Abstract
Aluminum is a widespread environmental neurotoxicant that can induce Alzheimer's disease (AD)-like damage, such as neuronal injury and impairment of learning and memory. Several studies have shown that aluminum could reduce the synaptic plasticity, but its molecular mechanism remains unclear. In this study, rats were treated with aluminum maltol (Al(mal)3) to establish a toxic animal model and PMA was used to interfere with the expression of PKC. The Morris water maze and open field test were used to investigate the behavioral changes of the rats. Western blotting and RT-PCR were used to detect the expression levels of NMDAR subunits, PKC and CaMKII. The results showed that Al(mal)3 damaged learning and memory function and reduced anxiety in rats. During this process, the expression of PKC was downregulated and it inhibited the expression of NMDARs through the phosphorylation of CaMKII.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) is the most abundant metal element in the Earth's crust and mainly exists in nature as aluminosilicate ore. However, it does not have any known beneficial effects on biological systems (Exley 2009; Kiss 2013; Shaw et al. 2013). During the past 200 years, the rapid development of the Al industry has made occupational aluminum exposure a serious occupational health problem (Bondy 2010). Additionally, because of its excellent properties, Al is widely used in daily life and medications, and it can enter and accumulate in the human body through food, water, medicines, vaccines, and cosmetics (Krewski et al. 2007). Al ions have a high charge and a small ion radius which makes it easy for Al ions to cross the blood–brain barrier (BBB) and accumulate in different areas of the brain (Tomljenovic 2011), thereby damaging brain tissue.
Since the nineteenth century, the toxicity of Al has attracted much attention from neurotoxicologists (Wenting et al. 2014). Research has estimated that adults directly or indirectly intake approximately 3–12 mg of Al per day (Shaw et al. 2014). Long-term Al exposure causes Al to accumulate in the brain, bones, muscles, kidneys, and other organs, thereby causing neurodegenerative diseases (Colomina and Peris-Sampedro 2017). Epidemiological studies have shown that areas with a higher burden of Al in drinking water had a higher incidence of Alzheimer's disease (AD) (Krewski et al. 2007). Animal experiments have shown that excessive intake of Al could damage the learning and memory ability of rats or mice and cause changes in learning and memory-related proteins (Farhat et al. 2017; Thippeswamy et al. 2013). Scholars have proposed that Al might be involved in glutamate-mediated nerve damage and cause neurotransmitter changes (Matyja 2000). Al is widely exposed to the population and has certain neurotoxicity, which makes the study of Al neurotoxicity of great public health significance.
N-methyl-d-aspartate receptor (NMDAR) is the most important excitatory glutamate ionotropic receptor in the central nervous system which is highly expressed in the cerebral cortex and hippocampus and plays a vital role in neurodevelopment, learning and memory, sensory perception, and synaptic plasticity (Kumar 2015). Synaptic plasticity is divided into long-term potentiation (LTP) and long-term inhibition (LTD), which are considered as electrophysiological manifestation of possible mechanisms of learning and memory. NMDARs and other ionotropic receptors (AMPARs and KAs) play an important role in the regulation of synaptic plasticity. Functional NMDARs are composed of NMDAR1 and one or more NMDAR2 subunits. With increasing age, the expression of NMDAR1 and NMDAR2 decreases (Mota et al. 2014), and the overexpression of NMDAR2B improves the synaptic plasticity and memory ability of aged mice (Brim et al. 2013; Cao et al. 2007). Burnashev found that LTP in the hippocampus of NMDAR1 knockout mice was significantly reduced, and the escape latency was significantly prolonged in a water maze experiment (Burnashev and Szepetowski 2015). NMDAR is necessary for the induction of LTP, and studies have found that NMDAR antagonists could inhibit the occurrence of LTP (Villarreal et al. 2002). These findings suggest that there is an inevitable connection between NMDARs and neural function, and changes in NMDAR expression cause learning and memory dysfunction.
Studies have shown that NMDAR is dynamically regulated, and its subunit composition and number change with increasing brain activity; so it plays an important role in NMDAR-dependent brain function (Bu et al. 2015; Hunt and Castillo 2012). This regulation of NMDAR is mainly dependent on the PKC–Src signaling pathway (Horak et al. 2014). Protein kinase C (PKC) is a serine/threonine protein kinase involved in the formation of synaptic plasticity and LTP. Li HB found that PKC could relieve the blocking effect of Mg2+ on NMDAR channels, increase Ca2+ influx, and cause changes in synaptic plasticity (Li et al. 2011). It was found that the tyrosine kinase Src could phosphorylate NMDARs and promote their further opening (Weilinger et al. 2016; Yang et al. 2012). PKC promoted the transfer of NMDARs to the cell membrane through interactions with NMDAR-related proteins (Yan et al. 2011). PKC first causes the autophosphorylation of CaMKII, thereby increasing the interaction between CaMKII and NMDAR2, which is transported to the postsynaptic membrane in the form of the NMDAR2–CaMKII complex, thus playing a role in the formation of LTP. In summary, PKC significantly increases the opening of NMDAR channels through the PKC–Src signaling pathway. PKC can phosphorylate CaMKII to promote the binding of CaMKII and NMDAR2 to the membrane.
Therefore, we speculated that the learning and memory impairment of Al-exposed rats might be related to the regulation of NMDAR expression by PKC, and the phosphorylation of CaMKII is involved in this process. In this study, the Morris water maze was used to test the learning and memory ability of Al-exposed rats, and an open field test was used to evaluate their anxiety and tension. NMDARs were detected by western blotting and PCR. PKC and CaMKII, which are involved in synaptic plasticity-related pathways, were measured to explore whether PKC and CaMKII play a role in learning and memory impairment caused by Al.
Materials and Methods
Laboratory Animals
Seventy 2-month-old healthy male Sprague–Dawley (SD) rats without specific pathogens were provided by the Experimental Animal Center of the Chinese Academy of Military Medical Sciences (Experimental Animal Production License Number: SCXK (Army) 2012-0004), weighing 180–220 g. Rats were raised in a barrier environment that met the requirements of GB14925-2010 "Laboratory Animal—Requirements of environment and housing facilities." During the entire Al exposure period, the rats were fed standard fodder and water ad libitum. The temperature was controlled at 22–24 ℃, and the relative humidity was 40–60% with day and night natural rhythm lighting. This study was reviewed and approved by the Medical Animal Ethics Committee of Shanxi Medical University.
Al(mal)3 and PMA Preparation
AlCl3 (Sigma-Aldrich, USA) was dissolved in normal saline to final concentrations of 8 mM, 32 mM, and 128 mM. Maltol (Sigma-Aldrich, USA) was dissolved in PBS to final concentrations of 24 mM, 96 mM, and 384 mM. AlCl3 and maltol solutions were mixed freshly when used in equal volumes to make Al(mal)3 solutions with concentrations of 4 mM, 16 mM, and 64 mM. The pH of the Al(mal)3 solution was adjusted to 7.4 with NaOH and filtered with a 0.22 mm filter membrane. The PKC agonist PMA (Sigma-Aldrich, USA) was dissolved in normal saline to a final concentration of 0.2 μM.
Surgery and Treatments
After 1 week of adaptive feeding, the rats were modeled by surgery. After the rats were anesthetized with 10% chloral hydrate, they were fixed in the prone position on a three-dimensional brain stereotaxic apparatus (RWD, China). The fur was cut to fully expose the skull. Starting from the fontanelle, 0.8 mm backward, and 1–1.5 mm laterally, the skull was drilled with a bone drill, and a cannula was placed. Screws were placed at 3 points on the skull, and dental cement was fixed. One week after the operation, the rats were divided randomly into 7 groups of 10 according to body weight: operation control group (lateral ventricle intubation only), saline control group (injected with 0.9% normal saline), Al(mal)3 exposure groups (4 mM, 16 mM, and 64 mM Al(mal)3, 5 μL), PMA (0.2 μM, 5 μL), and Al(mal)3 + PMA (64 mM/5 μL Al(mal)3 + 0.2 μM/5 μL PMA). The prepared 5 μL solution was slowly injected into the lateral ventricle with a microsyringe within 5 min for continuous injection for 7 days.
Morris Water Maze
The experimental device was mainly composed of two parts: a water maze and an automatic image monitoring system. The water maze was a circular pool with a diameter of 130 cm. There was a platform with a diameter of 10 cm that was 2 cm below the water surface, and the water temperature was controlled at 22–24 ℃. The four directional points S, W, N, and E were marked on the side of the pool, which divided the pool into four quadrants, namely, NW, NE, SW, and SE. When the rat entered the water maze, the upper camera monitored and recorded the rat's swimming track. When the rat found the platform and stayed for 10 s or failed to find the platform within 120 s, the computer automatically stopped recording.
The day before the experiment, the rats were put into the pool to swim freely for 120 s to adapt to the environment. In the 5-day positioning navigation experiment, the rats were trained 4 times a day, entering the water from different entry points (S, SE, NW, W). The platform was placed in the target quadrant (NE), and the time for each rat to find the platform was recorded. Then, the average value was calculated, which was the escape latency period of the rat on that day. If the rat did not find the platform within 120 s, it could be guided to the platform and held in place for 10 s. At this time, the escape latency was 120 s. On the second day after the positioning navigation experiment, the platform was removed, and the rat entered the water at the point (SW) farthest from the target quadrant. The camera recorded the swimming trajectory within 120 s and analyzed the first time arriving at the platform, the target quadrant dwell time, and the number of crossing the platform.
Open Field Test
The experimental device consisted of an open field reaction box (70 × 70 cm with 40 cm walls) and an automatic monitoring and acquisition system. The rat was placed in the center of the open field, and at the same time, the camera began to record the trajectory of the rat in the box for a total of 10 min. Time in the center area (30 × 30 cm), number of rearing (standing frequency of hind limbs in rats), and number of grooming (quick cleaning action of the front legs toward the face or body) were analyzed and quantified by a video monitoring system. After each rat was finished, the apparatus was cleaned with 70% alcohol to avoid the remaining urine, feces, and odor from affecting the next test.
Western Blotting
Total proteins were extracted from the hippocampus of rats with a tissue protein extraction reagent (CWBIO, China) and quantified with a BCA protein Assay Kit (CWBIO, China) according to the instructions. The samples were added to 5 × loading buffer at a ratio of 4:1 and boiled in water for 5 min. Forty-five micrograms of each sample was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the protein was transferred to a polyvinylidene difluoride membrane (PVDF). The membrane was blocked with 5% skimmed milk powder at room temperature for 2 h and then incubated with a specific primary antibody (GAPDH, 1:5000, CWBIO, China; Tublin, 1:5000, CWBIO, China; NMDAR1, 1:2000, Abcam, UK; NMDAR2A, 1:1000, Abcam, UK; NMDAR2B, 1:1000, CST, USA; PKC, 1:2000, Abcam, UK; CaMKII, 1:8000, Abcam, UK; p-CaMKII (Thr286), CST, USA) at 4 ℃ overnight. After rinsing 4 times with PBST, the membrane was incubated with the corresponding secondary antibody at 37 °C for 2 h. After rinsing with PBST, ECL-enhanced luminescent solution was developed. A gel electrophoresis image analysis system (Bio-Rad, USA) was used to detect the gray value of the protein, and the optical density ratio of the target protein to the internal reference protein was calculated (Quantity One-4.6.5 software).
Quantitative Real-Time PCR
Total RNA was extracted from the hippocampus of rats using TRIzol reagent (CWBIO, China) according to the instructions. RNA concentration and purity were detected by a UV spectrophotometer (Eppendorf, Germany). Complementary DNA (cDNA) was synthesized using Prime ScriptTM RT Master Mix (Takara, Japan) in a 10 μL reaction containing 500 ng of the total RNA, and the reaction system was as follows: 4 °C; 37 °C 15 min, 85 °C 5 s, 4 °C. To detect gene expression levels, cDNAs were amplified using specific primers (Table 1). Real-time PCR was performed using SYBR® Premix ExTaqTM II (Takara, Japan) on an Applied Biosystems 7500 system (ABI, USA) with 1.6 μL cDNA in a 20 μL reaction. PCR amplification conditions were as follows: 95℃ 30 s, 1 cycle; 95℃ 5 s, 60℃ 30 s, 40 cycles. β-actin was used as an endogenous control. The relative messenger RNA (mRNA) expression level was calculated according to the 2 − ∆∆Ct method.
Statistical Analysis
SPSS 22.0 was used for statistical analysis of the data, and all data are expressed as the mean ± standard deviation. One-way analysis of variance was used for comparisons between multiple groups. For pairwise comparisons, the SNK method was used when the variance was uniform, and the Dunnett T3 test was applied for uneven variance. Repeated measurement analysis of variance was used for repeated measures data. A p value of < 0.05 was considered statistically significant.
Results
Investigation of Acute Al-Induced Neurotoxicity in Rats
Al Exposure Damages the Learning and Memory Function of Rats
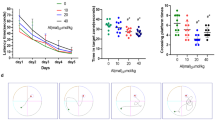
In this experiment, the swimming speeds of the rats in each group were similar, and there was no statistical difference, which ruled out the possibility of lateral ventricle surgery and aluminum exposure damaging the exercise ability of the rats (Fig. 1A). The positioning navigation experiment is mainly used to evaluate the spatial learning ability, and the index used is the escape latency period. As shown in Fig. 1B, as the number of training days increased, the escape latency period of rats in each group was gradually shortened, and the repeated measurement analysis of variance results showed that the difference in the escape latency period between the five time points was statistically significant (F = 281.362, p < 0.01). In the daily training, the escape latency period of each group increased with the increase of the exposure dose, and there were statistical differences between the five groups (F = 6.724, p < 0.01). The results of multiple comparison analysis showed that from the second day, the escape latency period in each Al-exposed group was significantly longer than that of the operation control group, and the difference was statistically significant (p < 0.01). On the fifth day, the escape latency period in the 64 mM Al(mal)3 group was significantly higher than that of the other groups, and there was no interaction between the exposure dose and the exposure time (F = 0.692, p = 0.833).
Morris water maze results of the operation group; saline group; and 4, 16, and 64 mM Al(mal)3 groups. A Swimming speed of rats in each group. B Comparison of the mean escape latency period per day in the navigation experiment. C The first mean time arriving at the platform. D Water maze space exploration results. E Mean crossing platform frequency. F Swimming track graphs of rats in each group on the fifth and sixth days. Compared with the operation group, ap < 0.05; compared with the saline group, bp < 0.05; compared with the 4 mM Al(mal)3 group, cp < 0.05
The space exploration experiment is mainly used to evaluate the spatial memory ability, and the indicators used are the first time arriving at the platform, the target quadrant dwell time, and the number of crossing the platform. As can be seen from Fig. 1C–E, with the increase of the exposure dose, the first time arriving at the platform was significantly prolonged (F = 5.406, p < 0.01), and the target quadrant dwell time was gradually shortened, and the difference was statistically significant (F = 2.414, p < 0.05). The first time arriving at the platform in the 64 mM Al(mal)3 group was approximately 70% longer than that in the operation control group. The target quadrant dwell time of the 64 mM Al(mal)3 group was reduced by 23.9% compared with the operation control group, and it was reduced by 21.7% compared with the 4 mM Al(mal)3 group. Although there was no significant difference in the number of crossing the platform in each group, a downward trend was observed (Fig. 1E). Figure 1F shows that through training, the control group could quickly find the platform and the swimming path was clear. With increasing exposure dose, although the rats finally reached the platform, their swimming time was prolonged and the track was random. After the platform was removed on the sixth day, the number of times the rats crossed the platform gradually decreased as the exposure dose increased. The water maze results showed that acute lateral ventricle exposure to Al could damage the learning and memory ability of rats.
Al Exposure Reduced Anxiety in Rats
The open field test is used to evaluate the anxiety and tension of animals in a novel environment. As shown in Fig. 2A, the time in the center area of rats in each group prolonged with the increase of the dose, and the 64 mM Al(mal)3 group was 56.5% longer than the operation control group (p < 0.05). With the increase of the exposure dose, the number of rearing and grooming decreased, and the 64 mM Al(mal)3 group had decreases of 36.8% and 37.9%, respectively, compared with the operation control group (p < 0.05) (Fig. 2B–C). The open field test results showed that Al exposure could make rats feel less nervous and anxious in a new environment.
The time in the center area (A), the number of rearing (B), and the number of grooming (C) in the open field test of rats in the operation group; saline group; and 4, 16, and 64 mM Al(mal)3 groups. D Trajectory diagram of each group of rats in the open field. Compared with the operation group, ap < 0.05; compared with the saline group, bp < 0.05
Effects of Al(mal)3 on the Protein Expression of NMDARs, PKC, CaMKII, and p-CaMKII(Thr286) in the Hippocampus
Western blotting is a protein detection technology that uses specific antibodies to detect a specific antigen. As shown in Fig. 3A–C, compared with the operation group, the protein expression of NMDAR1, NMDAR2A, and NMDAR2B in the saline group showed no significant difference (p > 0.05). In the 16 mM Al(mal)3 group, NMDAR1 and NMDAR2B protein expression decreased by 22% and 24.1%, respectively, compared with the operation control group (p < 0.05). In the 64 mM Al(mal)3 group, they decreased by 29.9% and 32.9% (p < 0.05). Although the protein expression of NMDAR2A also showed a downward trend, the difference was not statistically significant (p = 0.537). Each subunit of the NMDA receptor changed after aluminum exposure.
Changes in protein expression in rats exposed to Al(mal)3. A NMDAR1, B NMDAR2A, C NMDAR2B, D PKC, E CaMKII, F p-CaMKII (Thr286). Compared with the operation group, ap < 0.05; compared with the saline group, bp < 0.05; compared with the 4 mM Al(mal)3 group, cp < 0.05; compared with the 16 mM Al(mal)3 group, dp < 0.05
Next, we detected the relevant regulatory proteins of the NMDA receptors. As shown in Fig. 3D–F, the protein expression levels of PKC and p-CaMKII (Thr286) in the hippocampus of each group were significantly different (F = 8.980, p < 0.001; F = 5.290, p = 0.003). The expression level of PKC protein in the 16 mM Al(mal)3 group was lower than that in the operation control group and saline group, and the expression level of p-CaMKII (Thr286) in the 4 and 16 mM Al(mal)3 groups was lower than that in the operation control group, which was statistically significant. Compared with the operation control group, the protein expression of PKC and p-CaMKII (Thr286) in the 64 mM Al(mal)3 group decreased by 20.9% and 34.7% (p < 0.05). There was no significant difference in the protein expression level of CaMKII in each group of rats.
Effects of Al(mal)3 on the mRNA Expression of NMDARs in the Hippocampus
The RT-PCR experiment results (Fig. 4) showed that with increasing Al exposure dose, the mRNA expression levels of NMDAR1 and NMDAR2A in the hippocampus of each group gradually decreased, and the differences were statistically significant (p < 0.05). Compared with the operation control group, the NMDAR1 and NMDAR2A mRNA levels in the 64 mM Al(mal)3 group decreased by 48% and 38%, respectively (p < 0.05). NMDAR2B mRNA expression showed a downward trend, but the difference was not statistically significant (p > 0.05).
PKC Regulates Al-Induced Neurotoxicity in Rats
PKC Improved Learning and Memory Impairment Caused by Al
From the above results, it can be seen that acute Al exposure can cause a decrease in the level of NMDAR2B protein, but its mRNA level has no significant change, which reveals that the change of NMDAR2B does not occur in the transcription stage. Therefore, we speculate that the change in NMDA receptors may be caused by the modification of the interaction between proteins after transcription. PKC is an important protein kinase that plays an important role in the regulation of NMDA receptors. In this part, the PKC agonist PMA was used to treat rats to explore the main mechanism of PKC in Al-induced learning and memory impairment.
Similarly, there was no significant difference between the swimming speeds of rats in each group (Fig. 5A). In the positioning navigation experiment (Fig. 5B), there was no statistical difference in the escape latency period between the PMA group and the saline group (p < 0.05), suggesting that PMA did not affect the learning and memory of the rats. It was significantly lower in the Al(mal)3 + PMA group than that in the Al-exposed group on days 2, 4, and 5 (p < 0.05), indicating that PMA can appropriately improve the learning and memory impairment caused by Al. The results of the space exploration experiments (Fig. 5C–E) showed that there was no statistical difference between the Al(mal)3 + PMA group with the operation group and the saline group (p > 0.05). While compared with the Al-exposed group, the first time arriving at the platform was reduced by 34.8% (p < 0.05), and the target quadrant dwell time was increased by 26.9% (p < 0.05) in the Al(mal)3 + PMA group.
Morris water maze results of the operation group, saline group, PMA group, 64 mM Al(mal)3 group, and 64 mM Al(mal)3 + PMA groups. A Swimming speed of rats in each group. B Comparison of the mean escape latency period per day in the navigation experiment. C The first mean time arriving at the platform. D The water maze space exploration results. E Mean crossing platform frequency. Compared with the operation group, ap < 0.05; compared with the saline group, bp < 0.05; compared with the PMA group, cp < 0.05; compared with the 64 mM Al(mal)3 group, dp < 0.05
PMA Did Not Improve the Anxiety Suppression Caused by Al
Pairwise comparison results showed that compared with the Al-exposed group, the Al(mal)3 + PMA group had no significant difference in the time in the center area, number of rearing, and number of grooming (p > 0.05) (Fig. 6).
The time in the center area (A), the number of rearing (B), and the number of grooming (C) in the open field test of rats in the operation group, saline group, PMA group, 64 mM Al(mal)3 group, and 64 mM Al(mal)3 + PMA group. Compared with the operation group, ap < 0.05; compared with the saline group, bp < 0.05; compared with the PMA group, cp < 0.05
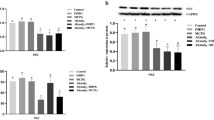
Effects of PKC Activation on the Inhibitory Effect of Al(mal)3 on the Protein Expression of p-CaMKII and NMDARs
In this experiment (Fig. 7), the protein expression level of NMDAR2A in each group did not change significantly (p > 0.05), and the protein expression of NMDAR1 and NMDAR2B was statistically different between the groups (p < 0.05). The use of PMA alone did not affect the subunits of the NMDA receptor. However, compared with the Al-exposed group, the expression levels of NMDAR1 and NMDAR2B in the Al(mal)3 + PMA group increased by 34.9% and 34.3%, respectively (p < 0.05). Western blotting results showed that after injection of the PKC agonist, PKC expression increased by 15.8%. Compared with the Al-exposed group, the PKC and p-CaMKII (Thr286) protein expression levels in the Al(mal)3 + PMA group recovered by 23.5% and 40.2%, respectively (p < 0.05).
Changes in protein expression in Al-exposed rats after injection with PMA. A NMDAR1, B NMDAR2A, C NMDAR2B, D PKC, E CaMKII, F p-CaMKII (Thr286). Compared with the operation group, ap < 0.05; compared with the saline group, bp < 0.05; compared with the PMA group, cp < 0.05; compared with the 64 mM Al(mal)3 group, dp < 0.05
Discussion
Al was once considered a harmless metal element and was widely used in daily life and production. Food is an important source of Al, so many countries have evaluated Al exposure in their diets. The 2006 UK total diet study showed that the population dietary exposure to Al was 5.4 mg/day, the Spanish report estimated that the Al intake was 10.2 mg/day, and the Greek Al intake was 3.7 mg/day, and Hong Kong intake was 0.6 mg/kg bw/week, mainly from tea and fried dough sticks (Bratakos et al. 2012; Chen et al. 2014; González-Weller et al. 2010; Rose et al. 2010). Since the neurotoxicity of Al was first reported, its neurotoxicity mechanism has attracted much attention. Epidemiology has shown that there is a certain correlation between Al content in drinking water and cognitive impairment, and occupational Al exposure also increases Al intake (Meyer-Baron et al. 2007). In vivo and in vitro experiments have confirmed that Al can enter the nervous system through the BBB, causing neurotoxicity such as decreased learning and memory and inattention. Paternain found that exposure to aluminum nitrate during pregnancy could cause weight loss in offspring and increase the incidence of visceral and bone deformities (Paternain et al. 1988). In this study, lateral ventricle injection was used to successfully establish a model of learning and memory impairment caused by Al in rats, and the mechanism of Al-induced synaptic plasticity damage was preliminarily studied.
Many studies have shown that, compared with AlCl3, Al(mal)3 could induce stronger neurotoxicity (Kawahara et al. 2001; Lévesque et al. 2000; Zeng et al. 2012). It may be that in biological systems, Al is more likely to form complexes with organic lipophilic substances, so organic lipophilic aluminum salt is a medium for studying Al neurotoxicity (Campbell et al. 2001). The Morris water maze experiment is a classic experiment of choice for conducting behavioral research, especially exploring learning and memory functions (Barnhart et al. 2015; Vorhees and Williams 2006). This study showed that with the increase of the exposure dose, the escape latency period of rats gradually increased, the target quadrant dwell time decreased, and the first time arriving at the platform also increased significantly. The rats in the 64 mM Al(mal)3 group showed significant impairment of learning and memory. Studies have shown that the escape latency period of rats in the 2 mg/mL and 3 mg/mL AlCl3 groups was significantly prolonged and the number of crossing the platform was also significantly reduced after drinking Al for 12 weeks (Wang et al. 2014). After intraperitoneal injection of Al(mal)3 for 60 days, the results of the water maze experiment showed that compared with the saline group, the learning and memory of rats in the middle- and high-dose Al-exposed groups was significantly impaired (Liang et al. 2012). These results were consistent with our study. The open field experiment is mainly used to detect animal exploration behavior and autonomous activity behavior. With the increase of the exposure dose, the time in the center area of rats gradually increased, the number of rearing and grooming decreased, and the difference was statistically significant, suggesting that acute lateral ventricle Al exposure could damage rats’ exploration behaviors in new environments and reduce their anxiety, which is consistent with the results of subchronic Al exposure.
Synaptic plasticity damage is one of the neurotoxic mechanisms of Al. Synaptic plasticity is a cellular mechanism of learning and memory, and changes in activity-dependent electricity and synaptic strength are closely related to LTP (Itoh et al. 2016). NMDARs play an important role in the occurrence of LTP, and the opening of their channels is the basis of learning and memory. The NR1/NR2 complex is widely distributed in the hippocampus, involved in learning and memory function, and can also cause excitotoxicity. It has been reported that the memory impairment of AD patients is not caused by Aβ deposition or neurofibrillary tangles but by synaptic loss, in which NMDARs play a major role (Terry et al. 1991). Studies have shown that the number and subunit composition of NMDARs in the postsynaptic membrane continue to change with the strength of synaptic activity. The release of glutamate activates AMPARs, leading to membrane depolarization. Ca2+ enters the postsynaptic membrane through activated NMDARs, which initiates various metabolic pathways and finally affects synaptic strength. Therefore, AMPARs and NMDARs have different control mechanisms to ensure the appropriate number and type of synaptic receptors on specific excitatory synapses (Bu et al. 2015; Horak et al. 2014). Increasing the expression of NMDARs could improve the symptoms of schizophrenia and improve memory function (Park et al. 2014), while inhibiting NMDARs in the hippocampus resulted in impairment of spatial learning and memory function and motor activity (Dai et al. 2015). The excitatory postsynaptic potential mediated by NMDARs decreased by 50–60% after stimulation with Schaeffer collateral in the hippocampus of aged rats (Kumar 2015; Lee et al. 2014). In this study, we first found that with increasing Al exposure dose, the protein expression levels of NMDAR1 and NMDAR2B decreased significantly, and there was no difference in NMDAR2A. However, RT-PCR results showed that the NMDAR2B gene level did not change significantly, indicating that the main reason for the decrease in NMDAR2B protein level was protein–protein interactions and multiple modifications. Therefore, we next focused on the regulation of NMDARs by PKC.
The regulation of NMDARs in neurons is the key to synaptic transmission and plasticity, and PKC can promote the regulation of NMDAR transport by interacting with NMDAR-related proteins (NAPs) on the cell surface (Yan et al. 2011). Immunofluorescence showed that PKC could increase the expression of NMDARs on the cell surface, and the PKC activator TPA could activate NMDAR channels (Lan et al. 2001; Lin et al. 2006). However, the transport of NMDARs to the membrane was not caused by direct phosphorylation of PKC. PKC promotes the transport of NMDARs through the autophosphorylation of CaMKII and through the phosphorylation of NMDARs through the tyrosine kinase Src (Yan et al. 2011). CaMKII is a very abundant brain protein concentrated in the postsynaptic compact (PSD) and a key molecule in the occurrence of LTP (Lisman et al. 2012). CaMKII knockout or mutant mice showed impaired learning ability and cognitive dysfunction (Bachstetter et al. 2014). The activation of CaMKII causes the autophosphorylation of T286, which promotes the effective binding of CaMKII and GluN2B and regulates the transport of NMDARs (Easton et al. 2011). At the same time, many studies have shown that the CaMKII/NMDAR complex is important not only for hippocampal LTP but also for the formation of hippocampal-dependent spatial learning and memory (Sanhueza and Lisman 2013; Stein et al. 2014). Increased synaptic activity leads to an increase in the CaMKII/NMDAR complex (Appleby et al. 2011). If the combination of CaMKII and GluN2B is destroyed, the LTP observed in the CA1 area is reduced by 50% by stimulating Shaffer collateral, and learning ability is also impaired in the water maze experiment (Halt et al. 2012). Sanhueza M found that the CaMKII/NMDAR complex was very important in maintaining synaptic strength (Sanhueza et al. 2011). PKC and CaMKII play an essential role in regulating NMDARs. Therefore, this study detected the protein expression levels of PKC, CaMKII, and p-CaMKII (Thr286) in the rat hippocampus. The results showed that the protein levels of PKC and p-CaMKII (Thr286) decreased significantly as the exposure dose increased, suggesting that PKC was involved in Al-induced learning and memory impairment. To further explore its mechanism, this experiment used the PKC agonist PMA to treat rats. The results showed that PMA alone could not improve the learning and memory ability of rats, but it could improve the learning and memory impairment caused by Al. The results of western blotting showed that adding PMA to the Al treatment group could increase the expression levels of PKC, p-CaMKII (Thr286), and NMDAR subunits.
In this study, we found that acute lateral ventricle Al exposure could damage learning and memory in rats and cause changes in the expression of NMDARs, while the activation of NMDARs was involved in AD-related synaptic dysfunction. AD is the most common form of dementia and characterized by the accumulation of β-amyloid (Aβ) and tau protein. Tau can cause abnormal phosphorylation of NMDARs (Ittner et al. 2010), and both Aβ and tau can change synaptic plasticity, leading to synapse loss, neurological dysfunction, and ultimately neuronal damage. Studies have shown that interference with synaptic transmission of glutamate might be the basis of the pathogenesis of AD (Rudy et al. 2015). An acute Al exposure rat model was used in this experiment; however, human exposure to Al is a chronic and long-lasting process. Therefore, the decreased expression of the NMDAR subunits PKC and p-CaMKII (Thr286) observed in the experiment needs to be further verified in other models, such as subchronic and chronic exposure models and even in the general population. In this experiment, it was observed that NMDAR2A had a decreasing trend, but there was no significant difference between the groups. This may be due to insufficient Al exposure time or dose, so the specific mechanism needs to be further studied with other models.
This study analyzed the effects of PKC on the expression of NMDAR subunits in Al-exposed rats, and the phosphorylation of CaMKII was involved in this process. We conducted a preliminary exploration of the mechanism of learning and memory impairment caused by Al. After Al enters nerve cells, it affects the translocation and activation of PKC, thereby reducing the expression and opening of NMDARs. It also reduces the influx of Ca2+ and the activation of CaMKII, thereby destroying the combination of NMDARs and CaMKII, further affecting the opening of NMDARs, and ultimately damaging learning and memory. NMDARs are also regulated by many other molecules, and their regulatory mechanism still requires further exploration.
Abbreviations
- Al(mal)3 :
-
Aluminum maltol
- PKC:
-
Protein kinase C
- NMDAR:
-
N-methyl-d-aspartic acid receptor
- LTP:
-
Long-term potentiation
References
Appleby VJ et al (2011) LTP in hippocampal neurons is associated with a CaMKII-mediated increase in GluA1 surface expression. J Neurochem 116:530–543. https://doi.org/10.1111/j.1471-4159.2010.07133.x
Bachstetter AD, Webster SJ, Tu T, Goulding DS, Haiech J, Watterson DM, Van Eldik LJ (2014) Generation and behavior characterization of CaMKIIβ knockout mice. PLoS ONE 9:e105191. https://doi.org/10.1371/journal.pone.0105191
Barnhart CD, Yang D, Lein PJ (2015) Using the Morris water maze to assess spatial learning and memory in weanling mice. PLoS ONE 10:e0124521. https://doi.org/10.1371/journal.pone.0124521
Bondy SC (2010) The neurotoxicity of environmental aluminum is still an issue. Neurotoxicology 31:575–581. https://doi.org/10.1016/j.neuro.2010.05.009
Bratakos SM, Lazou AE, Bratakos MS, Lazos ES (2012) Aluminium in food and daily dietary intake estimate in Greece. Food Addit Contam Part B Surveill 5:33–44. https://doi.org/10.1080/19393210.2012.656289
Brim BL et al (2013) Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behav Brain Res 238:211–226. https://doi.org/10.1016/j.bbr.2012.10.026
Bu Y et al (2015) Myosin IIb-dependent regulation of actin dynamics is required for N-Methyl-D-aspartate receptor trafficking during synaptic plasticity. J Biol Chem 290:25395–25410. https://doi.org/10.1074/jbc.M115.644229
Burnashev N, Szepetowski P (2015) NMDA receptor subunit mutations in neurodevelopmental disorders. Curr Opin Pharmacol 20:73–82. https://doi.org/10.1016/j.coph.2014.11.008
Campbell A, Hamai D, Bondy SC (2001) Differential toxicity of aluminum salts in human cell lines of neural origin: implications for neurodegeneration. Neurotoxicology 22:63–71. https://doi.org/10.1016/s0161-813x(00)00007-3
Cao X, Cui Z, Feng R, Tang YP, Qin Z, Mei B, Tsien JZ (2007) Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci 25:1815–1822. https://doi.org/10.1111/j.1460-9568.2007.05431.x
Chen MY, Chan BT, Lam CH, Chung SW, Ho YY, Xiao Y (2014) Dietary exposures to eight metallic contaminants of the Hong Kong adult population from a total diet study. Food Addit Contam Part A Chem Anal Control Expos Risk Assess 31:1539–1549. https://doi.org/10.1080/19440049.2014.935963
Colomina MT, Peris-Sampedro F (2017) Aluminum and Alzheimer’s disease. Adv Neurobiol 18:183–197. https://doi.org/10.1007/978-3-319-60189-2_9
Dai Y, Yang Y, Xu X, Hu Y (2015) Effects of uterine and lactational exposure to di-(2-ethylhexyl) phthalate on spatial memory and NMDA receptor of hippocampus in mice. Horm Behav 71:41–48. https://doi.org/10.1016/j.yhbeh.2015.03.008
Easton AC, Lucchesi W, Schumann G, Giese KP, Müller CP, Fernandes C (2011) αCaMKII autophosphorylation controls exploratory activity to threatening novel stimuli. Neuropharmacology 61:1424–1431. https://doi.org/10.1016/j.neuropharm.2011.08.036
Exley C (2009) Darwin, natural selection and the biological essentiality of aluminium and silicon. Trends Biochem Sci 34:589–593. https://doi.org/10.1016/j.tibs.2009.07.006
Farhat SM, Mahboob A, Ahmed T (2017) Cortex- and amygdala-dependent learning and nicotinic acetylcholine receptor gene expression is severely impaired in mice orally treated with AlCl(3). Biol Trace Elem Res 179:91–101. https://doi.org/10.1007/s12011-017-0942-1
González-Weller D, Gutiérrez AJ, Rubio C, Revert C, Hardisson A (2010) Dietary intake of aluminum in a Spanish population (Canary Islands). J Agric Food Chem 58:10452–10457. https://doi.org/10.1021/jf102779t
Halt AR et al (2012) CaMKII binding to GluN2B is critical during memory consolidation. EMBO J 31:1203–1216. https://doi.org/10.1038/emboj.2011.482
Horak M, Petralia RS, Kaniakova M, Sans N (2014) ER to synapse trafficking of NMDA receptors. Front Cell Neurosci 8:394. https://doi.org/10.3389/fncel.2014.00394
Hunt DL, Castillo PE (2012) Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol 22:496–508. https://doi.org/10.1016/j.conb.2012.01.007
Itoh N, Enomoto A, Nagai T, Takahashi M, Yamada K (2016) Molecular mechanism linking BDNF/TrkB signaling with the NMDA receptor in memory: the role of Girdin in the CNS. Rev Neurosci 27:481–490. https://doi.org/10.1515/revneuro-2015-0072
Ittner LM et al (2010) Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 142:387–397. https://doi.org/10.1016/j.cell.2010.06.036
Kawahara M, Kato M, Kuroda Y (2001) Effects of aluminum on the neurotoxicity of primary cultured neurons and on the aggregation of beta-amyloid protein. Brain Res Bull 55:211–217. https://doi.org/10.1016/s0361-9230(01)00475-0
Kiss T (2013) From coordination chemistry to biological chemistry of aluminium. J Inorg Biochem 128:156–163
Krewski D et al (2007) Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health Part B Crit Rev 10(Suppl 1):1–269. https://doi.org/10.1080/10937400701597766
Kumar A (2015) NMDA receptor function during senescence: implication on cognitive performance. Front Neurosci 9:473. https://doi.org/10.3389/fnins.2015.00473
Lan JY et al (2001) Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci 4:382–390. https://doi.org/10.1038/86028
Lee WH, Kumar A, Rani A, Foster TC (2014) Role of antioxidant enzymes in redox regulation of N-methyl-D-aspartate receptor function and memory in middle-aged rats. Neurobiol Aging 35:1459–1468. https://doi.org/10.1016/j.neurobiolaging.2013.12.002
Lévesque L, Mizzen CA, McLachlan DR, Fraser PE (2000) Ligand specific effects on aluminum incorporation and toxicity in neurons and astrocytes. Brain Res 877:191–202. https://doi.org/10.1016/s0006-8993(00)02637-8
Li HB, Jackson MF, Yang K, Trepanier C, Salter MW, Orser BA, Macdonald JF (2011) Plasticity of synaptic GluN receptors is required for the Src-dependent induction of long-term potentiation at CA3-CA1 synapses. Hippocampus 21:1053–1061. https://doi.org/10.1002/hipo.20818
Liang RF et al (2012) Aluminium-maltolate-induced impairment of learning, memory and hippocampal long-term potentiation in rats. Ind Health 50:428–436. https://doi.org/10.2486/indhealth.ms1330
Lin Y, Jover-Mengual T, Wong J, Bennett MV, Zukin RS (2006) PSD-95 and PKC converge in regulating NMDA receptor trafficking and gating. Proc Natl Acad Sci USA 103:19902–19907. https://doi.org/10.1073/pnas.0609924104
Lisman J, Yasuda R, Raghavachari S (2012) Mechanisms of CaMKII action in long-term potentiation Nature reviews. Neuroscience 13:169–182. https://doi.org/10.1038/nrn3192
Matyja E (2000) Aluminum enhances glutamate-mediated neurotoxicity in organotypic cultures of rat hippocampus. Folia Neuropathol 38:47–53
Meyer-Baron M, Schäper M, Knapp G, van Thriel C (2007) Occupational aluminum exposure: evidence in support of its neurobehavioral impact. Neurotoxicology 28:1068–1078. https://doi.org/10.1016/j.neuro.2007.07.001
Mota SI, Ferreira IL, Rego AC (2014) Dysfunctional synapse in Alzheimer’s disease—a focus on NMDA receptors. Neuropharmacology 76(Pt A):16–26. https://doi.org/10.1016/j.neuropharm.2013.08.013
Park JK, Lee SJ, Kim TW (2014) Treadmill exercise enhances NMDA receptor expression in schizophrenia mice. J Exerc Rehabil 10:15–21. https://doi.org/10.12965/jer.140088
Paternain JL, Domingo JL, Llobet JM, Corbella J (1988) Embryotoxic and teratogenic effects of aluminum nitrate in rats upon oral administration. Teratology 38:253–257. https://doi.org/10.1002/tera.1420380309
Rose M, Baxter M, Brereton N, Baskaran C (2010) Dietary exposure to metals and other elements in the 2006 UK Total Diet Study and some trends over the last 30 years. Food Addit Contam Part A Chem Anal Control Expos Risk Assess 27:1380–1404. https://doi.org/10.1080/19440049.2010.496794
Rudy CC, Hunsberger HC, Weitzner DS, Reed MN (2015) The role of the tripartite glutamatergic synapse in the pathophysiology of Alzheimer’s disease. Aging Dis 6:131–148. https://doi.org/10.14336/ad.2014.0423
Sanhueza M, Lisman J (2013) The CaMKII/NMDAR complex as a molecular memory. Mol Brain 6:10. https://doi.org/10.1186/1756-6606-6-10
Sanhueza M et al (2011) Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J Neurosci 31:9170–9178. https://doi.org/10.1523/jneurosci.1250-11.2011
Shaw CA, Li Y, Tomljenovic L (2013) Administration of aluminium to neonatal mice in vaccine-relevant amounts is associated with adverse long term neurological outcomes. J Inorg Biochem 128:237–244. https://doi.org/10.1016/j.jinorgbio.2013.07.022
Shaw CA, Seneff S, Kette SD, Tomljenovic L, Oller JW Jr, Davidson RM (2014) Aluminum-induced entropy in biological systems: implications for neurological disease. J Toxicol 2014:491316. https://doi.org/10.1155/2014/491316
Stein IS, Donaldson MS, Hell JW (2014) CaMKII binding to GluN2B is important for massed spatial learning in the Morris water maze. F1000Research 3:193
Terry RD et al (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30:572–580. https://doi.org/10.1002/ana.410300410
Thippeswamy AH, Rafiq M, Viswantha GL, Kavya KJ, Anturlikar SD, Patki PS (2013) Evaluation of Bacopa monniera for its synergistic activity with rivastigmine in reversing aluminum-induced memory loss and learning deficit in rats. J Acupunct Meridian Stud 6:208–213. https://doi.org/10.1016/j.jams.2013.02.004
Tomljenovic L (2011) Aluminum and Alzheimer’s disease: after a century of controversy, is there a plausible link? J Alzheimer’s Dis 23:567–598. https://doi.org/10.3233/jad-2010-101494
Villarreal DM, Do V, Haddad E, Derrick BE (2002) NMDA receptor antagonists sustain LTP and spatial memory: active processes mediate LTP decay. Nat Neurosci 5:48–52. https://doi.org/10.1038/nn776
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858. https://doi.org/10.1038/nprot.2006.116
Wang B et al (2014) Disturbance of intracellular calcium homeostasis and CaMKII/CREB signaling is associated with learning and memory impairments induced by chronic aluminum exposure. Neurotox Res 26:52–63. https://doi.org/10.1007/s12640-013-9451-y
Weilinger NL et al (2016) Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat Neurosci 19:432–442. https://doi.org/10.1038/nn.4236
Wenting L, Ping L, Haitao J, Meng Q, Xiaofei R (2014) Therapeutic effect of taurine against aluminum-induced impairment on learning, memory and brain neurotransmitters in rats. Neurol Sci 35:1579–1584. https://doi.org/10.1007/s10072-014-1801-x
Yan JZ et al (2011) Protein kinase C promotes N-methyl-D-aspartate (NMDA) receptor trafficking by indirectly triggering calcium/calmodulin-dependent protein kinase II (CaMKII) autophosphorylation. J Biol Chem 286:25187–25200. https://doi.org/10.1074/jbc.M110.192708
Yang K et al (2012) Metaplasticity gated through differential regulation of GluN2A versus GluN2B receptors by Src family kinases. EMBO J 31:805–816. https://doi.org/10.1038/emboj.2011.453
Zeng KW, Fu H, Liu GX, Wang XM (2012) Aluminum maltolate induces primary rat astrocyte apoptosis via overactivation of the class III PI3K/Beclin 1-dependent autophagy signal. Toxicol in Vitro: Int J Publ Assoc BIBRA 26:215–220. https://doi.org/10.1016/j.tiv.2011.11.010
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China [No. 81430078, 81872599]. We sincerely thank colleagues for their help and work on the research.
Funding
This work was supported by the National Natural Science Foundation of China [No. 81430078, 81872599].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, C., Ji, J., Zhao, X. et al. The Role of PKC in Regulating NMDARs in Aluminum-Induced Learning and Memory Impairment in Rats. Neurotox Res 39, 2042–2055 (2021). https://doi.org/10.1007/s12640-021-00407-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00407-0