Abstract
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide test and alkaline phosphatase activity assay were employed to assess the effects of mixed trace elements including Zn2+, Ca2+, and Mn2+ plus total flavonoids or icariin from Epimedium koreanum on the proliferation and differentiation of primary osteoblasts in vitro. The results indicated that icariin (0.1, 1, and 10 μmol/L) and total flavonoids (0.06, 0.6, and 6 μg/mL) inhibited the proliferation and promoted the differentiation of primary osteoblasts. Mixed trace elements including Zn2+, Ca2+, and Mn2+ (0.1, 1, and 10 μmol/L) inhibited the proliferation and promoted the differentiation at 0.1 and 1 μmol/L, but inhibited the differentiation at 10 μmol/L. The effects of mixed trace elements including Zn2+, Ca2+, and Mn2+ plus total flavonoids or icariin from E. koreanum on the proliferation and differentiation of primary osteoblasts in vitro are complicated, and both synergistic and antagonistic effects are generated. The results suggest that there may be a potential cooperative action between flavonoids and trace metal elements on the proliferation and differentiation of primary osteoblasts by forming metal complexes. The combination model between flavonoids and trace metal elements is a pivotal factor for switching the biological effects from toxicity to activity, from damage to protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the general population is aging, osteoporosis is becoming more prevalent, not just in China but worldwide. The rationale for prevention and treatment of osteoporosis is directed along two basic approaches, namely agents preventing bone resorption (estrogen, calcitonin, bisphosphonates, calcium, vitamin D, and raloxifene) and those stimulating bone formation (fluoride and anabolic steroids). Estrogen replacement therapy (ERT) was a popular regime in prevention and treatment of postmenopausal osteoporosis. However, recent evidence suggests that the therapy may generate serious side effects including increased risk of breast and ovarian cancer [1]. In addition, the most frequently used antiosteoporosis drugs are developed in affluent countries, and the costs are too high to benefit a large population in developing or even developed countries for prevention and treatment of osteoporosis. Thus, alternative treatment or prevention regimes for osteoporosis are urgently needed [2].
Mineral elements have been found to play an important role in bone metabolism. Zinc has been demonstrated to be a physiological activator in the regulation of bone formation due to stimulating bone protein synthesis in vivo and in vitro [3]. Recently, a clinical relationship between osteoporosis and Zn deficiency was established in elderly subjects [4]. Zinc supplementation was shown to inhibit postmenopausal bone loss [5]. It has been reported that calcium acted as activating factor of calmodulin-dependent kinase II, and intervened in osteogenic signaling pathways [6]. Calcium intake is positively related to calcium balance, and calcium supplementation benefits appendicular cortical bone mass [7]. Manganese deficiency results in abnormal skeletal development in a number of animal species. Manganese is a preferred cofactor of enzymes called glycosyltransferases, which are required for the synthesis of proteoglycans that are needed for the formation of healthy cartilage and bone [8].
Several traditional Chinese herbs have been reported to have therapeutic effects on osteoporosis and bone fracture in animal studies [9]. Accumulating evidence showed that the crude extract and flavonoids from Herba epimedii have been found effective in preventing osteoporosis in animal studies [10], yet no appreciable effect was observed when osteoblasts (OBs) were exposed to flavonoids in vitro [11]. These results suggested that traditional Chinese herbs may work by the synergism or additive effect of diverse chemical constituents. There are studies indicating that trace metal elements in traditional Chinese medicines may have potential effect on the biological process of human body. Chen et al. [12] reported that the virtue of Chinese medicine had something to do with the contents of Mn and Zn, and with the proportion of contents of Mn, Zn, and Fe. It has been reported that V, Ti, Mo, Co, Cr, Cu, Fe, Mn, Ni, Sr, and Zn had a significant influence on the properties and tastes of medicinal herbs, and suggested that the possibility of the anti-HIV properties of medicinal herbs was based on their trace element content [13]. It was reported that Zn2+, Ca2+, or Mn2+ are significantly high in H. epimedii. So, it is very important to explore whether the combination icariin or total flavonoids (TF) from H. epimedii with mixed trace elements (MTE) has a synergistic or antagonistic effect on the proliferation and differentiation of primary OBs. In this paper, this study was undertaken to determine whether the combination of MTE with TF or icariin from Epimedium koreanum has a synergistic or antagonistic effect on the proliferation and differentiation of primary OBs in vitro.
Materials and Methods
Materials
E. koreanum herb was collected in June–July 2003 in a valley, located in Xinbin, Liaoning Province, and authenticated by Q.-S. Sun, Professor of Pharmacognosy, Shenyang Pharmaceutical University, China. A voucher specimen (No. 19980816-1) has been deposited in the Herbarium of Shenzhen Research Center of Traditional Chinese Medicine and Natural Products.
NIH mice (SCXK2008-1-003) were obtained from the Animal Center of Hebei Medical University. Dulbecco's Modified Eagle's Medium (DMEM) and trypsin were purchased from Gibco (Grand Island, NY, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), penicillin, streptomycin, and collagen II were from Sigma (St. Louis, MO, USA). Neonatal bovine serum (NBS) was from Hangzhou Sijiqing Organism Engineering Institute. An alkaline phosphatase (ALP) activity kit was obtained from the Nanjing Jiancheng Biological Engineering Institute (Nanjing, China), and microprotein assay kit was from Beyotime Biotechnology (Haimen, China). Zinc chloride (ZnCl2), calcium chloride (CaCl2), manganese chloride (MnCl2), and all the other chemical reagents were of analytical grade.
Preparation of Test Samples
Icariin and TF were isolated as in a previous study [14]. Icariin and TF were dissolved in dimethylsulfoxide (DMSO) at concentrations of 10 mmol/L and 6 mg/mL, respectively, and diluted in culture medium to the working-solution. NaF was dissolved in phosphate buffer solution (PBS) as positive control. ZnCl2, CaCl2, and MnCl2 were dissolved as NaF. To avoid DMSO toxicity, the concentration of DMSO was less than 0.1% (v/v) in all experiments.
Isolation and Culture of Primary OBs
OBs were isolated enzymatically from newborn mouse skull as described previously [15]. Briefly, the skulls were dissected, the endosteum and periosteum were stripped off, and the bone was cut into approximately 1–2-mm2 pieces and sequentially digested with trypsin (2.5 mg/mL) for 30 min and collagenase II (1.0 mg/mL) twice for 1 h each time. The cells were collected and cultured in DMEM with 10% NBS, benzylpenicillin (100 U/mL), and streptomycin (100 μg/mL) for 24 h in a humidified atmosphere of 5% CO2 in air at 37°C, following which the used medium was changed. The grown cells were released from the surface of the culture dish with 0.25% trypsin at 80% confluence. Cells were divided in tissue culture flasks and referred to as first passage cells. After growing near confluence, the cells were released as described above, counted, plated in 75-cm2 flasks at a density of 2 × 106 cells/flask, and evenly distributed. These cells are referred to as second passage cells. For effects of steroids on growth, medium was charcoal stripped and without phenol red. Fresh medium was supplied to cells at 3-day intervals.
Assay for OB Proliferation
The protocol described by Mosmann was followed with some modifications [16]. Briefly, the second passage OBs were plated in 96-well culture plates (1 × 104 cells per well), and cultures were controlled every day to establish the stage of confluence. At a confluence of 50%, the cells were washed twice with incubation medium (DMEM with 1% BSA), MgSO4 (0.02%), penicillin (100 U/mL), and streptomycin (100 μg/mL) to remove NBS. Tested samples were added to achieve final concentrations. Control wells were prepared by addition of DMEM. Wells containing DMEM without cells were used as blanks. NaF (1 × 10−6 mol/L) were used as positive control. The plates were incubated at 37°C in a 5% CO2 incubator for 44 h. Cells were treated with MTT (20 μL, 5 mg/mL) for 4 h prior to the end of the experiment. At the end of this experiment, the supernatant was removed, and DMSO was added to dissolve formazan, and optical density (OD) at 570 nm was measured on a microplate spectrophotometer (MD VersaMax, USA). The viability rate (%) was calculated according to the formula: (ODsample − ODblank) / (ODcontrol – ODblank) × 100.
Assay for ALP Activity
The protocol described by Gray was followed [17]. Briefly, the second passage OBs were plated in 48-well culture plates (1 × 104 cells per well), and cultures were controlled every day to establish the stage of confluence. At a confluence of 100%, the cells were washed twice with incubation medium (DMEM with 1% BSA), MgSO4 (0.02%), penicillin (100 U/mL), and streptomycin (100 μg/mL) to remove the NBS. Tested samples were added to achieve final concentrations. Control wells were prepared by addition of DMEM. Wells containing DMEM without cells were used as blanks. NaF (1 × 10−6 mol/L) were used as positive control. The plates were incubated at 37°C in a 5% CO2 incubator for 3 days. The plates were washed twice with an ice-cold PBS and lysed by two cycles of freezing and thawing. Aliquots of supernatants were subjected to the ALP activity and the protein content measurement performed using an ALP activity kit and a microprotein assay kit. All results were normalized by protein content. The ALP activity (%) was calculated according to the formula: (ALP activitysample − ALP activityblank) / (ALP activitycontrol − ALP activityblank) × 100.
Statistical Analysis
Data were collected from at least three separate experiments. The results were expressed as mean ± SD. The statistical differences were analyzed using SPSS' t test. P values <0. 05 were regarded as indicating statistical differences.
Results
Effects of Icariin and TF on Proliferation of Primary OBs
As shown in Fig. 1, both icariin (0.1, 1, and 10 μmol/L) and TF (0.06, 0.6, and 6 μg/mL) inhibited the proliferation of primary OBs.
Effects of MTE on Proliferation of Primary OBs
As shown in Fig. 2, MTE inhibited the proliferation of primary OBs. The average inhibitory rate is about 15%.
Effects of Icariin plus MTE and TF plus MTE on the Proliferation of Primary OBs
As shown in Fig. 3, the antagonistic effect was generated by the treatment of icariin plus MTE. The most antagonistic effect was generated by the treatment of 0.1 μmol/L MTE plus 0.1 μmol/L icariin. The cell viability was resulted in 92.79%, 96.79%, and 94.69% by the treatment of 0.1, 1, and 10 μmol/L MTE plus 1 μmol/L icariin as compared with that of MTE or icariin treatment showing 86.31% (0.1 μmol/L MTE), 81.89% (1 μmol/L MTE), 82.20% (10 μmol/L MTE), and 89.27% (1 μmol/L icariin) cell viability. The cell viability was resulted in 100.47% and 102.23% by the treatment of 1 and 10 μmol/L MTE plus 10 μmol/L icariin as compared with that of MTE or icariin treatment showing 81.89% (1 μmol/L MTE), 82.20% (10 μmol/L MTE), and 93.37% (10 μmol/L icariin) cell viability. In addition, no effect was generated by the treatment of 0.1 μmol/L MTE plus 10 μmol/L icariin as compared with that of MTE treatment, but the synergistic effect was generated by the treatment of 0.1 μmol/L MTE plus 10 μmol/L icariin as compared with that of icariin treatment.
As shown in Fig. 4, both the antagonistic and synergistic effects were generated by the treatment of TF plus MTE. The antagonistic effect was generated by the treatment of 0.06, 0.6, or 6 μg/mL TF plus 0.1 μmol/L MTE, and the cell viability was resulted in 104.51%, 102.05%, and 91.54% as compared with that of TF or MTE treatment showing 78.74% (0.06 μg/mL TF), 80.21% (0.6 μg/mL TF), 79.12% (6 μg/mL TF), and 86.31% (0.1 μmol/L MTE) cell viability. The synergistic effect was generated by the treatment of 0.06, 0.6, or 6 μg/mL TF plus 10 μmol/L MTE, and the cell viability was resulted in 72.75%, 71.94%, and 72.61% as compared with that of TF or MTE treatment showing 78.74% (0.06 μg/mL TF), 80.21% (0.6 μg/mL TF), 79.12% (6 μg/mL TF), and 82.20% (10 μmol/L MTE) cell viability.
Effects of Icariin and TF on the ALP Activity of Primary OBs
As shown in Fig. 5, both icariin (0.1, 1, and 10 μmol/L) and TF (0.06, 0.6, and 6 μg/mL) promoted the differentiation of primary OBs.
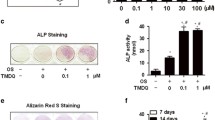
Effects of MTE on the ALP Activity of Primary OBs
As shown in Fig. 6, MTE (0.1 and 1 μmol/L) promoted the differentiation of primary OBs, but turned to inhibit the differentiation of primary OBs at a concentration of 10 μmol/L.
Effects of Icariin plus MTE and TF plus MTE on the ALP Activity of Primary OBs
As shown in Fig. 7, the synergistic effect was generated by the treatment of icariin plus MTE at most combinations. For instance, the ALP activity was increased by the treatment of 0.1, 1, or 10 μmol/L MTE plus 10 μmol/L icariin as compared with that of MTE treatment, and the ALP activity was increased by 1.36-, 1.40-, and 2.79-fold by the treatment of 0.1, 1, or 10 μmol/L MTE plus 10 μmol/L icariin as compared with that of 10 μmol/L icariin treatment. But the antagonistic effect was generated by the treatment of 1 μmol/L MTE plus 1 μmol/L icariin as compared with that of MTE or icariin treatment.
As shown in Fig. 8, the antagonistic effect was generated by the treatment of TF plus MTE at most combinations. For instance, the ALP activity was decreased by the treatment of 0.06, 0.6, or 6 μg/mL TF plus 0.1 μmol/L MTE as compared with that of MTE or TF treatment. But the ALP activity was increased by the treatment of 0.6 μg/mL TF plus 10 μmol/L MTE as compared with that of MTE or TF treatment.
Discussion
The progressive differentiation of OBs in culture is associated with the expression of ALP, an early marker of the OB phenotype. It was reported that degree of cell confluence had important effect on the growth and differentiation of primary human OBs in vitro. Siggelkow et al. [18] selected cells at a confluence of 50% and 75% as being at the stage of fast proliferation, and cells at 100% and 7 days after 100% confluence were used to describe the period of diminished proliferation and increasing differentiation. They reported that the effect of 1,25-(OH)2D3 on the growth and differentiation of primary osteoblast-like cell at four stages of cell confluence (stage I 50%, stage II 75%, stage III 100%, and stage IV 7 days postconfluence). So, the viability and ALP activity were studied by primary mouse OBs at two stages of cell confluence (stage I 50% and stage III 100%) in the present work. Our results demonstrated that icariin (0.1, 1, and 10 μmol/L) and TF (0.06, 0.6, and 6 μg/mL) inhibited the proliferation and promoted the differentiation of primary OBs. MTE inhibited the proliferation of primary OBs at tested concentrations, MTE (0.1 and 1 μmol/L) promoted the differentiation of primary OBs, but turned to inhibit at a concentration of 10 μmol/L. The combination of icariin or TF with MTE showed either synergistic or antagonistic effect on the viability and ALP activity of OBs. Moreover, the combination model is a pivotal factor for switching the biological effects toxicity to activity, from damage to protection.
In addition, it was reported that flavonoids (i.e., rutin and quercetin) have been used in clinical practice as pharmaceutical products to reduce the permeability of the capillary walls and decrease their fragility. Later, it was shown that rutin can be applied for the treatment of Fanconi anemia patients. These effects of flavonoids are supposedly due to their free radical scavenging and chelating activities. Kostyuk et al. [19] reported the influence of metal ions (Fe2+, Fe3+, Cu2+, Zn2+) on the protective effect of rutin, dihydroquercetin, and green tea epicatechins against asbestos-induced cell injury in vitro. Metals have been found to increase the capacity of rutin and dihydroquercetin to protect peritoneal macrophages against chrysotile asbestos-induced injury. This effect is due to the formation of flavonoid metal complexes, which turned out to be more effective radical scavengers than uncomplexed flavonoids. Epicatechins and their metal complexes have similar antiradical properties and protective capacities against the asbestos-induced injury of macrophages. Metal complexes of all flavonoids were found to be considerably more potent than parent flavonoids in protecting red blood cells against asbestos-induced injury. So, our results suggested that there might be a potential cooperative action between icariin or TF and MTE by the formation of metal complexes.
In summary, the combination of MTE with TF or icariin from E. koreanum generated synergistic or antagonistic effects on the proliferation and differentiation of primary OBs in vitro. The combination model is a pivotal factor for switching the biological effects from toxicity to activity, from damage to protection. The mechanism remains to be further studied.
Abbreviations
- ALP:
-
Alkaline phosphatase
- DMEM:
-
Dulbecco's Modified Eagle's Medium
- DMSO:
-
Dimethylsulfoxide
- ERT:
-
Estrogen replacement therapy
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- MTE:
-
Mixed trace elements including Zn2+, Ca2+, and Mn2+
- NBS:
-
Neonatal bovine serum
- NIH:
-
National Institutes of Health
- OBs:
-
Osteoblasts
- OD:
-
Optical density
- PBS:
-
Phosphate buffer solution
- SD:
-
Standard deviation
- TF:
-
Total flavonoids
References
Davison S, Davis SR (2003) Hormone replacement therapy: current controversies. Clin Endocrin 58:249–261
Sakamoto S, Sassa S, Kudo H et al (2000) Preventive effects of a herbal medicine on bone loss in rats treated with a GnRH agonist. Euro J Endocrinol 143:139–142
Yamaguchi M, Hashizume M (1994) Effect of beta-alanyl-L-histidinato zinc on protein components in osteoblastic MC3T3-El cells: increase in osteocalcin, insulin-like growth factor-I and transforming growth factor-beta. Mol Cell Biochem 136:163–169
Relea P, Revilla M, Ripoll E et al (1995) Zinc, biochemical markers of nutrition, and type I osteoporosis. Age Aging 24:303–307
Saltman PD, Strause LG (1993) The role of trace minerals in osteoporosis. J Am Coll Nutr 12:384–389
Seo JH, Jin YH, Jeong HM et al (2009) Calmodulin-dependent kinase II regulates Dlx5 during osteoblast differentiation. Biochem Biophys Res Commun 384:100–104
Reid IR, Ames RW, Evans MC et al (1993) Effect of calcium supplementation on bone loss in postmenopausal women. N Engl J Med 328:460–464
Keen CL, Zidenberg-Cherr S (1996) Manganese. In: Ziegler EE, Filer LJ (ed) Present knowledge in nutrition, 7th edn. ILSI Press, Washington D.C, pp 334–343
Hidaka S, Okamoto Y, Nakajima K et al (1997) Preventive effects of traditional chinese (Kampo) medicines on experimental osteoporosis induced by ovariectomy in rats. Calcif Tissue Int 61:239–246
Xie F, Wu CF, Lai WP et al (2005) The osteoprotective effect of Herba epimedii (HEP) extract in vivo and in vitro. Evid Based Complement Alternat Med 2:353–361
Chen KM, Ge BF, Ma HP et al (2004) The serum of rats administered flavonoid extract from Epimedium sagittatum but not the extract itself enhances the development of rat calvarial osteoblast-like cells in vitro. Pharmazie 59:61–64
Chen HL, Liu XY (1989) A probe into the relationship between virtue of Chinese medicine and four trace elements. Chin J Chin Mater Med 14:36–39
Zhao J, Jin RG, Luo L (2007) A correlation between the anti-HIV properties of Chinese medicinal herbs and their trace element content. J Beijing Univ Chem Technol 34:467–471
Cheng Y, Wang NL, Wang XL et al (2006) Chemical constituents from Epimedium koreanum Nakai. Journal of Shenyang Pharmaceutical University 23:644–647
Li XH, Zhang JC, Sui SF et al (2005) Effect of daidzin, genistin and glycitin on the osteogenic and adipogenic differentiation of bone marrow stromal cells and the adipocytic trans-differentiation of osteoblasts. Acta Pharmacol Sin 26:1081–1086
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth 65:55–63
Gray TK, Flynn TC, Gray KM et al (1987) 17 beta-estradiol acts directly on the clonal osteoblastic cell line UMR106. Proc Natl Acad Sci USA 84:6267–6271
Siggelkow H, Schulz H, Kaesler S et al (1999) 1, 25 Dihydroxyvitamin-D3 attenuates the confluence-dependent differences in the osteoblast characteristic proteins alkaline phosphatase, procollagen I peptide, and osteocalcin. Calcif Tissue Int 64:414–421
Kostyuk VA, Potapovich AI, Vladykovskaya EN et al (2001) Influence of metal ions on flavonoid protection against asbestos-induced cell injury. Arch Biochem Biophys 385:129–137
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 20971034), the Natural Science Key Foundation of Hebei Province (No. B2009000161), the Foundation for Key Program of the Ministry of Education of China (No. 208018), the Returned Scholars of Hebei Province (No. 207041), and the Natural Science Foundation of Hebei University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Li, Y., Sun, J. et al. Synergistic or Antagonistic Effect of MTE plus TF or Icariin from Epimedium koreanum on the Proliferation and Differentiation of Primary Osteoblasts In Vitro. Biol Trace Elem Res 143, 1746–1757 (2011). https://doi.org/10.1007/s12011-011-8987-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-8987-z