Abstract
Chronic excessive fluoride intake is known to be toxic, and effects of long-term fluorosis on different organ systems have been examined. However, there are few studies about the effects of fluorosis on cardiovascular systems. Here, we studied the fluoride-induced apoptosis in H9c2 cells and determined the underlying molecular mechanisms including the cell viability, intracellular reactive oxygen species (ROS) level, the changes of mitochondrial membrane potential (ΔΨm), and the cell apoptosis. Sodium fluoride (NaF) at concentrations of 0, 2, 4, 8, and 16 mg/L was administered to cultured H9c2 cells for up to 48 h. After the treatment, H9c2 cells were collected and the associated parameters were measured by flow cytometry. Our study found that fluoride not only inhibited H9c2 cell proliferation but also induced cell apoptosis. With the increment of NaF concentration, the apoptotic rates and ROS generation were increased, while the ΔΨm was decreased. In summary, these data suggested that NaF-induced H9c2 cell apoptosis is mediated by direct increased intracellular ROS and downregulated ΔΨm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The natural background fluoride levels of surface water is continuously increasing due to rapid industrialization, use of fluoride-containing pesticides, and discharges of fluoridated municipal water [1]. Fluoride is reported to prevent caries [2]. But long-term excessive intake of fluoride can cause chronic fluorosis [3], characterized by tooth discoloration and skeletal manifestations such as crippling, osteoporosis, and osteosclerosis. The latest report showed that around 200 million people from 25 countries around the world are affected by fluoride toxicity [4], and China is one of the most populous countries in the world that threatened by the dreadful fate of fluorosis [5].
It is well known that fluoride can cause the different effects on different organ systems, and the cardiovascular system is susceptible to be disrupted by a high concentration of fluoride. Fluorine is an active nonmetallic element, which easily interacts with calcium and magnesium in the blood, thus causing blood calcium metabolism disorder, leading to the functional and organic damage to the cardiovascular and other systems [6]. Studies showed that fluoride has severe detrimental effects on cardiac function in atherosclerosis-related myocardial injury and pathophysiological remodeling [7–11]. However, very few studies have ever addressed the mechanism of action of fluorosis in cardiovascular system. Our investigations have previously demonstrated that fluoride is a cytotoxic agent inducing damage in myocardial tissues by oxidative stress through the Bcl-2/Bax signaling pathway [12]. Fluoride-induced oxidative stress plays an important role in the progression of a variety of cardiac disorders such as cardiac failure and ischemia [13]. Knowing that reactive oxygen species (ROS) plays a crucial role in fluoride-induced cardiotoxicity and oxidative stress, studies have been carried out on the cardioprotective action of antioxidants against fluoride-induced toxicity in cardiac tissues [13].
As “power plants” of the cell, mitochondria provide with more than 90 % energy of the body. Not only does it generate energy, but also it moderates cell aging and death. Mitochondrial dysfunction has been shown to participate in the induction of apoptosis and has even been suggested to be central to the apoptotic pathway [14]. In some apoptotic systems, loss of mitochondrial membrane potential (∆ψm) may be an early event in the apoptotic process. However, emerging data suggest that, depending on the model of apoptosis, the loss of ∆ψm may not be an early requirement for apoptosis, but on the contrary, may be a consequence of the apoptotic-signaling pathway [14]. Mitochondrial permeability transition (MPT), which involves a sudden increase of the mitochondrial membrane permeability, is a central coordination event of apoptosis [15–17]. It has been postulated that MPT could be caused by exogenous and endogenous ROS [15]. Some studies suggest that mitochondria are the main source of ROS, and that MPT contributes the O2 − generation and subsequent H2O2 generation [18–20].
The toxicity of fluoride on cardiac system can be evaluated directly on in vitro cultured cardiomyocytes. The H9c2 cell line derived from embryonic rat hearts maintains the features of cardiomyocytes and has been used as an in vitro model to study the effects of NaF on the heart [21]. Therefore, we used H9c2 cells as an in vitro model in the present study to determine the underlying molecular mechanisms including the cell viability, intracellular ROS level, mitochondrial membrane potential, and the cell apoptosis, which provides information for further elucidating the cardiac toxicity of fluoride.
Materials and Methods
Chemicals and Reagents
Dulbecco’s modified Eagle’s medium (DMEM) and methyl thiazolyl tetrazolium (MTT) kit were purchased from BOSTER (Wuhan, China). Fetal bovine serum (FBS) was obtained from Hangzhou Sijiqing Biological Engineering Material Company (Hangzhou, China). The apoptosis detection kit containing Annexin V-FITC and propidium iodide (PI) was from Nanjing KeyGen Biotechnology Company (Nanjing, China). Penicillin-streptomycin solution was purchased from Solarbio Biological Technology Company (Shanghai, China). Mitochondrial membrane potential assay kit with JC-1 and reactive oxygen species assay kit were obtained from Beyotime Institute of Biotechnology (Shanghai, China). NaF was purchased from Sigma (St. Louis, MO, USA). All other reagents were of analytical grade.
Cell Culture
H9c2 cell lines were purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. The H9c2 cells were cultured with DMEM supplemented with 10 % FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin at 37 °C in 5 % CO2 incubator. Medium was changed every 2 days. Cells were subcultured at a ratio of 1:3 every 3 days using 0.2 % trypsin plus 0.02 % EDTA. For experiments, when the cells had again grown to 70–80 % confluence, they were exposed to different NaF concentrations (0, 2, 4, 8, and 16 mg/L) incubated in 5 % FCS-containing medium for 48 h. At the end of 48 h, cells were subjected to ROS, mitochondrial membrane potential, and cell apoptosis assays with FACS calibur flow cytometry.
Cell Survival Assay
To study the effects of fluoride on the survival of H9c2 cells, the viability of cells was evaluated at 24 and 48 h by utilizing the MTT assay. The cells were treated with different NaF concentrations (0, 2, 4, 8, and 16 mg/L). Then, 10 μL MTT solution was added to the media for another 4 h at 37 °C. Later, 100 μL formazan-dissolved solution was added to dissolve the formazan crystals. Finally, the optical density in each well was determined by spectrophotometry at a wavelength of 570 nm.
HE staining
Hematoxylin-eosin staining (HE staining) was used for morphological examination. The H9c2 cells were subcultured on the cover-slips at a concentration of 1 × 104 cells/mL in 24-well plates. DMEM complete medium containing 0, 2, 4, 8, and 16 mg/L NaF were added in each well for 48 h. After treatment, the cover-slips were took out of 24-well plates and then washed three times with PBS solution. The H9c2 cells were fixed in 95 % ethanol for 20 min followed by a rinse with PBS solution (1 min every time). After washing in PBS, the cells were stained with hematoxylin for 15 min and washed with tap water. Then the H9c2 cells were stained with eosin for 1 min followed by a rinse with tap water. After drying open-air, the cover-slips were mounted on glass slides with neutral gum for microscopic examination.
Detection of Intracellular ROS Level
To determine ROS generation within fluoride-treated cells, fluorescence-activated cell sorting (FACS) analysis was performed. After trypsinized to collect, H9c2 cells were centrifugated and then suspended again in 10 μmol/L diluted 2′,7′-dichlorofluorescin diacetate (DCFH-DA). The suspension cells were incubated for 20 min in 5 % CO2 incubator, blending them every 3–5 min. After treatment, cells were washed three times with DMEM without FBS, and after centrifugation, 250 μL binding buffer was added to each sample. The labeled cells were detected by FACS calibur flow cytometry.
Mitochondrial Membrane Potential Assay
Mitochondrial membrane potential assay kit with JC-1 was used to detect the ΔΨm within fluoride-treated cells. H9c2 cells were cultured in DMEM complete medium containing 0, 2, and 16 mg/L NaF for 48 h. After treatment, cells were trypsinized to collect, after centrifugated and then suspended again in DMEM complete medium at a concentration of 1 ~ 6 × 105 cells/mL. The cells were incubated for 20 min in 5 % CO2 incubator after blending with the 1ml JC-1 stain liquid. Then cells were washed two times with JC-1 stain buffer solution (1×), after centrifugation, cells were suspended again in 500 μL JC-1 stain buffer solution (1×) and then detected by FACS calibur flow cytometry.
Flow Cytometry Analysis of Cell Apoptosis
For apoptosis analysis, Annexin V-FITC/propidium iodide (PI) staining was performed using flow cytometry according to the manufacturer’s guidelines. H9c2 cells were cultured in DMEM complete medium containing 0, 2, and 16 mg/L NaF for 48 h. After treatment, cells were trypsinized and washed two times with PBS (pH 7.4) and then suspended again in binding buffer at a concentration of 1 × 106cells/mL. The suspension cells were incubated with 5 μL Annexin V-FITC and 5 μL PI for 15 min in the dark at 37 °C, then, 250 μL binding buffer was added to each sample. The labeled cells were detected directly by FACS calibur flow cytometry.
Statistical Analysis
All data were expressed as means ± standard error (SE). Differences between groups were determined by the one-way ANOVA with P < 0.05 considered as statistically significant.
Results
Effect of NaF Treatment on H9c2 Cell Viability
H9c2 cell viability was measured after NaF treatments for 24 and 48 h by cell viability assays. Compared with the control group, numbers of viable cells were moderately increased at low concentrations but decreased with high NaF concentration. For 48-h incubation, 16 mg/L NaF treatment dramatically depressed H9c2 cell proliferation. These results indicate that long time exposure to NaF inhibits H9c2 cell proliferation (Fig. 1).
HE Staining of H9c2 Cell after NaF Treatment
After incubation with serial concentrations NaF for 48 h, we evaluated the cellular morphology by using an inverted microscope. In the control group without fluoride, cells were presented to fusiform, lined up in order, and the intercellular space were tight. With the increase of NaF concentrations, the density of H9c2 cells was decreased. The majority of H9c2 cells became curled, flaked, and contracted with a dosage of 16 mg/L of NaF.
NaF-Induced Intracellular ROS Production in H9c2 Cells
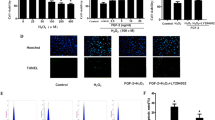
The effects of NaF on the production of intracellular ROS were examined by DCFH-DA staining. Following exposure to 0, 2, and 16 mg/L of NaF for 48 h, the number of cells with positive fluorescence indicating ROS generation was higher than that of the control group, and it shows a dose-dependent fashion. These results demonstrate that NaF upregulates the intracellular ROS level in H9c2 cells (Fig. 2).
NaF-induced intracellular ROS production in H9c2 cells after 48 h of treatment. a–c The intracellular ROS production was measured by a fluorescent dye DCFH-DA after 48 h of exposure to 0, 2, and 16 mg/L NaF concentrations. d The mean fluorescence intensity of ROS in control, 2 mg/L NaF, and 16 mg/L NaF group for 48 h. Values represent mean ± SEM of three independent experiments performed. (**P < 0.01 vs. control, ## P < 0.01 vs. control)
Effect of NaF on Mitochondrial Membrane Potential
The ΔΨm of H9c2 cells was analyzed by a fluorescent dye JC-1. JC-1 is capable of selectively entering mitochondria, where it forms monomers and emits green fluorescence when ΔΨm is relatively low. At high ΔΨm, JC-1 aggregates and gives a red fluorescence. When H9c2 cells were treated with different concentrations of NaF for 48 h, we found that the percentage of cells with green fluorescence increased with the increment of NaF concentration. This suggests that ΔΨm in NaF-induced H9c2 cells was down-regulated (Fig. 3).
Effects of NaF on ΔΨm in H9c2 cells by flow cytometry assay. a–c The ΔΨm was measured by a fluorescent dye JC-1 after 48 h of exposure to 0, 2, and 16 mg/L NaF concentrations. Cells in R2 area represented ratio of JC-1 from polymer to monomer. d The percentage of green fluorescence of JC-1 in control, 2 mg/L NaF, and 16 mg/L NaF group for 48 h. Values represent mean ± SEM of three independent experiments performed. (## P < 0.01 vs. control)
NaF Induces H9c2 Cell Apoptosis
The H9c2 cell apoptosis in response to NaF treatment was assessed by flow cytometry after staining with Annexin V-FITC and PI. Cells in the lower left quadrant of each picture correspond to normal cells (Annexin V /PI). Cells in right lower quadrant correspond to early apoptotic cells (Annexin V+/PI−). Cells in the right upper quadrant correspond to late apoptotic/dead cells (Annexin V+/PI+). NaF treatment induces both early and late stage cell apoptosis in a dose-dependent fashion. At the end of 48 h, the total apoptotic cells were increased by 23.16 and 28.27 % in the presence of 2 and 16 mg/L NaF, respectively (Fig. 4).
Effects of NaF on cell apoptosis in H9c2 cells by flow cytometry assay. a–c Cell apoptosis was measured by Annexin V-FITC and PI double-staining after 48 h of exposure to 0, 2, and 16 mg/L NaF. d The percentage of early apoptosis, late apoptosis, and total cell death in control, 2 mg/L NaF, and 16 mg/L NaF group for 48 h. Values represent mean ± SEM of three independent experiments performed. (**P < 0.01 vs. control; ## P < 0.01 vs. control)
Discussion
Taking fluoride at recommended concentration is suggested to be beneficial to health [22–24]. However, more and more evidence showed that fluoride intoxication produces oxidative stress and damages various tissues [25–28]. The cardiovascular systems are susceptible to a high concentration of fluoride, because many cardiovascular dysfunctions have been observed, such as the decreasing of cardiac output, the occurrence of arrhythmias, and heart block [7, 8, 29]. However, the mechanisms of fluorosis in the cardiovascular system need to be further illustrated.
In our study, we detected the level of intracellular ROS increased with the increasing of NaF for 48 h. It is showed that NaF causes cardiac cell oxidative metabolic abnormalities, breaks the ROS dynamic balance, and leads to the body system in oxidative stress condition [30]. This produces cytotoxic effect and eventually causing irreversible damage to cells [31, 32]. NaF treatment leads to cardiomyocyte dysfunction in which cells become curled, flaked, and contracted.
As one type of signaling molecule, ROS have participated in signal transduction of cell proliferation, differentiation, and apoptosis. Our results indicated that the ΔΨm in NaF-induced H9c2 cells were downregulated compared with the control group. Furthermore, the NaF induces the H9c2 cells apoptosis even at low dose (2 mg/L). ROS formation may cause the disruption of the mitochondrial membrane potential and subsequent release of cytochrome C, which leads to caspase-3 cleavage and finally cell apoptosis [33, 34].
Apoptosis is mediated by two central pathways including mitochondrial pathway and death receptor pathway [35]. Mitochondria are a target of ROS and a source for the additional ROS generation. In situ generated ROS can cause the opening of mitochondrial permeability transition pore (mPTP), with subsequent ΔΨ loss and cell apoptosis. Excessive ROS are the trigger in the early stages of apoptosis to induce the depolarization of the ΔΨm. This eventually results in an increase in the level of ROS along with other proapoptotic molecules in the cytosol [36–38].
In conclusion, our study suggested that NaF exerts a toxic effect on apoptosis in H9C2 cells via regulating the mitochondrial pathway, such as increasing of ROS generation and decreasing of ΔΨm. Simultaneously, NaF inhibited the cells proliferation and induced morphological damage in H9C2 cells. This research provides insightful evidences for the complexity and severity of fluoride’s effects on cellular functions because the effects of fluoride are closely related to exposure time and concentration. In addition, a more precise action mechanism of fluoride on these molecular targets should be delineated in further studies.
References
Aguirre-Sierra A, Alonso A, Camargo JA (2013) Fluoride bioaccumulation and toxic effects on the survival and behavior of the endangered white-clawed crayfish Austropotamobius pallipes (Lereboullet). Arch Environ Contam Toxicol 65:244–250
Mukhopadhyay D, Chattopadhyay A (2014) Induction of oxidative stress and related transcriptional effects of sodium fluoride in female zebrafish liver. Bull Environ Contam Toxicol 93:64–70
Adali MK, Varol E, Aksoy F, Icli A, Ersoy IH, Ozaydin M, Erdogan D, Dogan A (2013) Impaired heart rate recovery in patients with endemic fluorosis. Biol Trace Elem Res 152:310–315
Trevors JT, Saier MH Jr (2009) Where is the global environmental bailout? Water Air Soil Pollut 198:1–3
Ayoob S, Gupta A (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 55:433–487
Wang Y, Gu ZW (1996) Modern occupational medicine. People's medical publishing house, Beijing
Cicek E, Aydin G, Akdogan M, Okutan H (2005) Effects of chronic ingestion of sodium fluoride on myocardium in a second generation of rats. Hum Exp Toxicol 24:79–87
Basha MP, Sujitha NS (2011) Chronic fluoride toxicity and myocardial damage: antioxidant offered protection in second generation rats. Toxicol Int 18:99–104
Zhan WC, He DJ (1988) Ultrastructural findings in liver, kidneys, thyroid gland and myocardium of rabbits following sodium fluoride administration. J Guiyang Med Coll 13:129–132
Bian J, Lin X, Yang X, Fan T, Zhu Q (2010) Changes of certain oxidative, anti-oxidative and vascular function indexes of New Zealand rabbit exposed by high-fluoride. Wei Sheng Yan Jiu 39:751–754
Shen XY, Ruan Q, Zhang ZG, Xu XL (2003) Influence of fluorosis on hemodynamics of rats. Chin J Control Endemic Dis 18:208–209
Cheng RY, Nie QL, Sun HF, Zhang YJ, Wu LH, Ma YQ, Yan XY (2013) Fluoride-induced oxidative stress in rat myocardium through the Bax/Bcl-2 signalling pathway. Fluoride 46(4):198–203
Sinha M, Manna P, Sil PC (2008) Terminalia arjuna protects mouse hearts against sodium fluoride-induced oxidative stress. J Med Food 11:733–740
Ly JD, Grubb DR, Lawen A (2003) The mitochondrial membrane potential (∆ψm) in apoptosis; an update. Apoptosis 8:115–128
Kroemer G, Zazami N, Susin SA (1997) Immunol Today 18:44–51
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Kowaltowski AJ, Netto LE, Vercesi AE (1998) The thiol-specific antioxidant enzyme prevents mitochondrial permeability transition. Evidence for the participation of reactive oxygen species in this mechanism. J Biol Chem 273:12766–12769
Cai J, Jones DP (1998) Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem 273:11401–11404
Kim CN, Wang X, Huang Y, Ibrado AM, Liu L, Fang G, Bhalla K (1997) Overexpression of Bcl-X (L) inhibits Ara-C-induced mitochondrial loss of cytochrome C and other perturbations that activate the molecular cascade of apoptosis. Cancer Res 57:3115–3120
Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT (1998) Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273:11619–11624
Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ (2002) Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome c-mediated caspase-3 activation pathway. Diabetes 51:1938–1948
Sun Z, Niu R, Wang B, Jiao Z, Wang J, Zhang J, Wang S, Wang J (2011) Fluoride-induced apoptosis and gene expression profiling in mice sperm in vivo. Arch Toxicol 85:1441–1452
Ge Y, Niu R, Zhang J, Wang J (2011) Proteomic analysis of brain proteins of rats exposed to high fluoride and low iodine. Arch Toxicol 85:27–33
Yan X, Feng C, Chen Q, Li W, Wang H, Lv L, Smith GW, Wang J (2009) Effects of sodium fluoride treatment in vitro on cell proliferation, apoptosis and caspase-3 and caspase-9 mRNA expression by neonatal rat osteoblasts. Arch Toxicol 83:451–458
Dubey N, Khan AM, Raina R (2013) Sub-acute deltamethrin and fluoride toxicity induced hepatic oxidative stress and biochemical alterations in rats. Bull Environ Contam Toxicol 91:334–338
Shivashankara AR, Shivarajashankara YM, Bhat PG, Rao SH (2002) Lipid peroxidation and antioxidant defence systems in liver of rats in chronic fluoride toxicity. Bull Environ Contam Toxicol 68:612–616
Hassan HA, Yousef MI (2009) Mitigating effects of antioxidant properties of black berry juice on sodium fluoride induced hepatotoxicity and oxidative stress in rats. Food Chem Toxicol 47:2332–2337
Ranjan R, Swarup D, Patra RC (2009) Oxidative stress indices in erythrocytes, liver, and kidneys of fluoride-exposed rabbits. Fluoride 42:88–93
Dönmez N, Çinar A (2003) Effects of chronic fluorosis on electrocardiogram in sheep. Biol Trace Elem Res 92:115–121
Miyamoto H, Doita M, Nishida K, Yamamoto T, Sumi M, Kurosaka M (2006) Effects of cyclic mechanical stress on the production of inflammatory agents by nucleus pulposus and anulus fibrosus derived cells in vitro. Spine 31:4–9
Sohal RS, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273:59–63
Beatty S, Koh H, Phil M et al (2000) The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 45:115–134
Sang H, Zhang L, Li J (2012) Anti-benzopyrene-7, 8-diol-9, 10-epoxide induces apoptosis via mitochondrial pathway in human bronchiolar epithelium cells independent of the mitochondria permeability transition pore. Food Chem Toxicol 50:2417–2423
Khan M, Ding C, Rasul A, Yi F, Li T, Gao H, Gao R, Zhong L, Zhang K, Fang X, Ma T (2012) Isoalantolactone induces reactive oxygen species mediated apoptosis in pancreatic carcinomapanc-1 cells. Int J Biol Sci 8:533–547
Barbier O, Arreola-Mendoza L, Del Razo LM (2010) Molecular mechanisms of fluoride toxicity. Chem Biol Interact 188:319–333
Carmody RJ, Cotter TG (2001) Signalling apoptosis: a radical approach. Redox Reports 6:77–90
Chandra J, Samali A, Orrenius S (2000) Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 29:323–333
Shih CM, Ko WC, Wu JS, Wei YH, Wang LF, Chang EE, Lo TY, Cheng HH, Chen CT (2004) Mediating of caspase-independent apoptosis by cadmium through the mitochondria-ROS pathway in MRC-5 fibroblasts. J Cell Biochem 91:384–397
Acknowledgments
This research was sponsored by the China National Natural Science Foundation (Grant Nos. 31240009, 31302158); Shanxi Scholarship Council of China (Grant No. 2012–079); Shanxi Province Science and Technology Bureau Program (Grant Nos. 2011021030-1, No. 2013011059-1) and Shanxi Province Soft Science (Grant No. 2013041084-03)
Conflicts of Interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yan, X., Yang, X., Hao, X. et al. Sodium Fluoride Induces Apoptosis in H9c2 Cardiomyocytes by Altering Mitochondrial Membrane Potential and Intracellular ROS Level. Biol Trace Elem Res 166, 210–215 (2015). https://doi.org/10.1007/s12011-015-0273-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0273-z