Abstract
The biological effect of Se and Cu2+ on Escherichia coli (E. coli) growth was studied by using a 3114/3236 TAM Air Isothermal Calorimeter, ampoule method, at 37°C. From the thermogenesis curves, the thermokinetic equations were established under different conditions. The kinetics showed that a low concentration of Se (1–10 μg/mL) promoted the growth of E. coli, and a high concentration of Se (>10 μg/mL) inhibited the growth, but the Cu2+ was always inhibiting the growth of E. coli. Moreover, there was an antagonistic or positive synergistic effect of Se and Cu2+ on E. coli in the different culture medium when Se was 1–10 μg/ml and Cu2+ was 1–20 μg/ml. There was a negative synergistic effect of Se and Cu2+ on E. coli when Se was higher than 10 μg/ml and Cu2+ was higher than 20 μg/ml. The antagonistic or synergistic effect between Se and Cu2+ on E. coli was related to the formation of Cu–Se complexes under the different experimental conditions chosen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) and copper (Cu) are essential elements for animals and human [1, 2]. The physiological functions of Se mainly play an antioxidant in the form of glutathione peroxidase (GSH-Px). The activity of glutathione peroxidase and antioxidant enzyme in body both decreases when Se is deficient in body. With Se concentration increasing, the activity of GSH-Px is elevated while the mutagenic effect decreases [3, 4]. Recent research revealed that Se has been not only the element for life but also the defensive reagent that can protect organism from toxicants’ damage. For example, Hg discharged in the muscle and kidney in fish increases when Se is supplemented as a nutrition for fish [5]. As a toxicant, Cu will disturb the function of indispensable elements in body [6]. Abdel Rhaim [7] studied the effect of Cu2+ (1.3–200 mg/kg body weight) on the Se in food fed to rat according to experiments. The result revealed that there is an antagonism between Se and Cu. However, the interaction mechanism of Se and Cu is not clear completely.
Se and Cu are also the component part of a kind of biofluoresence probe Cu–ZnSe and CdSe etc. quantum dots (QDs) [8–10]. These QDs, e.g., CdSe QDs, with their broad excitation spectra, sharp emission, and easily tunable emission properties, have been widely used in light-emitting diodes, thin-film transistors, solar cells, especially in biodetection assays for replacing conventional fluorescent markers, etc. [11–13]. In the long run, this material will enter the environment, even the human body. So the study on the effect of Cu and Se is important for the toxicology of QDs.
Microcalorimetry can be used to study the metabolism of cell and the effect of the toxicants on cellular metabolism [14–17]. Beer demonstrated microcalorimetric measurements of bacterial growth revealed by temporal details not observed by other techniques [18]. One of the most prominent features of the microbial growth process is the production of heat. If heat is monitored by microcalorimeter, much useful information, both qualitative and quantitative, may be obtained [19].
Microbe has a unique power–time curve, as recorded by the microcalorimeter, under given growth conditions. Any substance that can affect the metabolic growth processes of microbe, including cells, will change the power–time curve obtained from the microcalorimeter. From the power–time curves, we can obtain corresponding kinetic data by studying the effect of toxic agents on microbe. The purpose of this paper was to report the effect of Se and Cu2+ on the growth of E. coli respectively by microcalorimetry, and meanwhile investigate the antagonism and synergism between Se and Cu2+ on the growth of E. coli.
Materials and Methods
Cells and Reagents
E. coli (CCTCC AB91112) was provided by China Center of Type Culture Collection, Wuhan University, China. Analytical reagent grade Cu(NO3)2 was supplied by Nankai Chemical Reagent Factory of Tianjin. Selenium morpholine was synthesized and characterized by the Department of Chemistry, Wuhan University.
Culture Medium
E. coli was grown in the peptone culture medium containing NaCl 5 g, peptone 10 g, beef extract 3 g in 1,000 mL de-ionized water, the pH of the medium was adjusted to 7.0 with NaOH and HCl, finally the medium was sterilized by autoclaving at 120°C for 30 min.
Instrument and Operating Principle
In this paper, the 3114/3236 TAM Air Isothermal Calorimeter, manufactured by Thermometric Corporation of Sweden, was used to research the action of Se and Cu of different concentrations on the growth metabolism of E. coli. The working principle of the TAM Air Isothermal Calorimeter could be obtained from Liu P [20]. The production heat of E. coli growth at 37°C was measured by the isothermal calorimeter. From these thermogenesis curves (log phase), the growth rate constant, k, was calculated. According to the k–c relationship, we discussed and compared the action of Se and Cu2+ on E. coli.
Methods
The E. coli was cultivated under different conditions.
First, the E. coli was cultivated at control experiment; the values of k of E. coli growth metabolism at different control experiment times can be obtained (Table 1).
Second, the E. coli was cultivated only in the presence of different amounts of Se or Cu2+. The thermogenic curves and the values of k of E. coli growth metabolism can be obtained (Figs. 1, 2, Tables 2, 3).
Finally, the E. coli was cultivated at different amounts of Se and Cu2+. Two methods were applied in the experiment. One (method 1) was that the bacteria was cultivated in the peptone culture medium in the absence of Se. The values of k and the relationship curves of the k of E. coli can be obtained [Table 4, Fig. 3a]. The other (method 2) was that the bacteria was cultivated in the peptone culture medium in the presence of Se [Table 5, Fig. 3b]. The detailed procedures were basically similar to those described by Ref. [15].
Results
Thermogenesis Curves and the Growth Rate Constant of E. coli
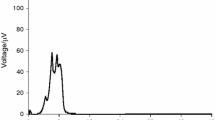
The thermogenesis curves of E. coli growth metabolism affected by Se, Cu2+, were obtained by microcalorimetry. These experiments had good reproducibility (Fig. 1).
In the log phase of growth, the cell number and culture time correspond to exponential law [21]. If the cell number is n 0 at time 0, and n t at time t, then
where, k is the growth rate constant. If the power output of each cell is w, then
We write P 0 = N 0 w and P t = n t w giving
The thermogenetic curves of the log phase of growth obey Eq. 3. So, making use of the data lnP t and t taken from the curves to fit a linear equation, can obtain the growth rate constant (k).
The power–time curves of the E. coli growth only containing Se of different concentrations were shown in Fig. 1. When the culture of E. coli containing different Se and Cu2+ was inoculated, the power–time curves were shown in Fig. 2. The values of rate constant (k) of E. coli growth under different experiment conditions were shown in Tables 1, 2, 3, 4, and 5.
Discussion and Conclusion
The Effect of Se on the Growth of E. coli
As seen from Table 2, the growth rate of E. coli in the presence of Se increased with the Se concentration increasing. When Se was 10 μg/mL, the maximal growth rate of E. coli was obtained. Subsequently, the growth rate of E. coli decreased with increasing of Se concentration. When Se ranged from 1 to 10 μg/mL, Se was mainly used to sustain the integrality of cell, and to act on the enzyme needed in biological body. The results showed that the low-concentration (1–10 μg/mL) Se promoted the growth of bacteria, high-concentration (>10 μg/mL) Se inhibited the growth. The biochemistry function of Se is mainly showed in the interaction between Se and enzyme [22–24].
In the investigation of effect of trace elements on activity of E. coli formate dehydrogenase, Se is one of the indispensable trace elements to sustain the activity of the enzyme [25]. Furthermore, Se is one of the necessary component of formate dehydrogenate of anaerobion, and it exists in the form of Se-cysteine. If the suitable amount of Se is added to peptone culture medium, not only can one enzyme without Se be formed, but also the enzymes of much higher activity containing Se be produced. Under this condition, the growth rate of a lot of microbes can increase evidently. When the concentration of Se is much higher, however, Se compounds have toxic action on microbe by attacking the special dehydrogenate system, especially amber acid dehydrogenate, including methionine adenosine transferase and cytochrome oxidase. The reason of the toxic action of Se compounds is that Se inhibits the activity of enzymes and other functional proteins with the mitochondria through the catalysis of oxidation reactions of S–H groups to S–S or S–Se–S bonds. Consequently, the growth rate of E. coli decreases because the normal division or multiplication of the cell is inhibited. The changes of the growth rate of the microbe are closely related to Se.
The Effect of Cu on the Growth of E. coli
Cu is an essential trace element for human and animals. It is a necessary component for over 30 kinds of metal enzymes in the biological body, and one of the indispensable trace elements to sustain the activity of some enzymes. It has been proven that tyrosine hydroxylase, cytochrome C oxidase, and peroxide dismutase are vital enzymes containing Cu [26]. In the absence of Cu, the activity of the above kinds of enzymes will decrease obviously. As a toxicant, Cu can inhibit the part activity of enzymes. Subsequently, the many-sided dysfunction also comes into being.
As seen from Table 3, the growth rate of E. coli decreased with increasing of Cu-only containing Cu. During this process, Cu inhibited the growth of E. coli, which might result from the action of Cu2+ on the enzymes in E. coli. Moreover, heavy metal ions, such as Hg2+ and Ag+, have the higher affinity to the ligand containing sulfur, which can result in the inhibiting action of heavy metal ions on S–H enzyme.
The Antagonism and Synergism Between Se and Cu2+ on the Growth of E. coli
The effect of Se and Cu2+ on some living organism has been studied. Research revealed there was antagonistic or synergistic action between Se and Cu2+ in living organism [27]. Cu2+ can inhibit the toxicity effect of Se on mice. The endurance ability of mice to Cu will decrease when the amount of Se is deficient in mice body. From Fig. 2, we found the shapes of the power–time curves of E. coli growth were almost similar. The higher the concentration of Cu is, the later the metabolism peak of E. coli advents when the Se keeps invariability.
Comparing Table 3 with Tables 4 and 5, the strong antagonism of Se to Cu2+ was found in method 1 (Fig. 3a) when the concentration of Se was 1–10 μg/ml and Cu2+ was 1–10 μg/ml. The inhibiting action of Cu2+ treated by Se on the growth of E. coli was less than that of Cu alone. However, the positive synergism of Se to Cu2+ was found in method 2 (Fig. 3b) when the concentration of Se was 1–10 μg/ml and Cu2+ was 1-20 μg/ml. Thus, when Se was 1–10 μg/ml and Cu2+ was 1–20 μg/ml, the growth rate of E. coli increased, and the k value 0.0131 is even much higher than the average value 0.00687 of the control experiment (Table 1). When the concentration of Se was higher than 10 μg/ml and the concentration of Cu was higher than 20 μg/ml, the growth rate of E. coli always decreased. This showed there was a negative synergism of Se and Cu2+ on E. coli. What is more, from Fig. 3, we saw that the all growth rate constant values of E. coli in the culture medium presence of Se were higher obviously than that of E. coli in the culture medium absence of Se. This showed the effect of Se and Cu2+ on E. coli growth was related to the different experimental conditions.
The results also revealed that the Se and Cu2+ had toxic action on the metabolism of E. coli under above conditions. The injury induced by Cu2+ could not be prevented completely by adding Se to Cu2+ under any circumstances. The effect of Se was thought to be related to either the formation of Cu–Se complexes in association with metallothioneins, or changes of the distribution of Cu2+ in tissue. According to the results, the protective effect of Se resulted from the production of Se–Cu complex compounds. Cu2+ can combine with the S–H of proteins and enzymes to change their activity. Se is a softer alkali than sulfur. According to the hard and soft acids and bases rule, Se can have the advantage over sulfur to combine with soft acid Cu2+ and produce the Se–Cu complex compounds. Accordingly, the Cu2+ becomes the stable and nontoxic Se–Cu complex compounds. The complex compounds may be vented through the body by metabolizing. The S–H target molecule can be protected from Cu2+. So, the organisms can be kept from being injured.
This study shows that microcalorimetric method is a straight-forward and simple method in the studies of interaction between trace elements and organism. The microcalorimetric method requires only an observable difference between the power production in the treated and controlled incubations. Unlike many other procedures, transparent solution is not required. Colored or turbid solutions, even suspensions can be put into the calorimeter [28]. That approximates more closely the vivo state than many other techniques do. Through the technique and other methods, the reasonable effect concentrations of Se and Cu2+ on the growth of E. coli and the maximum growth rate constant k of E. coli are obtained easily by microcalorimetry. It also shows that the study is important for the toxicology detection of QDs. All of these are significant to synthesize and select drug, ecotoxicological tests, and biocompatibility estimate, and so on.
References
Mohammad AH, Bahram F, Jesus T, Norma L, Rodriguez RC (2009) Maternal milk concentration of zinc, iron, selenium, and iodine and its relationship to dietary intakes. Biol Trace Elem Res 127:6–15
Dervis O, Hafize U (2009) Copper intoxication; antioxidant defenses and oxidative damage in rat brain. Biol Trace Elem Res 127:45–52
Zhang L, Wan N (2006) Advances in the research of sport fatigue caused by the action of free radical lipid oxidation. Chinese J Laboratory Diagn 10(9):1104–11081
Liu Z-D (1990) Genetics. Higher Education Press, Beijing, pp 365–372
Bjerregaard P, Andersen BW, Rankin JC (1999) Retention of methyl mercury and inorganic mercury in rainbow trout Oncorhynchus mykiss (W): effect of dietary selenium. Aquat Toxicol 45:171–180
Qian Z, Sun J, Tie B-Q, Mao X-Q, Zhan L-Z (2006) The toxic effect of Cu, Cd, As and their compound pollution on the duckweed. Chinese J Eco Agric 14(3):135–137
Wang K (1996) Trace element in life science. Chinese Measure, Beijing, 523
Liu P, Wang Q-S, Li X (2009) Studies on CdSe/l-cysteine quantum dots synthesized in aqueous solution for biological labeling. J Phys Chem C 113:7670–7676
Narayan P, X-G P (2007) Efficient and color-tunable Mn-doped ZnSe nanocrystal emitters: control of optical performance via greener synthetic chemistry. J Am Chem Soc 129:3339–3347
Narayan P, David G, Jason T, X-G P (2005) An alternative of CdSe nanocrystal emitters: pure and tunable impurity emissions in ZnSe nanocrystals. J Am Chem Soc 127:17586–17587
Igor LM, Tetsuo UH, Goldman ER, Mattoussi H et al (2005) Quantum dot bioconjugates for imaging, labelling and sensing. Nature Materials 4:435–446
Nathan IH, Todd E, Michael DB (2007) Quantum dots coordinated with conjugated organic ligands: new nanomaterials with novel photophysics. Nanoscale Res Lett 2:282–290
Akiyoshi H, Kouki F, Taisuke O, Shun N, Masakazu S (2004) Quantum dots targeted to the assigned organelle in living cells. Microbiol Immunol 48(2):985–994
Xu X-J, Xue Z, Xiao Q, Hou A-X, Liu Y (2008) Antibacterial activities of novel diselenide-bridged bis (porphyrin)s on Staphylococcus aureus investigated by microcalorimetry. Biol Trace Elem Res 125(2):185–192
Li X, Liu Y, Deng F-J, Wang C-X, Qu S-S (2000) Microcalorimetric study of the toxic effect of sodium selenite on the mitochondria metabolism of Carassius auratus liver. Biol Trace Elem Res 77:261–271
Li X, Liu Y, Zhao R-M, Wu J, Shen X-S, Qu S-S (2000) Microcalorimetric study of E. coli growth inhibited by the selenomorpholine complexes. Biol Trace Elem Res 75(1-3):167–175
Buikema AL Jr, Niederlehner BR, Cairns J Jr (1982) Biological monitoring. Part IV. Toxicity testing. Water Res 16(3):239–262
Buckion G, Russell SJ, Beezer AE (1991) Pharmaceutical calorimetry: A selective review. Thermochim Acta 193:195–214
Chen X-J, Shen Y-F, Feng W-S, Liu Y, Miao W (2006) Advances on Isothermal microcalorimetric studies of microbial communities. Chemistry in Chinese 69:1–8
Liu P, Liu Y, Chen Y-G, Qu S-S (2002) The Study of isothermal microcalorimetry method and its application in life science. Chin J Chem (10):682–687
Liu Y (1997) Thermochemical Studies of Metabolic Characteristics of Bacteria and Mitochondria. [Ph.D.Thesis], Wuhan University, Wuhan, the People’s Republic of China
Bayse CA, Allison BD (2007) Activation energies of selenoxide elimination from Se-substituted selenocysteine. J Mol Model 13:47–53
Cui Q-H, Tang J-C, Huang Y-G (2002) Effects of lead and selenium on telomere binding protein Rap1p, telomerase and telomeric DNA in Saccharomyces cerevisiae. Acta Biochimica Et Biophysica Sinica 34(2):240–244
Liu Q, Wang H, Hu D-C, Ding C-J, Xiao H et al (2003) Effects of sodium selenite on telomerase activity and telomere length. Acta Biochimica Et Biophysica Sinica 35(12):1117–1122
Li X, Liu Y, Wu J, Qu S-S (2003) Microcalorimetric study of the associated action of Se4+ to arsenic on the growth of E. coli. Chem J Chin Univ 24(1):91–94
Fu Y-H (1997) Trace element and clinic. Chinese Medicine Science and Technology, Beijing
Jensen LS (1975) Proc Soc Exp Biol Med 149:113
Wadso I (1997) Chem Soc Rev:79–86
Acknowledgments
This study was supported by the National Nature Science Foundation of China (No. 5020310).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, L., Li, X., Liu, P. et al. Study of the Action of Se and Cu on the Growth Metabolism of Escherichia coli by Microcalorimetry. Biol Trace Elem Res 137, 364–372 (2010). https://doi.org/10.1007/s12011-009-8583-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-009-8583-7