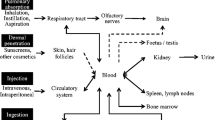
Abstract
A number of dermal toxicological studies using TiO2 nanoparticles exist which are based on the study of various animal models like mice, rabbits etc. However, a well-defined study is lacking on the dermal toxic effects of TiO2 nanoparticles on rats, which are the appropriate model for systemic absorption study of nanoparticles. Furthermore, toxicity of TiO2 nanoparticles varies widely depending upon the size, concentration, crystallinity, synthesis method etc. This study was conducted to synthesize TiO2 nanoparticles of different sizes (∼15 to ∼30 nm) by aqueous method, thereby evaluating the concentration-dependent toxicological effects of the ∼20-nm sized nanoparticles on Wistar rats. Characterization of the particles was done by transmission electron microscope, dynamic light scattering instrument, X-ray diffractrometer, and ultraviolet spectrophotometer. The toxicity study was conducted for 14 days (acute), and it is observed that TiO2 nanoparticles (∼20 nm) at a concentration of 42 mg/kg, when applied topically showed toxicity on rat skin at the biochemical level. However, the histopathological studies did not show any observable effects at tissue level. Our data suggest that well-crystallized spherical-shaped ∼20 nm anatase TiO2 nanoparticles synthesized in aqueous medium can induce concentration-dependent biochemical alteration in rat skin during short-term exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Titanium dioxide (TiO2) is one of the nanomaterials that have attracted a great attention due to its unique properties. Titanium dioxide is classified as being physiologically inert in both humans and animals [1], hence it has been widely used in many ways; as an additive, including as a white pigment in paint, as food colorant, in sunscreens and in cosmetic creams. It is also a well-known photocatalyst [2, 3], due to which it is applied in environment application and in waste water as a disinfectant [4]. Recently, TiO2 was used as a photosensitizer for photodynamic therapy of endobronchial and esophageal cancers [5]. Despite these bright outlooks, there is an increasing concern that nanosized TiO2 particles may adversely affect the health of humans and his environment. It is expected that the minute size of nanomaterials which gives them unusual properties of strength and reactivity, the same property would give them the unpredicted properties of toxicity also [6]. The toxicological concern is due to the distinct properties of nanoparticles, such as small size, high number per given mass and large specific surface area. It has been reported that the biological responses to nanoparticles may exceed those elicited by micron-sized particles [7, 8]. The physical properties of these nanomaterials would allow them to catalyze a number of bio-molecular interactions, which potentially could produce adverse toxicological effects.
Many in vivo studies on generation of inflammatory response due to accumulation of nanosized TiO2 particles in the liver, kidney, spleen, lung, heart, and brain has been reported [9–16]. For instance, intraperitonial administration of TiO2 nanoparticles of smaller size (∼5 nm) can easily enter mouse liver cells and bond to liver DNA, at higher doses [17]. Intragastric administration of TiO2 nanoparticles for 30 consecutive days could lead to liver function damage in mice [18] and induces liver hepatitis when administered at a dose of 10 and 50 mg/kg body weight (BW) [19]. Studies had shown that by intra-tracheal instillation of TiO2 nanoparticles in rats, pulmonary inflammation and cytotoxicity is observed [20–22]. TiO2 nanoparticles with oral gavages increased the activity of lactate dehydrogenase causing hepatocyte necrosis in mice [23]. Intraperitoneal injection of various doses of TiO2 nanoparticles can lead to its accumulation in the mouse spleen [9]. Higher doses of anatase TiO2 by intragastric administration exert toxicity through oxidative stress [24].
A few studies have investigated dermal toxicity and systemic exposure of nanoscale TiO2 particles by topical application on rabbits and mice [25–30]. However, there are no reported toxicity studies on TiO2 nanoparticles when rat skin is used as a portal entry for whole body exposure. The ability of TiO2 nanomaterials to traverse the skin is a primary determinant of its dermato-toxic potential. That is, TiO2 nanoparticles must penetrate the stratum corneum in order to exert toxicity in lower cell layers. The quantitative prediction of toxicity is carried out in the current research and is studied by biochemical alteration and histological changes in the rat skin. This research aims to synthesize TiO2 nanoparticles of different sizes (∼15 to ∼30 nm) by aqueous method, characterize its property and assessing its concentration based toxicological properties by topical application on Wistar rats.
Materials and Methods
Chemicals
Titanium tetrachloride (TiCl4; 1 M in Toluene) was purchased from Spectrochem (India). Tri-sodium citrate 2-hydrate GR (>99% purity) was purchased from Merck (India). Acetic acid glacial extra-pure was purchased from SD-Fine Chem Ltd. (India). Reduced glutathione, 1-chloro-2,4-dinitrobenzene (CDNB), thiobarbituric acid, and bovine serum albumin (BSA) were obtained from Sigma-Aldrich, USA. Trichloroacetic acid was purchased from Central Drug House (CDH), India. Biological Assay kit for the estimation of lactate dehydrogenase (LDH) was purchased from Reckon (India). All other chemicals were of analytical grade. Milli-Q water was used for the whole experiment.
Synthesis of TiO2 Nanoparticles
Titanium dioxide nanoparticles of different sizes were prepared by aqueous method in the following manner. Tri-sodium citrate at varying concentration (0.01, 0.05, 0.08, and 0.1 M, respectively) was dissolved in 100 ml of Milli-Q water. The solution was vortexed thoroughly and then sonicated for 5 min. The solution was then kept under constant stirring under magnetic stirrer. To this solution, 500 μL of TiCl4 was added dropwise under vigorous stirring. The reaction was performed at thermostatically controlled temperature and stirring was continued for 24 h. Since the particle size depends on the concentration of capping agent and the metal ion, we prepared TiO2 nanoparticles of a broad range of size (∼15, ∼20, ∼25, and ∼30 nm) following the above protocol. The prepared particles were studied further for characterization.
Characterization of TiO2 Nanoparticles
Transmission Electron Microscopy
The size of the nanoparticles was measured by Transmission Electron Microscope (TEM; Philips Morgagni). Measurements were made using computerized image analyzer and the average size of nanoparticles was noted.
Dynamic Light Scattering
Dynamic light scattering (photon correlation spectroscopy) is a technique used to determine the size distribution profile of small particles in suspension. The technique was performed on “Malvern ZS” instrument.
X-ray Diffractrometry
For determining the crystallographic nature of the sample, X-ray diffraction (XRD) measurements were performed on PANalytical instrument, using powdered samples of the TiO2 nanoparticles.
Ultraviolet Spectroscopy
All the spectrophotometer studies were done using a Spectroscan 80 DV spectrophotometer.
Animal Model
Both male and female rats of Wistar strain, approximately 8 weeks old (weights in the range of 150–200 g) were used in this study. The rats were obtained from the Central Animal House facility of Jamia Hamdard, New Delhi and were housed in a well-ventilated room at ±22°C, under a 12-h light/dark cycle. Research on the experimental animals was conducted in accordance with the internationally accepted principles for laboratory animal’s use and care as found in the guidelines laid down by the Indian Ethical Committee, Committee for the Purpose of Control and Supervision of Experiments on Animals. The rats, in groups of five, were kept hygienically in separate polypropylene cages. They were acclimatized for 1 week before the start of the study and were allowed free access to standard laboratory feed (Hindustan Lever Ltd, India) and water ad libitum. The dorsal portions of the rat’s skin were shaven with an electric clipper (Oster A2) followed by the application of hair removing cream (Anne French, Geoffrey Manners, India) 2 days before treatment. Excess cream was washed off with cotton sorbs dipped in lukewarm water. Only rats that showed no signs of hair regrowth were included in the experiment.
Treatment Regimen
Effects of TiO2 nanoparticles (∼20 nm) on rat’s skin was studied by randomly allocating 25 Wistar rats (both male and female) into five groups, each having five rats. All animals of the experimental study received topical application of nanoparticles for a period of 14 consecutive days (acute toxicity study).
At the end of the experiment, animals of all the groups were sacrificed under mild anesthesia. Blood was taken for various serological parameters. Skin from dorsal area was removed and cleaned off extraneous tissue. A piece of skin was preserved in 10% neutral buffered formalin for histopathological investigation. Skin homogenates were prepared in chilled phosphate buffer (0.1 M, pH 7.4) using polytron homogenizer and then filtered through muslin cloth. The homogenized tissue was centrifuged at 10,500 rpm for 30 min at 4°C to obtain post-mitochondrial supernatant (PMS). PMS was used in various biochemical measurements as detailed in Biochemical assays.
Biochemical Assays
Glutathione-S-transferase (GST) activity was measured by the method of Habig et al [31], and expressed as nanomoles of CDNB conjugates formed per minute per milligram of protein. Catalase (CAT) activity was assayed by the method of Claiborne et al [32] and expressed as nanomoles of H2O2 consumed per minute per milligrams of protein. Superoxide dismutase (SOD) was examined by a modified method of Misra and Fridovich [33], and the activity was expressed in micormolars of epinephrine oxidized per minute per milligram of protein. Estimation of lipid peroxidation was done according to the method of Wright et al. [34]. The results were expressed as nanomoles of Malon dialdehyde (MDA) formed per hour per gram of tissue at 37°C using a molar extinction coefficient of 1.56 × 105M−1 cm−1. LDH activity was estimated in serum by the standard protocol method mentioned in the biological assay kit. Protein estimation in all samples was done using the method of Lowry et al. [35] using BSA as standard.
Histopathological Investigation
The skin samples were processed with haematoxylin and eosin stain for gross tissue histo-architecture evaluation. The formalin fixed skin samples were dehydrated with graded ethanol (Merck) and embedded in paraffin (Hi-Media Labs, India) after rinsing with distilled water. The samples were cut by microtome at 5-mm thick and mounted on glass slides. The slides were studied using “Olympus DP71 Biological” microscope.
Statistical Analysis
The level of significance between different groups is based on analysis of variance test followed by Dunnett’s t test.
Results and Discussions
Characterization of Nano-TiO2 Samples
Transmission Electron Microscope
TEM images of TiO2 nanoparticles synthesized at varying concentrations (0.01, 0.05, 0.08, and 0.1 M, respectively) are shown in Fig. 1a–d. Well-defined nanoscale size distribution of highly monodisperse TiO2 nanoparticles with spherical shape was obtained.
Dynamic Light Scattering
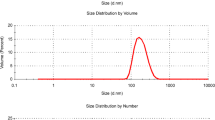
The size distribution of TiO2 particles (0.05 M) suspended in water was taken by dynamic light scattering (DLS) and shown in Fig. 2. TiO2 nanoparticles have a negative surface charge, thus stabilizing the suspensions via repulsive forces. The figure reveals that the size of synthesized TiO2 is less than 50 nm.
X-Ray Diffraction
XRD addresses the structural information of a large portion of nanosized sample. Sharp peaks at 101 of the nanosized TiO2 particles (0.05 M) were observed, which indicate the crystalline nature of the sample. All prominent peaks show the tetragonal crystal structure of anatase-TiO2 (Fig. 3). Calculation by the Scherrer’s equation showed that the average crystal size of synthesized TiO2 was ∼20 nm.
UV Spectroscopy
TiO2 nanoparticles exhibit broad absorption bands in the ultraviolet visible range. These are due to the excitation of plasma resonance or interband transition and are a characteristic property of the metallic nature of the nanosized TiO2 particle. Nanosized spherical-shaped TiO2 particles exhibit a surface plasmon peak at around 230 nm, characterizing the synthesized samples to be anatase. In Fig. 4a–d represent the respective peaks for ∼15, ∼20, ∼25, and ∼30 nm, respectively.
Toxicological Study
Biochemical Estimation
The effects of topical treatment of ∼20 nm TiO2 nanoparticles on the skin of rats of different groups at different concentration are studied and shown in the figures below. Group I is the control group while the remaining groups II, III, IV, and V are the nanoparticles-treated group with a concentration level of 14, 28, 42, and 56 mg/kg BW, respectively.
The result depicts the induced oxidative stress and alteration in the rat skin on exposure to concentration of 42 mg/kg BW of TiO2 nanoparticles through topical application. There is a significant depletion in the activity of the antioxidant enzyme, catalase (Fig. 5), and SOD (Fig. 6) in group IV (p < 0.05) when compared with acetone-treated control (group I) animals. No significant change was observed in the other groups.
There is also depletion in the level of an important phase II enzyme, glutathione-S-transferase (p < 0.05) as compared with the control-treated group (Fig. 7). Groups II, III, and V do not show any significant alterations and the results are close to normal.
Group IV animals showed slight significant enhancement in levels of LDH (Fig. 8) and MDA (Fig. 9) formation (p < 0.05), when compared with group I (control group).No significant difference was observed in other treated groups (groups II, III, and V).
Histopathological Findings
When treated with ∼20 nm TiO2 nanoparticles, the rat skin did not show any marked alterations. The histo-architecture of rat skin of group I rats showed normal histology of well differentiated dermal layer and thin wavy epidermis with basal levels of neutrophils. Among the treatment groups, group IV showed very slight changes in cutaneous architectures as compared with control group which are not significantly visible. All the other treatment groups (groups II, III, and V) do not show any significant differences when compared with the control-treated group (group I)
Discussion
There are many advantages in the dedicated synthesis of nanoparticles for toxicity studies. A good control of particle-size range within each batch is also important if reliable links between toxicity and size are to be made. Our synthesis focused primarily on the methodology of preparing TiO2 nanoparticles by aqueous method under controlled condition. Since particle size depends on the concentration, we synthesized nanosized TiO2 of different sizes ranging from approximately ∼15 to ∼30 nm using the aqueous method. The synthesized particles were further characterized by TEM, XRD, DLS, and UV spectrophotometer. We then followed this synthesis work with in vivo toxicological studies. Because of their diminutive size, nanoparticles carry several inherent properties. Firstly, ultrafine particles have larger surface areas per unit mass. Secondly, particle toxicity is determined by surface reactivity. Thus, given their structure, nanoparticles exhibit greater harm compared with larger particles because of their proportionally increased surface area. The large surface area also provides a distinctive interface for catalytic reactions of surface-located mediators with biological targets such as proteins.
Since the skin is an important interface between man and his environment, it is a significant portal of entry of hazardous agents and a vulnerable target organ system. The skin is endowed with a versatile group of adaptive and defensive mechanism. The penetration of materials into the stratum corneum is limited by molecular size. The intercellular space between the cells composing the stratum corneum measures approximately 100 nm3 and may be widened with topical application of various products [36]. This raises the question of whether the particles used in TiO2-based sunscreens have the potential to penetrate the stratum corneum. Studies reporting the dermal toxicity concluded that TiO2 do not reach the viable cells [6]. These findings are based on the study on various animal models like mice, rabbits etc.; and that too for a period of 3 days maximum. However, a well-defined documentary report is lacking in the dermal toxicity of TiO2 nanoparticles on rats, which are the appropriate model for system absorption study of nanoparticles [37].
Size, crystal structure, and surface chemistry (such as coating) are among the factors that influence the effects of nano-TiO2 particles. Other physicochemical properties, such as shape [38], manufacturing process, doping, and purity (or impurities) could also play a role in the toxicity of nano-TiO2, but such information is usually not reported in toxicological studies. Physicochemical properties, experimental conditions, and the immediate environment can all influence the ecological and health effects of nano-TiO2 particles. Another important parameter of nanoparticles’ toxicity study is the purity of water used for synthesis. It affects the degree of aggregation, which in turn may affect exposure-dose and toxicity. The degree of aggregation generally increases with the presence of salt, minerals, and organic matter in water [39, 40]. Although the influences of media and vehicle and dispersion methods on particle aggregation and distribution have been reported, information on these influences on health effects is very scarce [41].
In our present study, we formulated the TiO2 nanoparticles via aqueous route and further studied to reveal its characteristic properties for in vivo toxicological studies. We investigated the effect of TiO2 on a number of biochemical parameters mainly SOD, CAT, GST, LPO, and LDH. Enzymes SOD and CAT remove reactive oxygen species (ROS) generated by the free radicals. SOD converts O −2 into H2O2 and O2 while CAT reduces H2O2 into H2O and O2. Thus SOD and CAT prevent further ROS generation in cells. GST family of phase II detoxification enzymes catalyzes the conjugation of glutathione to a wide variety of endogenous and exogenous electrophilic compounds, such as therapeutic drugs, environmental toxins, and products of oxidative stress. Lipid peroxidation is the oxidative degradation of lipids in which free radicals steal electrons from the lipids in cell membranes, resulting in cell damage. LDH is an enzyme present in body tissues that get released during tissue damage. With the experimental setting of the current study, we aim to address the current concern that once exposed to TiO2 nanoparticles for consecutive days; it may become systemically available and cause toxic effects. This can be seen from the results of the biochemical assay carried out on rat skin exposed to nanosized TiO2 particles. There was a significant depletion in the activities of catalase, SOD, and GST with a concomitant increase in the LPO and LDH activity in group IV, at a concentration of 42 mg/kg BW. The toxicity level at a concentration of 42 mg/kg BW vis-à-vis control group is observed to be significant as compared with other concentrations. The decrease in level of toxicity at 56 mg/kg BW is attributed to the coagulation of the nanosized TiO2 particles as the concentration increased.
The most discussed aspect at present is the induction of reactive oxygen species due to the chemical properties of TiO2. Overall results of this study indicate that rats exposed to TiO2 nanoparticles by topical route showed significant health effects at the dose level of 42 mg/kg BW, even though no marked histological alteration was seen. It is evident from our study that at lower concentration of TiO2 nanoparticles, there was no noticeable level of toxicity. This study will provide valuable information regarding the safety evaluation of TiO2 nanoparticles as the synthesis protocol and the species studied (rat model) to test the toxicological properties of TiO2 nanoparticles is different from the previously reported studies [37].
The science of toxicology has always provided the foundation for understanding the interactions between chemistry and biology. Consequently, the unique physical-chemical characteristics of engineered nanomaterials that lead to their distinctive properties will likely contribute to the hazards associated with these materials. Therefore, the approach to addressing the safety of these materials will best be conducted via multidisciplinary teams.
Conclusions
The inferences drawn from this study highlight the toxicity parameters in experimental rat model after topical application of nanosized TiO2 nanoparticles of ∼20 nm size at a defined concentration of 42 mg/kg, for 14 consecutive days. The study also confirms that nanoparticles’ toxicity is due to the oxidant generation and the resultant oxidant stress to cells. Oxidative stress has been clearly shown to occur during TiO2 dermal application on rats. Superoxide anions are generated which will lead to the formation of highly reactive species, hydroxyl radicals that attack proteins and lipid membranes causing cell damage or genetic alterations. LPO and SOD are therefore, the markers for assessing the extent of damage in cells. With the application of TiO2 nanoparticles, the level of the cellular antioxidant enzyme and glutathione is depleted. Additionally, the levels of MDA increased which indicated increased peroxidation of lipids. The histopathology study showed only slight changes in cutaneous architectures. The data suggest that well-crystallized spherical-shaped anatase TiO2 synthesized in aqueous medium induced concentration-dependent biochemical alteration in rat model with no marked alteration on the histology of skin tissue.
References
Bernard BK, Osheroff MR, Hofmann A et al (1990) Toxicology and carcinogenesis studies of dietary titanium dioxide-coated mica in male and female fischer 344 rats. J Toxicol Environ Health 29(4):417–429
Hurum DC, Agrios AG, Gray KA (2003) Explaining the enhanced photocatalyt activity of degussa P25 mixed-phase TiO2 using EPR. J Phys Chem 107:4545–4549
Hurum DC, Gray KA (2005) Recombination pathways in the degussa P2 formulation of TiO2: surface versus lattice mechanisms. J Phys Chem 109:977–980
Cho M, Chung H, Choi W et al (2004) Linear correlation between inactivation of e coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res 38(4):1069–1077
Ackroyd R, Kelty C, Brown N et al (2001) The history of photodetection and photodynamic therapy. Photochem Photobiol 74:656–669
Davis JM, Wang A, Shatkin JA et al. (2009) External review draft nanomaterial case studies: nanoscale titanium dioxide in water treatment and in topical sunscreen. US Environmental Protection Agency, Research Triangle Park. pp 5–31
Borm PJ, Robbins D, Haubold S et al (2006) The potential risks of nanomaterials: a review carried out for ECETOC. Particle Fibre Toxicol 3:11
Ne A, Xia T, Madler L et al (2006) Toxic potential of materials at the nanolevel. Science 311:622–627
Wang JX, Zhou GQ, Chen CY et al (2007) Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168:176–185
Brown JS, Zeman KL, Bennett WD (2002) Ultrafine particle deposition and clearance in the healthy and obstructed lung. Am J Respir Crit Care Med 166:1240–1247
Kreyling WG, Semmler M, Erbe F et al (2002) Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health 65:1513–1530
Oberdoerster G, Sharp Z, Atudorei V et al (2004) Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 16:437–445
Oberdorster G, Oberdorster E, Oberdorster J (2005) Nanotoxicology:an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839
Muller J, Huaux F, Moreau N et al (2005) Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol 207:221–231
Chen HW, Su SF, Chien CT et al (2006) Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. FASEB J 20:1732–1741
Liu HT, Ma LL, Zhao JF et al (2009) Biochemical toxicity of nano-anatase TiO2 particles in mice. Biol Trace Elem Res 129(1):170–180
Ma LL, Zhao JF, Wang J et al (2009) The acute liver injury in mice caused by nano-anatase TiO2. Nanoscale Res Lett 4:1275–2128
Duan YM, Liu J, Ma LL et al (2010) Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice. Biomaterials 31:894–899
Cui Y, Liu H, Zhou M et al (2010) Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J Biomed Mater Res A 96(1):221–229. doi:10.1002/jbm.a.32976
Afaq F, Abidi P, Matin R et al (1998) Cytotoxicity, pro-oxidant effects and antioxidant depletion in rat lung alveolar macrophages exposed to ultrafine titanium dioxide. J Appl Toxicol 18:307–312
Warheit DB, Webb TR, Reeda KL et al (2007) Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: differential responses related to surface properties. Toxicology 230:90–104
Warheit DB, Hoke RA, Finlay C et al (2007) Development of a base of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol Lett 171:99–110
Liu R, Yin LH, Pu YP et al (2009) Pulmonary toxicity induced by three forms of titanium dioxide nanoparticles via intra-tracheal instillation in rats. Prog Nat Sci 19(5):573–579
Wang J, Li N, Zheng L et al (2011) P38-Nrf-2 signaling pathway of oxidative stress in mice caused by nanoparticulate TiO2. Biol Trace Elem Res, doi: 10.1007/s12011-010-8663-8.
Gamer AO, Leibold E, Van Ravenzwaay B (2006) The in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skin. Toxicol In Vitro 20:301–307
Kiss B, Biro T, Czifra G et al (2008) Investigation ofmicronized titanium dioxide penetration in human skin xeno-grafts and its effect on cellular functions of human skin-derived cells. Exp Dermatol. doi:10.1111/j.1600-0625.2007.00683
NANODERM (2007) Quality of skin as a barrier to ultra-fine particles. Final Report. (Project Number: QLK4-CT-2002-02678). Available at: http://www.uni-leipzig.de/*nanoderm/
Menzel F, Reinert T, Vogt J, Butz T (2004) Investigations of percutaneous uptake of ultrafine TiO2 particles at the high energy ion nanoprobe LIPSION. Nucl Instrum Methods Phys Res B 219–220:82–86
Kertesz ZS, Szikszai Z, Gontier E et al (2005) Nuclear microprobe study of TiO2-penetration in the epidermis of human skin xenografts. Nucl Instrum Methods Phys Res B 231:280–285
Bennat C, Muller-Goymann CC (2000) Skin penetration and stabilization of formulations containing microfine titanium dioxide as physical UV filter. Int J Cosmet Sci 22:271–283
Habig WH, Pabst MJ, Jokoby WB (1974) Glutathione-S-transferase—the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) Handbook of methods for oxygen free radical research. CRC Press, Boca Raton, pp 283–284
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Wright JR, Colby HD, Miles PR (1981) Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys 206:296–304
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Newman MD, Stotland M, Ellis J (2009) The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J Am Acad Dermatol 61:685–692
Shayne CG (2006) Animal models in toxicology, 2nd edn. Taylor & Francis, New York, p 177
Warheit DB, Hoke RA, Finlay C et al (2006) CM Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: toxicity is not dependent upon particle size and surface area. Toxicol Sci 91:227–236
Domingos RF, Tufenkji N, Wilkinson KJ et al (2009) Aggregation of titanium dioxide nanoparticles: role of a fulvic acid. Environ Sci Technol 43:1282–1286
French RA, Jacobson AR, Kim B et al (2009) Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ Sci Technol 43:1354–1359
Jemec A, Drobne D, Remskar M et al (2008) T Effects of ingested nanosized titanium dioxide on terrestrial isopods porcellio scaber. Environ Toxicol Chem 27:1904–1914
Acknowledgments
Authors are thankful to University Grants Commission, Government of India, New Delhi, for providing meritorious research fellowship and funding to carry out the experiment. The authors are also thankful to Prof. GN Qazi Vice-Chancellor, Hamdard University for providing infrastructure for this research.
Conflicts of Interest Statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Unnithan, J., Rehman, M.U., Ahmad, F.J. et al. Aqueous Synthesis and Concentration-Dependent Dermal Toxicity of TiO2 Nanoparticles in Wistar Rats. Biol Trace Elem Res 143, 1682–1694 (2011). https://doi.org/10.1007/s12011-011-9010-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9010-4