Abstract
Aluminum is associated with etiology of many neurodegenerative diseases specially Alzheimer’s disease. Chronic exposure to aluminum via drinking water results in aluminum deposition in the brain that leads to cognitive deficits. The study aimed to determine the effects of aluminum on cholinergic biomarkers, i.e., acetylcholine level, free choline level, and choline acetyltransferase gene expression, and how cholinergic deficit affects novel object recognition and sociability in mice. Mice were treated with AlCl3 (250 mg/kg). Acetylcholine level, free choline level, and choline acetyltransferase gene expression were determined in cortex, hippocampus, and amygdala. The mice were subjected to behavior tests (novel object recognition and social novelty preference) to assess memory deficits. The acetylcholine level in cortex and hippocampus was significantly reduced in aluminum-treated animals, as compared to cortex and hippocampus of control animals. Acetylcholine level in amygdala of aluminum-treated animals remained unchanged. Free choline level in all the three brain parts was found unaltered in aluminum-treated mice. The novel object recognition memory was severely impaired in aluminum-treated mice, as compared to the control group. Similarly, animals treated with aluminum showed reduced sociability compared to the control mice group. Our study demonstrates that aluminum exposure via drinking water causes reduced acetylcholine synthesis in spite of normal free choline availability. This deficit is caused by reduced recycling of acetylcholine due to lower choline acetyltransferase level. This cholinergic hypofunction leads to cognitive and memory deficits. Moreover, hippocampus is the most affected brain part after aluminum intoxication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurotoxicity is a pathological condition that is caused by exposure to certain chemicals that cause deleterious effects on nervous system [1]. Neurotoxicity results from the exposure to synthetic or environmental toxic substances, referred to as neurotoxins, which can alter the nervous system’s normal activity and cause neurodegeneration [2]. Aluminum is a known neurotoxin for over a century [3] and has been implicated in the pathogenesis of many neurodegenerative disorders including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis [4], and senile dementia of Alzheimer’s type [5]. Aluminum is abundantly present in the Earth’s crust with unknown biological functions [6]. Human exposure to this metal is via drinking water, food, and certain drugs such as antacids [4]. The aluminum solubility in drinking water is negligible at neutral pH, but increased acidification of water bodies due to pollution, industrial development, and acid rain has considerably increased aluminum concentration in water bodies raising concerns about its toxicity [7].
Due to small size of aluminum, it rapidly crosses the blood brain barrier and has ability to accumulate there [8]. In the brain, aluminum may interfere with many biochemical functions including acetylcholine synthesis [9]. Very little is known about the effect of aluminum on the cholinergic system, particularly the effect on acetylcholine (Ach) synthesis and its recycling, and this needs to be elaborated.
Aluminum-induced toxic effects on acetylcholine system may be the cause of behavioral deficits observed in aluminum-treated animals and the humans suffering from diseases, for which aluminum is considered a contributing factor, such as dialysis encephalopathy and Alzheimer’s disease. Acetylcholine is a very important neurotransmitter because of its wide distribution in the brain and its critical role in arousal, sleep-wake cycle, modulation of cognitive performance, and in learning and memory processes [10, 11].
Lack of sociability is commonly observed as cognitive deficit, observed in patients suffering from neurodegenerative disorders, but this behavioral parameter is not well studied in aluminum-induced neurotoxicity. Therefore, the aim of this study was to investigate the underlying mechanism that how aluminum can affect acetylcholine system and its implications on behavioral parameters (using novel object recognition test and social novelty preference test).
Materials and Methods
Chemicals
Aluminum chloride hexahydrate (AL0770) was obtained from Scharlau, Spain. Taq polymerase, 10 mM dNTPs and reverse transcriptase enzyme were obtained from Fermentas® while Tri-reagent was obtained from Invitrogen™.
Animals
The behavior test experiments complied with the rulings of the Institute of Laboratory Animal Research, Division on Earth and Life Sciences, National Institute of Health, USA (Guide for the Care and Use of Laboratory Animals). The research protocol was approved by the Internal Review Board (IRB), Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology. Animals of 3 months of age weighing 28–35 g were used for behavior tests and analysis. Mice were kept under controlled environmental conditions at 25 ± 2 °C, and the natural light and dark cycle was followed.
Aluminum Chloride Administration
Animals were divided into two groups designated as the control group (received distilled water) and AlCl3-treated group (received AlCl3 250 mg/kg in drinking water for 42 days).
Determination of Acetylcholine and Free Choline Level in Brain
Measurement of acetylcholine and free choline level in the brain was carried out in cortex, hippocampus, and amygdala using choline/acetylcholine assay kit ab65345 (Abcam, USA). The assay was performed according to the instructions provided with the kit.
Gene Expression Studies for Choline Acetyltransferase
Animals were sacrificed on the 43rd day of aluminum treatment. Cortex, hippocampus, and amygdala were isolated from brain according to the method described previously [12], and RNA was extracted from these regions using Tri-reagent according to manufacturer’s instructions. RNA samples were checked quantitatively (spectrophotometrically) and qualitatively (on 2 % agarose gel). One microgram of extracted RNA was processed for RT-PCR to make 40 μl cDNA which was later used to carry out PCR for choline acetyltransferase (ChAT) gene using forward primer 5′-CTGGTGGAGAGAATAAACCG-3′ and reverse primer 5′-CTGGTGGAGAGAATAAACCG-3′. The housekeeping gene β-actin was amplified using forward primer 5′-GCCTTCCTTCTTGGGTATGG-3′ and reverse primer 5′-CAGCTCAGTAACAGTCCGC-3′. The ChAT amplicon size was 351 bp. PCR conditions were initial denaturation for 5 min at 95 °C, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s. The PCR cycle was terminated by a final extension at 72 °C for 10 min. The PCR product was run at 2 % agarose gel and visualized via ethidium bromide staining. Each sample was normalized with housekeeping gene Actin. Densitometric quantification of each band was done via NIH software “ImageJ.”
Behavior Tests
Behavior tests were performed from 10 a.m. to 5 p.m. on the 42nd day of aluminum treatment. Animals were transferred to the behavioral testing room 30 min prior to beginning of behavior test. The behavior test was recorded with a video camera, in the absence of experimenter and analyzed later on.
Novel Object Recognition Test
The test was conducted as described earlier [13]. Briefly, animals were allowed to acclimatize with the box (40 cm × 40 cm × 40 cm) for 5 min, followed by familiarization session and test session (10 min each with 20-min interval between sessions). In the familiarization session, two objects were placed in two opposite corners of the box, 5 cm away from the respective corners, and test animal was allowed to explore box and interact with both the objects. In the test session, one of the objects was replaced by a novel object and the test animal was allowed to interact and recognize the novel object. The time when animal was physically touching and sniffing the object was considered as exploration time. The recognition index was calculated for test session using the following formula
Social Novelty Preference Test
The test was conducted as previously described [14]. The test consisted of two sessions, i.e., social interaction session and social novelty preference session. Each session was of 10 min with 20 min inter-session interval. At the start of experiment, the animal was placed in the center of the square box (40 × 40 × 40 cm) and was given 5 min to acclimatize with this novel environment. After acclimatization time, social interaction session started by the introduction of a small cage, made of wire, carrying a mouse of same weight, age, and strain (mouse 1), while another empty cage of same dimensions was placed diagonal to first cage. After completion of first session, the animal was returned to its home cage. In social novelty preference session, a new mouse (mouse 2) was introduced in the empty cage, while familiar mouse (mouse 1) remained in the same cage as was in the previous session. In both the sessions, the interaction time was recorded as the time when test mouse touched the cage or physically contacted the mouse 1 or mouse 2.
Statistical Analysis
Data are expressed as mean ± standard error of mean (SEM). Results were analyzed using Graph pad Prism V5.0 for Windows (GraphPad software, San Diego, CA). Statistical tool was one-way ANOVA followed by Bonferroni’s multiple comparison test. Results were taken as significant only when P value was less than 0.05.
Results
Effect of Oral Aluminum on Acetylcholine and Free Choline Levels
Acetylcholine Levels
The acetylcholine level in cortex of aluminum-treated animals was significantly lower (4.42 ± 1.06), as compared to the acetylcholine levels in the cortex of control animals (22 ± 5.98, P < 0.01). Similarly, there was a highly significant difference between the acetylcholine levels in hippocampus of aluminum-treated animals (3.71 ± 1.48), as compared to the control animals (37.9 ± 2.59, P < 0.001; Fig. 1a). The acetylcholine level remained unaltered in amygdala of aluminum-treated animals (9.6 ± 1.11) and control animals (11.84 ± 2.2, P > 0.05; Fig. 1a).
Graph showing acetylcholine and free choline concentration. a Comparison of acetylcholine level in cortex, hippocampus, and amygdala of the control and aluminum-treated animals. b Comparison of free choline level in cortex, hippocampus, and amygdala of control and aluminum-treated animals. ***P < 0.001, **P < 0.01; n sample size
Free Choline Levels
The results indicated that free choline level remained the same in cortex, hippocampus, and amygdala of aluminum-treated animals (11.6 ± 1.34, 16.82 ± 3.9, and 13.59 ± 3.26, respectively), as compared to the free choline levels in cortex, hippocampus, and amygdala of the control animals (21.39 ± 5.89, 20.66 ± 5.07, and 21.25 ± 5.38, respectively, P > 0.05; Fig. 1b).
Effect of Aluminum on the Choline Acetyltransferase Gene Expression
The ChAT gene expression was significantly reduced in hippocampus after aluminum treatment (0.87 ± 0.13), as compared to control animals (1.9 ± 0.39, P < 0.01). While the ChAT expression remained same in cortex and amygdala of aluminum-treated animals (0.4 ± 0.12, 0.43 ± 0.1, respectively) and control animals (1 ± 0.19, 0.42 ± 0.08, respectively, P > 0.05; Fig. 2).
Effect of Aluminum on Behavior
Novel Object Recognition Test
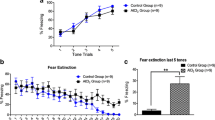
The results revealed that control animals prefer to spend significantly more time with novel object (82.20 ± 9.45, P < 0.05), as compared to aluminum-treated animals (52.20 ± 6.40; Fig. 3a, b) and object 1 (51 ± 6.03, P < 0.05). Aluminum-treated animals spent the same time with novel object (52.20 ± 6.40) and object 1 (42.8 ± 5.18, P > 0.05) showing impaired recognition for novel object after aluminum treatment. Comparison of recognition index of control (0.63 ± 0.031, P < 0.05) and aluminum-treated animals (0.48 ± 0.046) for known and novel object (Fig. 3c) showed that control group had higher recognition for novel object, as compared to aluminum-treated group.
Novel object recognition test. a Comparison of exploration time between control and aluminum-treated animals in first session (familiarization session). Graph represents the time spent, by control and aluminum-treated animals, in exploration of objects 1 and 2. b Comparison of novel object preference by control and aluminum-treated animals during second session (test session). c Graph showing the recognition index for novel object during test session by both the groups. *P < 0.05; n sample size
Effect of Aluminum on Social Novelty Preference Test
In social interaction session, the control animals spent significantly more time with mouse 1 (114.22 ± 17.41), as compared to aluminum-treated animals (68.55 ± 13.7, P < 0.05) than empty cage (40.44 ± 4.85, P < 0.001), while aluminum-treated animals spent the same time with mouse 1 (68.55 ± 13.7) and empty cage (41.55 ± 5.29, P > 0.05; Fig. 4a) showing impaired social interaction.
Social novelty preference test. a Comparison of social interaction in first session in the control and aluminum-treated animals. Graph showing the interaction time by control and aluminum-treated animals with empty cage and mouse 1. b Comparison of social novelty preference in the control and aluminum-treated animals. Graph showing the time spent by control and aluminum-treated animals interacting with mouse 1 and mouse 2 (stranger mouse) in second session of the test. *P < 0.05; n sample size
In social novelty preference session, control animals spent significantly more time with stranger mouse (mouse 2) (86.66 ± 11.66), as compared to aluminum-treated animals (53.11 ± 8.91, P < 0.05) and mouse 1 (47.66 ± 7.34, P < 0.01; Fig. 4b) revealing the intact memory for mouse 1 in control animals and also demonstrating social preference memory. The aluminum-treated animals spent similar time with stranger mouse (53.11 ± 8.91) and mouse 1 (37 ± 7.53).
Discussion
Aluminum is abundantly present in the Earth’s crust and its exposure beyond adaptive capability may result in the development of Alzheimer’s-like symptoms including dementia [15]. Human exposure to aluminum occurs via different routes. Aluminum is absorbed via gastrointestinal tract and is excreted through urine. In normal renal function, aluminum is eliminated almost completely from the body and very little accumulation occurs. For this reason, it is important to understand the neurotoxic effects of high aluminum consumption. This study aimed to elucidate aluminum neurotoxicity at high end of the human exposure resulting in high aluminum content in brain. Previously, it has been reported that 2000 μg Al/g (260 mg Al/kg) for a period of 5 weeks is within the order of magnitude of estimated maximal human intake [16, 17]. Keeping in view these facts and aluminum-induced neurotoxicity studies from our own laboratory [14, 18], we administered 250 mg/kg aluminum for a period of 6 weeks.
Chronic exposure to high level of aluminum results in its accumulation in brain, due to its ability to rapidly cross blood brain barrier, and it affects cholinergic system [19, 20]. The cholinergic system, which mainly depends on neurotransmitter acetylcholine, plays important role in memory formation, in memory retention, and in new learning processes [21, 22]. In the present study, the effects of aluminum on central cholinergic system, including its effects on gene expression of biosynthetic enzyme choline acetyltransferase and its product acetylcholine, were investigated. The results of our study show that there was a decrease in acetylcholine level in hippocampus and cortex, while in amygdala the acetylcholine concentration remains unaltered. The results obtained, for cortex and hippocampus, are in accordance to previously reported observation [23] but in contrary to increase in acetylcholine level observed by Yellamma et al. [8] after aluminum toxicity. The difference in our results from Yellamma et al. [8] might be due to the reason that they had provided 140 mg/kg of dose for 25 days. As aluminum is reported to accumulate slowly in brain [24], therefore, during treatment time reported by Yellamma et al., aluminum might not have accumulated in enough amounts to cause damage to acetylcholine level. While in our study, the dose of 250 mg/kg for a period of 42 days was designed to mimic the aluminum accumulation conditions at advanced stages of aluminum intoxication. Alzheimer’s disease, for which aluminum is considered an etiopathogenic factor, is also characterized by a decrease in the acetylcholine level [25].
Interestingly, in spite of the decrease in acetylcholine level, the free choline level in the brain tissues remained unchanged in aluminum-treated animals. This was surprising, as with previous reports of a decrease in the activity of acetylcholinesterase enzyme after aluminum treatment [6, 7, 23], one would expect a rise in the acetylcholine level and reduced level of free choline. It might be anticipated that the lower acetylcholine concentration, in spite of normal free choline availability, is either because of lower reuptake of choline in presynaptic terminal or, most probably, because of impaired synthesis of acetylcholine. Therefore, expression of choline acetyltransferase (ChAT) gene was measured. The effect of aluminum on activity of ChAT enzyme has been reported by various workers previously. Therefore, we wanted to determine whether the aluminum exposure has any effect on ChAT gene expression or not, which is not previously reported. There are controversial reports about the activity of ChAT enzyme in aluminum-induced neurotoxicity. A few studies report decrease in ChAT enzyme activity [5, 26], while others report no alteration in ChAT enzyme activity after aluminum treatment [27, 28]. Our results indicate that expression of ChAT gene was significantly reduced after aluminum treatment in hippocampus but remained unaltered in cortex and amygdala. Previously, Gulya et al. [5] reported decrease in ChAT activity in both cortex and hippocampus. These differences in the results in our study from that of Gulya et al. [5] is due to the reason that they have measured the activity of ChAT enzyme, while we have checked the expression of ChAT gene. According to our data for aluminum accumulation in the cortex and hippocampus (data not shown in this article), higher aluminum was accumulated in the hippocampus, as compared to cortex. Therefore, it is assumed that aluminum affects the activity of enzymes even at lesser concentrations but at high aluminum concentrations, the expression of genes is also affected. Therefore, higher aluminum levels in hippocampus have affected the ChAT gene expression along with its effects on ChAT enzyme (as reported by Gulya et al. [5]), whereas in the cortex, lower concentration of aluminum has not yet affected the gene expression, though a decreasing trend in the expression can be clearly seen in our study (Fig. 2).
It is evident from our results that hippocampus, which plays very important role in learning and memory, is the most affected brain region after aluminum treatment. This is substantiated by the fact that hippocampus receives maximum cholinergic innervations from forebrain [29]. There are several studies that report the degeneration of basal forebrain cholinergic neurons after aluminum treatment [20, 30, 31], but the region-specific degeneration is not studied in basal forebrain after aluminum treatment. Cortex and amygdala are innervated by cholinergic projections coming from horizontal limb of diagonal band of basal forebrain, while hippocampus receives its cholinergic innervations from vertical limb of diagonal band [29]. Therefore, it can be speculated that damage caused to the hippocampus, more than other brain parts, might be due to the damage of selective basal forebrain structures but this needs to be further investigated.
Intriguingly, the expression of ChAT gene reduced only in hippocampus and was not significantly reduced in cortex of aluminum-treated animals, but a very significant reduction in levels of acetylcholine was observed in both cortex and hippocampus. The expression of ChAT gene in cortex was not significantly reduced, but a very clear trend for the reduction of ChAT gene expression can be seen. Moreover, the reduction in the activity of acetyl CoA after aluminum exposure has already been reported multiple times [32–34]; therefore, it is assumed that after aluminum exposure, reduction in ChAT gene expression along with previously reported reduction of acetyl-CoA activity results in a greater reduction in acetylcholine concentration in different brain parts which leads to behavioral deficits.
Based on the degenerative effect of aluminum on key players of cholinergic system, further studies were conducted to determine the extent of damage in terms of impairment in cognitive function. Behavioral functions are the result of net output of motor, sensory, and cognitive functions of nervous system. Therefore, the behavioral functions are a sensitive measure for neurodegeneration caused by xenobiotics [23]. The novel object recognition test is a widely used test for the measurement of memory alterations [35], and cholinergic system is known to play a very important role in object recognition [36]. Therefore, novel object recognition test (NOR) was done in our study to determine the memory impairment. The results of NOR showed that control animals had significantly higher recognition memory for the novel object, as compared to aluminum-treated group. Presentation of a novel object is further useful for the reason that recognition of novelty requires the involvement of more cognitive skills from subject than the exploration of a novel environment or a single object [37]. Moreover, novel object preference also means that familiar object still remains in the animal memory [38]. The NOR is dependent on both cortex and hippocampus, the most affected brain parts in our experiments, as the lesions in these two brain parts have impaired activity in NOR [39, 40]. Our results demonstrated that the cholinergic system in cortex and hippocampus was translated in neurobehavioral functions which manifest memory impairment in aluminum-induced neurotoxicity.
The results of the social novelty preference test showed that aluminum treatment resulted in impaired social interaction as animals treated with aluminum preferred to spend more time sitting in the corner of the test box and showed little interaction with mouse 1 or empty cage during social interaction trial. Similarly, in social novelty preference trial, the aluminum-treated animals spent similar time with mouse 1 and mouse 2 (stranger mouse) which manifested that aluminum-intoxicated animals could not discriminate between familiar and strange mice. Our results are in agreement to the previously reported lack of sociability in mice administered with AlCl3 intraperitoneally [13]. Similar low social activity has been observed in patients suffering from Alzheimer’s disease [41]. Therefore, aluminum has the ability to produce some of the symptoms that are associated with Alzheimer’s disease, and aluminum-intoxicated animals can be used as model for studying cholinergic system impairment.
Conclusion
Our study demonstrates that chronic aluminum exposure in drinking water results in decreased choline acetyltransferase gene expression. Reduced ChAT expression leads to decreased acetylcholine synthesis in spite of normal free choline availability. The cholinergic hypofunction results in neurobehavioral deficits in the form of reduced sociability and impaired recognition for novel object.
References
Abd-Elhady RM, Elsheikh AM, Khalifa AE (2013) Anti-amnestic properties of Ginkgo biloba extract on impaired memory function induced by aluminum in rats. Int J Dev Neurosci 31(7):598–607
Pohl HR, Roney N, Abadin HG (2011) Metal ions affecting the neurological system. Met Ions Life Sci 8(247):62
Zatta P, Ibn-Lkhayat-Idrissi M, Zambenedetti P, Kilyen M, Kiss T (2002) In vivo and in vitro effects of aluminum on the activity of mouse brain acetylcholinesterase. Brain Res Bull 59(1):41–45
Hu W-P, Li X-M, Chen J-G, Li Z-W (2007) Potentiation of the nicotinic acetylcholine receptor by aluminum in mammalian neurons. Neuroscience 149(1):1–6
Gulya K, Rakonczay Z, Kasa P (1990) Cholinotoxic effects of aluminum in rat brain. J Neurochem 54(3):1020–1026
Kaizer R, Correa M, Gris L, Da Rosa C, Bohrer D, Morsch V, Schetinger MRC (2008) Effect of long-term exposure to aluminum on the acetylcholinesterase activity in the central nervous system and erythrocytes. Neurochem Res 33(11):2294–2301
Brus R, Szkilnik R, Popieluch I, Kostrzewa RM, Mengel K (1997) Effect of aluminium exposure on central serotonine and muscarine receptors reactivity in rats. Med Sci Monit 3(5):BR631–BR636
Yellamma K, Saraswathamma S, Kumari BN (2010) Cholinergic system under aluminium toxicity in rat brain. Toxicol Int 17(2):106
Stevanović ID, Jovanović MD, Čolić M, Jelenković A, Bokonjić D, Ninković M (2010) Nitric oxide synthase inhibitors protect cholinergic neurons against AlCl 3 excitotoxicity in the rat brain. Brain Res Bull 81(6):641–646
Schliebs R, Arendt T (2006) The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm 113(11):1625–1644
Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A (2001) Ultrastructural distribution of the α7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci 21(20):7993–8003
Mahboob A, Farhat SM, Iqbal G, Babar MM, Nabavi SM, Ahmed T (2016) Alpha-lipoic acid-mediated activation of muscarinic receptors improves hippocampus-and amygdala-dependent memory. Brain Res Bull 122:19–28
AN Hashmi, Yaqinuddin A, Ahmed T (2014) Pharmacological effects of Ibuprofen on learning and memory, muscarinic receptors genes expression and APP isoforms levels in pre-frontal cortex of AlCl3-induced toxicity mouse model. Int J Neurosci (0):1–37
Syed H, Ikram MF, Yaqinuddin A, Ahmed T (2015) Cyclooxygenase I and II inhibitors distinctly enhance hippocampal-and cortex-dependent cognitive functions in mice. Mol Med Rep 12(5):7649–7656
Wills MR, Savory J (1985) Water content of aluminum, dialysis dementia, and osteomalacia. Environ Health Perspect 63:141
Commissaris R, Cordon J, Sprague S, Keiser J, Mayor G, Rech R (1982) Behavioral changes in rats after chronic aluminum and parathyroid hormone administration. Neurobehav Toxicol Teratol 4(3):403
Golub MS, Donald JM, Gershwin ME, Keen CL (1989) Effects of aluminum ingestion on spontaneous motor activity of mice. Neurotoxicol Teratol 11(3):231–235
Iqbal G, Iqbal A, Mahboob A, Farhat S, Ahmed T (2016) Memory enhancing effect of black pepper in the AlCl3-induced neurotoxicity mouse model is mediated through its active component chavicine. Curr Pharm Biotechnol
Harkany T, Lengyel Z, Kasa P, Gulya K (1995) Chronic aluminum treatment results in aluminum deposits and affects Ml muscarinic receptors in rat brain. Neurobiology (Budapest, Hungary) 4(1–2):35–43
Peng J-HF, Xu Z-C, Xu Z-X, Parker JC, Friedlander ER, Tang J-P, Melethil S (1992) Aluminum-induced acute cholinergic neurotoxicity in rat. Mol Chem Neuropathol 17(1):79–89
Shafer TJ, Mundy WR, Tilson HA (1993) Aluminum decreases muscarinic, adrenergic, and metabotropic receptor-stimulated phosphoinositide hydrolysis in hippocampal and cortical slices from rat brain. Brain Res 629(1):133–140
Maheswari S, Venkatakrishna Murali R, Balaji R (2014) Aluminium induced cholinotoxicity in zebra fish brain—a sequel of oxidative stress. Int J Adv Res 2:322–335
Julka D, Sandhir R, Gill KD (1995) Altered cholinergic metabolism in rat CNS following aluminum exposure: implications on learning performance. J Neurochem 65(5):2157–2164
Kumar S (1998) Biphasic effect of aluminium on cholinergic enzyme of rat brain. Neurosci Lett 248(2):121–123
Mayeux R, Stern Y (2012) Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med 2(8):a006239
Cherroret G, Desor D, Hutin M, Burnel D, Capolaghi B, Lehr P (1996) Effects of aluminum chloride on normal and uremic adult male rats. Biol Trace Elem Res 54(1):43–53
Connor DJ, Jope RS, Harrell LE (1988) Chronic, oral aluminum administration to rats: cognition and cholinergic parameters. Pharmacol Biochem Behav 31(2):467–474
Zhang R, Zhang J, Fang L, Li X, Zhao Y, Shi W, An L (2014) Neuroprotective effects of sulforaphane on cholinergic neurons in mice with Alzheimer’s disease-like lesions. Int J Mol Sci 15(8):14396–14410
Woolf NJ (1991) Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37(6):475–524
Stevanović ID, Jovanovic M, Colic M, Jelenkovic A, Bokonjic D, Ninkovic M, Stojanovic I (2011) N-nitro-L-arginine methyl ester influence on aluminium toxicity in the brain. Folia Neuropathol 49(3):219–229
Jelenković A, Jovanović MD, Stevanović I, Petronijević N, Bokonjić D, Živković J, Igić R (2014) Influence of the green tea leaf extract on neurotoxicity of aluminium chloride in rats. Phytother Res 28(1):82–87
Bielarczyk H, Tomaszewicz M, Szutowicz A (1998) Effect of aluminum on acetyl-CoA and acetylcholine metabolism in nerve terminals. J Neurochem 70(3):1175–1181
Jankowska A, Madziar B, Tomaszewicz M, Szutowicz A (2000) Acute and chronic effects of aluminum on acetyl-CoA and acetylcholine metabolism in differentiated and nondifferentiated SN56 cholinergic cells. J Neurosci Res 62(4):615–622
Szutowicz A, Bielarczyk H, Kisielevski Y, Jankowska A, Madziar B, Tomaszewicz M (1998) Effects of aluminum and calcium on acetyl-CoA metabolism in rat brain mitochondria. J Neurochem 71:2447–2453
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13(2):93–110
Kruk-Słomka M, Michalak A, Budzyńska B, Biała G (2014) A comparison of mecamylamine and bupropion effects on memory-related responses induced by nicotine and scopolamine in the novel object recognition test in mice. Pharmacol Rep 66(4):638–646
Silvers JM, Harrod SB, Mactutus CF, Booze RM (2007) Automation of the novel object recognition task for use in adolescent rats. J Neurosci Methods 166(1):99–103
Ennaceur A (2010) One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res 215(2):244–254
Buckmaster CA, Eichenbaum H, Amaral DG, Suzuki WA, Rapp PR (2004) Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. J Neurosci 24(44):9811–9825
Clark RE, Zola SM, Squire LR (2000) Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20(23):8853–8860
Isaev N, Stelmashook E, Genrikhs E, Oborina M, Kapkaeva M, Skulachev V (2015) Alzheimer’s disease: an exacerbation of senile phenoptosis. Biochem Mosc 80(12):1578–1581
Acknowledgments
We are thankful to the Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology, Islamabad, Pakistan for providing funding, support, and research facilities to carry out this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The behavior test experiments complied with the rulings of the Institute of Laboratory Animal Research, Division on Earth and Life Sciences, National Institute of Health, USA (Guide for the Care and Use of Laboratory Animals). The research protocol was approved by the Internal Review Board (IRB), Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Farhat, S.M., Mahboob, A., Iqbal, G. et al. Aluminum-Induced Cholinergic Deficits in Different Brain Parts and Its Implications on Sociability and Cognitive Functions in Mouse. Biol Trace Elem Res 177, 115–121 (2017). https://doi.org/10.1007/s12011-016-0856-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0856-3