, 18]. The elevated LDL-C and TG in the HFD group, whose results were indicative of progressive liver damage, induce the risk of coronary heart disease. LDL-C contributes to the buildup of fat deposits in the arteries (atherosclerosis), which can cause decreased blood flow and heart attack. The P, SS, and SP treatments reduced the serums AST, ALT, TC, TG, and LDL-C compared to the HFD group. Also, P, SS, and SP consumption significantly increased the TP and HDL-C levels (p < 0.05) compared with the HFD group. Both supplementation probiotics, namely inorganic selenium and selenium-enriched probiotics, significantly alleviated serumal lipid profile—a result which is in line with previous findings by other studies [16] suggesting that total cholesterol concentration in the serum was significantly lower in the probiotic group than in the control group throughout the experimental period. The mice fed with P showed reduced LDL concentration as compared to the mice fed with SS but SP supplementation had more effects on this aspect than P and SS; most likely, this was because organic forms of Se have less toxicity and greater bioavailability compared with inorganic forms. An earlier report showed that organic forms of Se are more digestible, better accumulated in tissue, and more biologically active than inorganic [19]. This finding also agrees with the result of earlier studies that the addition of probiotic mixture to the HFD reduced the fat deposition in mice fed a HFD [14]. It has also been shown that Se-enriched Agaricus blazei Murill (Se-AbM) on liver injury in mice induced obesity (p < 005) and decreased serum ALT, AST, and MDA levels [20]. The probiotic properties of L. acidophilus NS1, such as acid resistance, bile tolerance, and cholesterol assimilation activity including cholesterol and LDL cholesterol levels were significantly lower in mice fed a HFD with L. acidophilus NS1 than in those fed a HFD only [21]. When fed on a high-fat diet supplemented with Bifidobacterium breve B-3 at 10(8) or 10(9) CFU/day for 8 weeks. B. breve B-3 supplementation improved the serum levels of total cholesterol [15].
Oxidative stress is a continuous level of oxidative damage in animal cells, which is caused by an overabundance of reactive oxygen species or a decline in antioxidant ability against them;, these oxidative stress markers have been attenuated by administration of several antioxidants [22]. Catalase, traditionally considered a peroxisomal protein, was found to be present in cardiac mitochondria and significantly increased in content and activity during high-fat feeding [23]. Glutathione are well characterized as major components of the antioxidant defense, with roles in many cellular processes. In this study, high feeding of lipids promotes an oxidative stress in the liver of mice. The groups fed with P, SS, and SP supplementation improved SOD, GSH-Px, and CAT activities compared to the HFD group (p < 0.05; Fig. 4a–c). There were no significant differences in SOD activities between the SS and SP groups. However, the SP group showed a further increase compared with the SS supplementation (p < 0.05; Fig. 4a). MDA levels significantly increased in liver of the HFD group compared to the control group (p < 0.05). However, P, SS, and SP supplementation significantly lowered the MDA levels compared with the HFD-treated group, and this agreed with the earlier work of [24] that the Se-enriched Lactobacillus-protected liver homogenate GSH-Px and SOD activities were higher or significantly higher than those in the model group and were close to those in the control group. Thus, feeding a high-fat diet significantly increased the MDA content in liver homogenates, while administration of Se-enriched Lactobacillus prevented MDA elevation. Feeding mice with lactobacillus La-Dahi or LaBb-Dahi increased CAT and GPx activities [25].
The peroxidative status revealed inhibited by SP as shown by the lower lipid peroxide (MDA). The liver of all four experimental groups revealed ameliorated fatty liver induced by HFD [26]. Se, selenium-enriched or SePC significantly increased the plasma antioxidant capacity by 42 % compared with that of the controls. A sparing effect in liver glutathione peroxidase (87 % on average) and superoxide dismutase (56 % on average) activity was observed for all the groups compared to the controls [27].
An earlier report showed that carbohydrates and lipids may activate or inhibit lipogenic transcription factors, such as sterol regulatory element-binding proteins (SREBP) and peroxisome proliferator-activated receptor gamma (PPARγ). Peroxisome proliferator-activated receptors (PPARs) are transcription factors involved in the regulation of numerous metabolic processes. PPARalpha holds a fundamental role in the control of lipid homeostasis by directly regulating genes involved in fatty acid transport and oxidation. Importantly, PPARalpha agonists are effective in raising HDL-cholesterol and lowering triglycerides, properties that reduce the risk of cardiovascular diseases [28]. PPARs are transcription factors involved in the regulation of numerous metabolic processes. The PPARalpha isotype is abundant in liver and activated by fasting [29]. Accumulation of lipid in hepatocytes may cause a dysfunction in the synthesis of fatty acids. Transcription factors such as sterol-regulatory-element-binding protein-1c (SREBP1) and peroxisome proliferator-activated receptor alpha (PPARs) promote hepatic fatty acid synthesis [30, 31].
HFD feeding decreased lipolytic gene expression of (CPT1, CPT2, ACAT2, ACOX2, and PPARα) and up-regulated lipogenic gene expression (FAS, LPL, PPARγ, and SREBP1). The P, SS, and SP supplementation significantly up-regulated levels (p < 0.05) of CPT1, CPT2, ACAT2, ACOX2, and PPARα, compared to the HFD group. The P and SP showed better results than SS. Fatty acid oxidation-related genes (CPT1, CPT2, and ACOX1) were up-regulated in mice receiving probiotic treatment [7]; these results agree with [7] that HFD intake significantly decreased the expression of genes involved in fatty acid oxidation (CPT1) and increased the expression of genes involved in the regulation of adipogenesis (PPARγ, SREBP1) and lipogenesis (LPL). Carnitine supplementation has been used to reduce obesity caused by high-fat diets, which is beneficial for lowering blood and hepatic lipid levels and for ameliorating fatty liver [32]. NS Lactobacillus strains obviously alleviated hepatic injuries, decreased liver lipid deposition, and reduced adipocyte size of high cholesterol diet fed rats. NS Lactobacillus strains have been shown to regulate the mRNA expression levels of liver enzymes related to cholesterol metabolism, including the down-regulation of acyl-CoA: cholesterol acyltransferase (ACAT) [33]. Dietary energy increasing the activities of LPL, fatty acid synthase (FAS), and acetyl-CoA carboxylase significantly increased, and carnitine palmitoyltransferase-1 (CPT-1) significantly diminished. Peroxisome proliferator-activated receptor gamma (PPARgamma), LPL, FAS, and sterol regulatory element binding protein 1 (SREBP-1) expression were significantly increased by dietary energy increase. These results indicated that with dietary energy increasing, fat accumulation mainly increased due to adipose tissue lipogenic gene expression and decreased lipolytic gene expression [34, 35].
In short BS15, Lactobacillus exhibited a positive effect on liver lipid peroxidation through anti-oxidative stress activity by enhancing the liver antioxidant defense system thus decreasing the mRNA levels of acetyl-CoA carboxylase 1, fatty acid synthase, and peroxisome proliferator-activated receptor gamma and increased the expression of the fasting-induced adipose factor in livers [36]. In another study, [37] showed that the probiotic Lactobacillus reuteri strain prevented diet-induced obesity, possibly via a previously unknown mechanism of inducing liver expression of CPT1.
The PPARγ plays an important role in lipid capture by adipocytes. In fact, its high expression in mice fed the HFD was associated with an increased LPL activity and fat synthesis expression of sterol regulatory element-binding protein1 (Srebp1) in the liver. PPARγ was dramatically reduced in mice fed with a HFD compared with those fed on a normal diet.
Activation of the peroxisome proliferator-activated receptor (PPAR)-alpha which regulates lipid metabolism in tissues such as the liver decreases circulating lipid levels [38], and transgenic overexpression of SREBP-1 was associated with significantly higher hepatic triglycerides. These results provide evidence for important roles of SREBP-2 in regulation of lipid and glucose metabolism [39]. However, groups which received P, SS, and SP supplementation significantly decreased lipolytic gene expression and up-regulated the mRNA lipogenic gene expression (p < 0.01). Thus, SP, which combines the virtues of probiotics with the virtues of organic Se, can exert dual effects of organic Se and probiotics at the same time. In conclusion, we suggest that probiotics, inorganic selenium, and selenium-enriched probiotics may facilitate alleviating metabolic syndrome improved by dietary SP supplementation than SS and P group.
References
Vargas-Robles H, Rios A, Arellano-Mendoza M, Escalante BA, Schnoor M (2015) Antioxidative diet supplementation reverses high-fat diet-induced increases of cardiovascular risk factors in mice. Oxidative Med Cell Longev 2015:467471
Fan Y, Liu Y, Xue K, Gu G, Fan W, Xu Y, Ding Z (2015) Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS One 10(4), e0120775
Rebuffe-Scrive M, Surwit R, Feinglos M, Kuhn C, Rodin J (1993) Regional fat distribution and metabolism in a new mouse model (C57BL6J) of non-insulin-dependent diabetes mellitus. Metab Clin Exp 42(11):1405–1409
Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN (1988) Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37(9):1163–1167
Paccaud F, Schlüter-Fasmeyer V, Wietlisbach V, Bovet P (2000) Dyslipidemia and abdominal obesity: an assessment in three general populations. J Clin Epidemiol 53(4):393–400
Kumar S, Mahajan BB, Kamra N (2014) Future perspective of probiotics in dermatology: an old wine in new bottle. Dermatol Online J 20(9)
Park D-Y, Ahn Y-T, Park S-H, Huh C-S, Yoo S-R, Yu R, Sung M-K, McGregor RA, Choi M-S (2013) Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One 8(3), e59470
Alzate A, Canas B, Perez-Munguia S, Hernandez-Mendoza H, Perez-Conde C, Gutierrez AM, Camara C (2007) Evaluation of the inorganic selenium biotransformation in selenium-enriched yogurt by HPLC-ICP-MS. J Agric Food Chem 55(24):9776–9783
Behne D, Alber D, Kyriakopoulos A (2010) Long-term selenium supplementation of humans: selenium status and relationships between selenium concentrations in skeletal muscle and indicator materials. J Trace Elem Med Biol 24(2):99–105
Gao J, Huang K, Sn Q (2006) Determination of selenomethionine in selenium-enriched yeast by gas chromatography-mass spectrometry. Se pu = Chin J Chromatogr/Zhongguo hua xue hui 24(3):235–238
Pan C, Huang K, Zhao Y, Qin S, Chen F, Hu Q (2007) Effect of selenium source and level in hen’s diet on tissue selenium deposition and egg selenium concentrations. J Agric Food Chem 55(3):1027–1032
Bancroft NH, Designed By-Cunningham F (1996) Implementing SAP r/3: prentice hall PTR
Gan F, Chen X, Liao SF, Lv C, Ren F, Ye G, Pan C, Huang D, Shi J, Shi X (2014) Selenium-enriched probiotics improve antioxidant status, immune function, and selenoprotein gene expression of piglets raised under high ambient temperature. J Agric Food Chem 62(20):4502–4508
Ibrahim HA, Zhu Y, Wu C, Lu C, Ezekwe MO, Liao SF, Huang K (2012) Selenium-enriched probiotics improves murine male fertility compromised by high fat diet. Biol Trace Elem Res 147(1-3):251–260
Kondo S, Xiao JZ, Satoh T, Odamaki T, Takahashi S, Sugahara H, Yaeshima T, Iwatsuki K, Kamei A, Abe K (2010) Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci Biotechnol Biochem 74(8):1656–1661
Fukushima M, Nakano M (1995) The effect of a probiotic on faecal and liver lipid classes in rats. Br J Nutr 73(5):701–710
DiStefano MT, Danai LV, Roth Flach RJ, Chawla A, Pedersen DJ, Guilherme A, Czech MP (2015) The lipid droplet protein Hypoxia-inducible gene 2 promotes hepatic triglyceride deposition by inhibiting lipolysis. J Biol Chem
Zhu PL, Pan SY, Zhou SF, Zhang Y, Wang XY, Sun N, Chu ZS, Yu ZL, Ko KM (2015) Effects of combined dietary supplementation with fenofibrate and Schisandrae Fructus pulp on lipid and glucose levels and liver function in normal and hypercholesterolemic mice. Drug Des Devel Ther 9:923–935
Adebayo AO, Zandbergen F, Kozul-Horvath CD, Gruppuso PA, Hamilton JW (2015) Chronic exposure to low-dose arsenic modulates lipogenic gene expression in mice. J Biochem Mol Toxicol 29(1):1–9
Yu L, Yang S, Sun L, Jiang YF, Zhu LY (2014) Effects of selenium-enriched Agaricus blazei Murill on liver metabolic dysfunction in mice, a comparison with selenium-deficient Agaricus blazei Murill and sodium selenite. Biol Trace Elem Res 160(1):79–84
Song M, Park S, Lee H, Min B, Jung S, Park S, Kim E, Oh S (2015) Effect of Lactobacillus acidophilus NS1 on plasma cholesterol levels in diet-induced obese mice. J Dairy Sci 98(3):1492–1501
Kunitomo M (2007) Oxidative stress and atherosclerosis. Yakugaku zasshi : J Pharm Soc Jpn 127(12):1997–2014
Rindler PM, Plafker SM, Szweda LI, Kinter M (2013) High dietary fat selectively increases catalase expression within cardiac mitochondria. J Biol Chem 288(3):1979–1990
Chen L, Pan DD, Zhou J, Jiang YZ (2005) Protective effect of selenium-enriched Lactobacillus on CCl4-induced liver injury in mice and its possible mechanisms. World J Gastroenterol 11(37):5795–5800
Kaushal D, Kansal VK (2012) Probiotic Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum alleviates age-inflicted oxidative stress and improves expression of biomarkers of ageing in mice. Mol Biol Rep 39(2):1791–1799
Zhang B, Piao J, Gu L (2002) Effects of selenium-enriched garlic on blood lipids and lipid peroxidation in experimental hyperlipidemic rats. Wei sheng yan jiu = J Hyg 31(2):93–96
Riss J, Decorde K, Sutra T, Delage M, Baccou JC, Jouy N, Brune JP, Oreal H, Cristol JP, Rouanet JM (2007) Phycobiliprotein C-phycocyanin from Spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters. J Agric Food Chem 55(19):7962–7967
Konstandi M, Shah YM, Matsubara T, Gonzalez FJ (2013) Role of PPARalpha and HNF4alpha in stress-mediated alterations in lipid homeostasis. PLoS One 8(8), e70675
Patsouris D, Reddy JK, Muller M, Kersten S (2006) Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology 147(3):1508–1516
Peng Y, Rideout D, Rakita S, Lee J, Murr M (2012) Diet-induced obesity associated with steatosis, oxidative stress, and inflammation in liver. Surg Obes Relat Dis 8(1):73–81
Schultz A, Neil D, Aguila MB, Mandarim-de-Lacerda CA (2013) Hepatic adverse effects of fructose consumption independent of overweight/obesity. Int J Mol Sci 14(11):21873–21886
Wu T, Guo A, Shu Q, Qi Y, Kong Y, Sun Z, Sun S, Fu Z (2015) L-Carnitine intake prevents irregular feeding-induced obesity and lipid metabolism disorder. Gene 554(2):148–154
Hu X, Wang T, Li W, Jin F, Wang L (2013) Effects of NS Lactobacillus strains on lipid metabolism of rats fed a high-cholesterol diet. Lipids Health Dis 12:67
Zhang H, Zhang X, Wang Z, Dong X, Tan C, Zou H, Peng Q, Xue B, Wang L, Dong G (2015) Effects of dietary energy level on lipid metabolism-related gene expression in subcutaneous adipose tissue of Yellow breed x Simmental cattle. Anim Sci J = Nihon chikusan Gakkaiho 86(4):392–400
Goto T, Kim YI, Funakoshi K, Teraminami A, Uemura T, Hirai S, Lee JY, Makishima M, Nakata R, Inoue H (2011) Farnesol, an isoprenoid, improves metabolic abnormalities in mice via both PPARalpha-dependent and -independent pathways. Am J Physiol Endocrinol Metab 301(5):E1022–E1032
Xin J, Zeng D, Wang H, Ni X, Yi D, Pan K, Jing B (2014) Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl Microbiol Biotechnol 98(15):6817–6829
Fak F, Backhed F (2012) Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe-/- mice. PLoS One 7(10), e46837
Kimura R, Takahashi N, Murota K, Yamada Y, Niiya S, Kanzaki N, Murakami Y, Moriyama T, Goto T, Kawada T (2011) Activation of peroxisome proliferator-activated receptor-alpha (PPARalpha) suppresses postprandial lipidemia through fatty acid oxidation in enterocytes. Biochem Biophys Res Commun 410(1):1–6
Landa V, Zidek V, Mlejnek P, Simakova M, Silhavy J, Trnovska J, Kazdova L, Pravenec M (2014) Sterol regulatory element binding protein 2 overexpression is associated with reduced adipogenesis and ectopic fat accumulation in transgenic spontaneously hypertensive rats. Physiol Res 63(5):587–590
Acknowledgments
This work was funded by the National Natural Science Foundation of China (31272627, 31472253) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Jiangsu, China).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Nido, S.A., Shituleni, S.A., Mengistu, B.M. et al. Effects of Selenium-Enriched Probiotics on Lipid Metabolism, Antioxidative Status, Histopathological Lesions, and Related Gene Expression in Mice Fed a High-Fat Diet. Biol Trace Elem Res 171, 399–409 (2016). https://doi.org/10.1007/s12011-015-0552-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0552-8