Abstract
To investigate the effect of excessive fluoride on the mitochondrial function of cardiomyocytes, 20 healthy male mice were randomly divided into 2 groups of 10, as follows: control group (animals were provided with distilled water) and fluoride group (animals were provided with 150 mg/L F− drinking water). Ultrastructure and pathological morphological changes of myocardial tissue were observed under the transmission electron and light microscopes, respectively. The content of hydrolysis ATP enzyme was observed by ATP enzyme staining. The expression levels of ATP5J and ATP5H were measured by Western blot and quantitative real-time PCR. The morphology and ultrastructure of cardiomyocytes mitochondrial were seriously damaged by fluoride, including the following: concentration of cardiomyocytes and inflammatory infiltration, vague myofilaments, and mitochondrial ridge. The damage of mitochondrial structure was accompanied by the significant decrease in the content of ATP enzyme for ATP hydrolysis in the fluoride group. ATP5J and ATP5H expressions were significantly increased in the fluoride group. Thus, fluoride induced the mitochondrial dysfunction in cardiomyocytes by damaging the structure of mitochondrial and interfering with the synthesis of ATP. The proactive ATP5J and ATP5H expression levels were a good response to the mitochondrial dysfunction in cardiomyocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiomyocytes are an important part of the heart; when the cardiomyocyte contracts, the blood containing nutrients and oxygen is pushed into the arteries and flows throughout the body, cardiomyocyte diastolic blood from the vein flows into the heart [1, 2]. Adenosine triphosphate (ATP), an energy material produced by cardiomyocyte mitochondria, plays a vital role in the cardiomyocyte systolic and diastolic processes [3]. As crucial subcellular structures in cardiomyocyte, mitochondria synthesize different types of enzymes that allow sufficient energy synthesis and continuous aerobic respiration [4]. Mitochondria ATP synthase is a crucial enzyme in the mitochondrial internal membrane; it promotes oxidative phosphorylation to enhance the synthesis of ATP [5, 6]. ATP synthase consists of F0 and F1 subunits; the combination of F0 and F1 subunits promotes the synthesis of ATP [7, 8]. ATP5J and ATP5H are important nuclear-coding genes of F0 subunit; ATP5J and ATP5H expressions are related to the synthesis of ATP synthase and mitochondrial ATP [9–12].
Fluorine is a non-metallic element with lively chemical properties; it mainly exists as fluoride (F), such as CaF2, Na[AlF6], and Ca10(PO4)6F2 [13]. Fluorine plays an important role in the growth and development of the animal body, especially in the formation of mammalian enamel; the fluoride needed by the body is mainly obtained through drinking water and consuming food [14–16]. F is an anion that easily penetrates the cell membrane; excessive accumulation of fluorine ion in cells seriously affects the structure and function of the organelles, thereby resulting in different phenomena, such as mitochondrial swelling and endoplasmic reticulum vacuolization [15, 17, 18]. The heart is one of the most important organs in the cardiovascular system; cardiomyocytes contain abundant mitochondria that play an important role in the normal function of the heart [19]. Studies reported that the incidence of heart diseases is related to long-term exposure to fluoride in fluorosis areas; examples of these heart diseases are atherosclerosis, myocardial infarction, and hypertension [13, 20]. These diseases are related to the mitochondria dysfunction in the cardiomyocytes, because mitochondria are vulnerable to fluoride, and the synthesis of ATP in the mitochondria is blocked by fluoride [21]. The fluoride-induced mitochondrial dysfunction in cardiomyocytes was investigated in the present work by observing the structure of histomorphology and ultrastructure of cardiomyocyte mitochondria. The content of ATP hydrolytic enzyme was detected, and the expression levels of ATP5J and ATP5H were measured.
Material and Methods
Animals and Treatment
Twenty healthy male Kunming mice (4 weeks old; weighting about 18.6 g) were obtained from the Animal Center of Zhengzhou University and were randomly divided into the control and fluoride groups of 10 members after a week of balanced feeding. In the control group, the animals were given distilled water for 70 days. In the fluoride group, 150 mg/L F− was included in the drinking water for 70 days. All experimental mice were kept in the light (12 h light/dark cycle) and provided standard diet. The temperature of animal house was maintained at 22 °C˗25 °C under ventilation conditions. Mice were anesthetized via urethane intraperitoneal injection. After blood collection, the hearts were carefully removed. Three samples from each group were fixed for observation, and the rest of the samples were preserved in liquid nitrogen for messenger RNA (mRNA) and protein expression studies.
Histomorphological Observation
Myocardium tissue was fixed in 4% paraformaldehyde for 48 h and dehydrated in a series of alcohol solutions. After transferring into xylene, the heart tissue was embedded in paraffin. Sections of 5 μm were sliced and stained with hematoxylin-eosin and then observed under the light microscope.
TEM Examination
Myocardium tissue was fixed in 2.5% glutaraldehyde phosphate buffer (PB) (pH 7.4) at 4 °C and washed by PB. Afterward, it was fixed in 1% osmic acid and washed thrice in PB before dehydrating in different concentrations of alcohol. The tissue was finally embedded in araldite resin. Ultrathin sections of 50 nm were cut and stained with uranyl acetate and lead citrate. These samples were then observed under the TEM (H-7650, Hitachi, Japan).
ATP Enzyme Staining
Myocardium tissue was obtained from male mice, and the sections were prepared following the methods of Guth et al. [22] and Gollnick et al. [23]. The sections were observed under light microscope.
Quantitative Real-Time PCR Analysis
Total cellular RNA was isolated from the liquid nitrogen-frozen myocardium tissue using Trizol Reagent (Invitrogen, USA) following the manufacturer’s instruction. The mRNA expression levels of ATP5J and ATP5H were detected with Mx3000pTMQRT-PCR system (Stratagene, USA) real-time PCR RG-3000A and a SYBR® Permix Ex Taq™ (Perfect Real Time) Kit (Takara, China). The specific primer sequences for ATP5J, ATP5H and β-actin were designed (Table 1). Relative mRNA expression was determined using the △△cycle threshold (Ct) method. PCR was performed under the following conditions: 95 °C for 2 min and 30 cycles of amplification (95 °C for 10 s, 55 °C for 20 s, and 72 °C for 25 s).
Western Blot Analysis
Myocardium tissue was thoroughly washed in icecold PB before homogenization. The homogenate was subsequently centrifuged at 12,000×g for 5 min at 4 °C. The collected supernatant was used for protein detection and analysis. The supernatant protein was separated by SDS-PAGE and transferred onto polyvinylidene (PVDF) membrane. After blotting by using 5% non-fat milk, the membrane was incubated in primary antibody overnight at 4 °C. The primary antibodies were used: rabbit polyclonal anti-ATP5J (BA1778-2; Wuhan Boster Biological Technology Ltd., China), rabbit polyclonal anti-ATP5H (PB0281; Wuhan Boster Biological Technology Ltd., China), and rabbit polyclonal anti-β-actin (GB13001-1; Wuhan Guge Biological Technology, China). The secondary antibody was horseradish peroxidase-conjugated goat anti-rabbit IgG (GB23303; Wuhan Guge Biological Technology, China). PVDF membrane was covered with the developing solution and then placed in the BioRad developer. The positive bands were detected using enhanced chemiluminescent reagents. The gray densities of the protein bands were normalized by using β-actin density as the control.
Statistical Analysis
Experimental data were showed as mean ± SD. Statistical and data analyses were measured by the Software of SPSS13.0 version and Student’s t test. Values of p < 0.05 were statistically significant.
Results
Effects of Fluoride on the Morphology of Cardiomyocyte in Mice
Morphological changes in cardiomyocyte of mice are shown in Fig. 1. In the control group, considerable amounts of cardiomyocytes showed normal features. The intercellular spacing was clear, and the nuclear structure was normal (Fig. 1 (a1, a2)). However, in the fluoride group, the cardiomyocytes had irregular sequences, and a large number of inflammatory cell infiltration and nuclear condensation were observed (Fig. 1 (b1, b2)).
Effects of Fluoride on the Ultrastructure of Cardiomyocyte in Mice
The ultrastructure changes of cardiomyocyte were observed (Fig. 2). Normal ultrastructure of cardiomyocyte was observed in the control group, i.e., well-regulated myofilaments, clear mitochondrial ridge, and intercalated disks (Fig. 2 (a1, a2)). However, myocardial fiber breakage, mitochondrial ridge dissolution and breakage, and slight vacuolization of mitochondria were observed in the fluoride group (Fig. 2 (b1, b2)).
ATP Enzyme Stain Examinations
Figure 3 shows the results of ATP enzyme staining. In the control group, ATP enzyme was widely distributed in myocardial fibers, and the stain was brown black in color (Fig. 3 (a1, a2)). However, in the fluoride group, the ATP enzyme stain became khaki and pale in the myocardial fibers and enlarged interspace of myocardial fibers was observed with the decreasing content of ATP enzyme (Figs. 3 (b1, b2)).
Western Blot of ATP5J and ATP5H Protein Expression
The protein expression levels of ATP5J and ATP5H in myocardium tissue are shown in Fig. 4. In the fluoride group, ATP5J and ATP5H showed significant protein imprint expressions (Fig. 4a). The protein relative expression levels of ATP5J and ATP5H were significantly increased by 50% (p < 0.01) and 125.6% (p < 0.01), respectively, compared with the control group (Fig. 4b).
Quantitative Real-Time PCR of ATP5J and ATP5H mRNA Expression
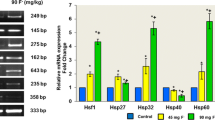
The mRNA expression levels of ATP5J and ATP5H are shown in Fig. 5. In the fluoride group, the mRNA expression levels of ATP5J and ATP5H were significantly increased by 136% (p < 0.01) and 194% (p < 0.01) respectively, compared with the control group (Fig. 5).
Discussion
Heart is the most important organ in the vertebrate and contains a special kind of myocardial fiber. The regular contraction and relaxation of the heart are caused by the myocardial fiber, which has an automatic rhythm of excitement, along with the assistance of ATP produced by cardiomyocyte mitochondria [2, 3, 24]. Incidence of atherosclerosis is related to the exposure to fluoride, such as myocardial infarction and hypertension [13, 20]. In the present study, the concentration of cardiomyocyte and inflammatory infiltration was observed in the fluoride group. The fluoride-induced breaking of the myocardial fibers and mitochondrial ridge was also observed under TEM. Numerous fluoride ions accumulate in the cardiomyocyte, thereby inducing oxidative stress and producing reactive oxygen species, which further cause mitochondrial damage and cell apoptosis [13, 25, 26]. Fluoride-induced damage through oxidative stress in the brain, liver, and kidney has been reported [27–29]. Zhou et al. found that excessive fluoride reduces the activities of SOD, GSH-Px, and the total anti-oxidizing capability (T-AOC), thereby further increasing the contents of ROS and RNS [15]. Some studies have suggested that the primary source of oxidative stress in mammalian cells is the mitochondria [30]. In the present study, fluoride-induced mitochondrial damage in cardiomyocyte is related to the balance of anti-oxidase activity.
Cardiomyocyte contains abundant mitochondria, which play an important role in the normal function of the heart. Excessive fluoride intake induces mitochondrial damage and reduces the production of ATP in the cardiomyocyte [31]. ATP content decreased after the fluoride-induced damaging of the cardiomyocytes. Mitochondrial ATP synthase plays a crucial role in ATP synthesis in the mitochondrial and is mainly composed of two parts: a water soluble protein complex F1 and a hydrophobic part F0 [32, 33]. ATP5J and ATP5H belong to the nuclear encoding genes of F0 subunits of ATP synthase [32] and are closely related to ATP synthesis; the change in their sequence and expression directly or indirectly reflects the changes of mitochondria and ATP synthase function [34, 35]. The expression of mRNA and protein levels of ATP5J and ATP5H was significantly increased, but the production of ATP decreased. We speculated that increasing ATP5J and ATP5H expressions is related to ATP decrease. Heart is an important blood pump in the body and demands substantial ATP for contraction and relaxation. When the content of ATP decreased due to fluoride-induced damage to cardiomyocyte mitochondria, ATP deficiency caused myocardial dysfunction and further induced myocardial ischemia and heart failure [36, 37]. The expressions of ATP5J and ATP5H increased to promote the synthesis of ATP in cardiomyocyte mitochondria and to ensure the normal function of the heart and the production of ATP supply. Thus, the increased expressions of ATP5J and ATP5H compensate for fluoride-induced mitochondrial dysfunction in cardiomyocytes that further lead to reduced synthesis of ATP.
In conclusion, excessive fluoride intake induces mitochondrial dysfunction of cardiomyocyte by damaging the ultrastructure of cardiomyocyte mitochondria and indirectly interfering with the synthesis of ATP. ATP5J and ATP5H proactive expressions are closely related to the fluoride-induced mitochondrial dysfunction in cardiomyocyte.
Reference
Williams GS, Smith GD, Sobie EA, Jafri MS (2010) Models of cardiac excitation-contraction coupling in ventricular myocytes. Math Biosci 226(1):1–15
Norman C, Rall JA, Tikunova SB, Davis JP (2007) Modulation of the rate of cardiac muscle contraction by troponin C constructs with various calcium binding affinities. Am J Physiol Heart Circ Physiol 293(4):H2580–H2587
Ren M, Liu Y, Zhao H, Dong S, Jiang Z, Li K, Tian J (2016) Adenosine triphosphate postconditioning is associated with better preserved global and regional cardiac function during myocardial ischemia and reperfusion: a speckle tracking imaging-based echocardiologic study. Cardiovasc Ther 34(5):343–351
Antico Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC (2012) Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal 16(10):1150–1180
Bernardi P, Di Lisa F, Fogolari F, Lippe G (2015) From ATP to PTP and back: a dual function for the mitochondrial ATP synthase. Circ Res 116(11):1850–1862
Beutner G, Alavian KN, Jonas EA, Porter GA Jr (2016) Erratum to: the mitochondrial permeability transition pore and ATP synthase. Handb Exp Pharmacol. doi:10.1007/164_2016_87
Nowak G, Bakajsova D (2015) Protein kinase C-α interaction with F0F1-ATPase promotes F0F1-ATPase activity and reduces energy deficits in injured renal cells. J Biol Chem 290(11):7054–7066
Hornung T, Volkov OA, Zaida TM, Delannoy S, Wise JG, Vogel PD (2008) Structure of the cytosolic part of the subunit b-dimer of Escherichia coli F0F1-ATP synthase. Biophys J 94(12):5053–5064
Rai AK, Spolaore B, Harris DA, Dabbeni-Sala F, Lippe G (2013) Ectopic F0F1 ATP synthase contains both nuclear and mitochondrially-encoded subunits. J Bioenerg Biomembr 45(6):569–579
Nesci S, Ventrella V, Trombetti F, Pirini M, Pagliarani A (2014) Thiol oxidation of mitochondrial F0-c subunits: a way to switch off antimicrobial drug targets of the mitochondrial ATP synthase. Med Hypotheses 83(2):160–165
Sawai H, Takai-Igarashi T, Tanaka H (2015) Identification of collaborative activities with oxidative phosphorylation in bipolar disorder. Bioinformation 11(4):207–216
Croston TL, Shepherd DL, Thapa D, Nichols CE, Lewis SE, Dabkowski ER, Jagannathan R, Baseler WA, Hollander JM (2013) Evaluation of the cardiolipin biosynthetic pathway and its interactions in the diabetic heart. Life Sci 93(8):313–322
Yan X, Yang X, Hao X, Ren Q, Gao J, Wang Y, Chang N, Qiu Y, Song G (2015) Sodium fluoride induces apoptosis in H9c2 cardiomyocytes by altering mitochondrial membrane potential and intracellular ROS level. Biol Trace Elem Res 166(2):210–215
Shim MY, Parr C, Pesti GM (2011) The effects of dietary fluoride on growth and bone mineralization in broiler chicks. Poult Sci 90(9):1967–1974
Zhou BH, Zhao J, Liu J, Zhang JL, Li J, Wang HW (2015) Fluoride-induced oxidative stress is involved in the morphological damage and dysfunction of liver in female mice. Chemosphere 139:504–511
Wang HW, Zhou BH, Cao JW, Zhao J, Zhao WP, Tan PP (2017a) Pro-inflammatory cytokines are involved in fluoride-induced cytotoxic potential in HeLa cells. Biol Trace Elem Res 175(1):98–102
Wu Z, Tang X (2015) Visualizing fluoride ion in mitochondria and lysosome of living cells and in living mice with positively charged ratiometric probes. Anal Chem 87(17):8613–8617
Wang HW, Zhao WP, Tan PP, Liu J, Zhao J, Zhou BH (2017b) The MMP-9/TIMP-1 system is involved in fluoride-induced reproductive dysfunctions in female mice. Biol Trace Elem Res. doi:10.1007/s12011-016-0929-3
El-Hattab AW, Scaglia F (2016) Mitochondrial cardiomyopathies. Front Cardiovasc Med 3:25
Cicek E, Aydin G, Akdogan M, Okutan H (2005) Effects of chronic ingestion of sodium fluoride on myocardium in a second generation of rats. Hum Exp Toxicol 24(2):79–87
Liang S, Zhao MH, Ock SA, Kim NH, Cui XS (2016) Fluoride impairs oocyte maturation and subsequent embryonic development in mice. Environ Toxicol 31(11):1486–1495
Guth L, Samaha FJ (1970) Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol 28(2):365–367
Gollnick PD, Parsons D, Oakley CR (1983) Differentiation of fiber types in skeletal muscle from the sequential inactivation of myofibrillar actomyosin ATPase during acid preincubation. Histochemistry 77(4):543–555
Dimitriu-Leen AC, Scholte AJ, Katsanos S, Hoogslag GE, van Rosendael AR, van Zwet EW, Bax JJ, Delgado V (2017) Influence of myocardial ischemia extent on left ventricular global longitudinal strain in patients after ST-segment elevation myocardial infarction. Am J Cardiol 119(1):1–6
Panneerselvam L, Govindarajan V, Ameeramja J, Nair HR, Perumal E (2015) Single oral acute fluoride exposure causes changes in cardiac expression of oxidant and antioxidant enzymes, apoptotic and necrotic markers in male rats. Biochimie 119:27–35
Panneerselvam L, Raghunath A, Perumal E (2017) Acute fluoride poisoning alters myocardial cytoskeletal and AMPK signaling proteins in rats. Int J Cardiol 229:96–101
Qin SL, Deng J, Lou DD, Yu WF, Pei J, Guan ZZ (2015) The decreased expression of mitofusin-1 and increased fission-1 together with alterations in mitochondrial morphology in the kidney of rats with chronic fluorosis may involve elevated oxidative stress. J Trace Elem Med Biol 29:263–268
Sarkar C, Pal S, Das N, Dinda B (2014) Ameliorative effects of oleanolic acid on fluoride induced metabolic and oxidative dysfunctions in rat brain: experimental and biochemical studies. Food Chem Toxicol 66:224–236
Nabavi SM, Nabavi SF, Eslami S, Moghaddam AH (2012) In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem 132(2):931–935
Starkov AA (2008) The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci 1147:37–52
Sun ZL, Zhang W, Xue XC, Zhang YL, Niu RY, Li XY, Li BJ, Wang XW, Wang JD (2016) Fluoride decreased the sperm ATP of mice through inhabiting mitochondrial respiration. Chemosphere 144:1012–1017
Wagner K, Rehling P, Sanjuán Szklarz LK, Taylor RD, Pfanner N, van der Laan M (2009) Mitochondrial F1Fo-ATP synthase: the small subunits e and g associate with monomeric complexes to trigger dimerization. J Mol Biol 392(4):855–861
Rühle T, Leister D (2015) Assembly of F1F0-ATP synthases. Biochim Biophys Acta 1847(9):849–860
Boada M, Antúnez C, Ramírez-Lorca R, AL DS, González-Pérez A, Gayán J, López-Arrieta J, Ikram MA, Hernández I, Marín J, Galán JJ, Bis JC, Mauleón A, Rosende-Roca M, Moreno-Rey C, Gudnasson V, Morón FJ, Velasco J, Carrasco JM, Alegret M, Espinosa A, Vinyes G, Lafuente A, Vargas L, Fitzpatrick AL, Alzheimer’s Disease Neuroimaging Initiative, Launer LJ, Sáez ME, Vázquez E, Becker JT, López OL, Serrano-Ríos M, Tárraga L, van Duijn CM, Real LM, Seshadri S, Ruiz A (2014) ATP5H/KCTD2 locus is associated with Alzheimer’s disease risk. Mol Psychiatry 19(6):682–687
Zhu H, Chen L, Zhou W, Huang Z, Hu J, Dai S, Wang X, Huang X, He C (2013) Over-expression of the ATP5J gene correlates with cell migration and 5-fluorouracil sensitivity in colorectal cancer. PLoS One 8(10):e76846
Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, Cleland JG, Colucci WS, Butler J, Voors AA, Anker SD, Pitt B, Pieske B, Filippatos G, Greene SJ, Gheorghiade M (2016) Expert consensus document: mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. doi:10.1038/nrcardio.2016.203
Lesnefsky EJ, Chen Q, Tandler B, Hoppel CL (2017) Mitochondrial dysfunction and myocardial ischemia-reperfusion: implications for novel therapies. Annu Rev Pharmacol Toxicol 57:535–565
Acknowledgements
This work is supported by the China National Nature Science Foundation (grant no. 31201963).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Hw., Zhao, Wp., Liu, J. et al. ATP5J and ATP5H Proactive Expression Correlates with Cardiomyocyte Mitochondrial Dysfunction Induced by Fluoride. Biol Trace Elem Res 180, 63–69 (2017). https://doi.org/10.1007/s12011-017-0983-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-0983-5