Abstract
Lead (Pb2+) is a well-known highly toxic element, and Spirodela polyrrhiza is one of the hydrophytes most sensitive to Pb2+, which might be highly toxic to photosynthesis. However, the mechanism by which Pb2+ inhibits energy transfer and conversion efficiency remains unclear. Here, we report the effects of Pb2+ on the secondary structure and function of photosystem II (PS II) of S. polyrrhiza using spectral methods. We found that Pb2+ accumulated in PS II and damaged its secondary structure, decreased the absorbance of visible light, inhibited energy transfer among amino acids within the PS II protein–pigment complex, and reduced energy transport from tyrosine residue to chlorophyll a. Taken together, Pb2+ exposure damaged the structure and function of S. polyrrhiza PS II.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Toxic levels of heavy metals, such as lead (Pb), occur in natural and agricultural systems as a result of environmental pollution from mining, smelting, manufacturing, agricultural, and waste disposal technologies (1), and there has been much interest in the toxic effects of heavy metals on plants (2–7). Spirodela polyrrhiza is a common species of duckweed in China; it has a larger leaf area and more roots than other duckweed species (8). It is also a species that is used in wastewater treatment systems and is one of the hydrophytes most sensitive to Pb2+. Furthermore, photosynthesis is one of the most Pb2+-sensitive processes in plants (9). The effects of Pb2+ are multifactorial, affecting both in vivo and in vitro photosynthetic CO2 fixation. Long-term exposure results in reduced leaf growth, decreased levels of photosynthetic pigments, altered chloroplast structure, and decreased enzyme activities for CO2 assimilation (10, 11). The total chlorophyll content and relative proportions of chlorophylls a and b are reduced through an inhibition of chlorophyll biosynthesis (12–16). The substitution of the central atom of chlorophyll, magnesium, by lead in vivo prevents photosynthetic light-harvesting in affected chlorophyll molecules, resulting in a breakdown of photosynthesis (17). Lead also significantly inhibits the Hill reaction activity of spinach chloroplast in addition to photophosphorylation, and lead has a more conspicuous effect on cyclic photophosphorylation than on noncyclic photophosphorylation (18). In vitro studies with isolated chloroplasts have shown that Pb inhibits photosynthetic electron transport and the photochemical activity of photosystem II (PS II) (9, 19); however, whether or not Pb inhibits energy transfer among amino acids within the PS II protein complex and from amino acid residues to chlorophyll a remains unclear.
PS II is located at the thylakoid membrane of the chloroplast, and its protein complex executes the PSII function. In the present study, we isolated and purified PS II particles from S. polyrrhiza exposed to different concentrations of PbCl2. We studied the effects of Pb2+ on secondary structure, energy transfer among amino acids and from amino acid residues to chlorophyll a in the PS II protein–pigment complex, as well as its impact on PS II photochemical activity, using various spectral methods.
Materials and Methods
Reagents
Triton-X-100, 2- (N-morpholino) ethanesulfonic acid (MES) were purchased from Sigma. Other reagents were acquired in China and were of analytical grade.
Material Treatment and Culture
The S. polyrrhiza used in this study were collected from a wetland near Tai Lake (located in Suzhou City, Jiang Province, China). The experiments were conducted under laboratory conditions with fluorescent lamps with a light intensity of 500 μM·m−2·s−1 (16 h light, 8 h dark). The temperature was 25°C. The growth medium used to culture duckweed was based on the Hoagland’s culture media (20). Cultivation of S. polyrrhiza was performed in 500-mL plastic breakers using 400 mL of Hoagland’s medium supplemented with 0, 25, 50, or 100 μM PbCl2. Forty healthy fronds of S. polyrrhiza were placed in the plastic containers at the beginning of each experiment. Hoagland medium was replaced once every 7 days so as to avoid nutrient exhaustion due to algal growth. The pH of each culture was always maintained at about 7 (6.5–7.5) in all experiments by the addition of 1 M HCl or KOH every day. Loss of water by evaporation was compensated for by the addition of demineralized water. Spirodela polyrrhiza was exposed to PbCl2 + Hoagland’s medium for 35 days, and each experiment had five replicates.
PS II Preparation
We prepared PS II particles from S. polyrrhiza using the method of Kuwabara and Murata et al. (21). Chloroplasts were extracted from S. polyrrhiza leaves with a medium containing 100 mM sucrose, 200 mM NaCl, and 50 mM K phosphate buffer (pH 7.4). The chloroplasts were then suspended in a second medium containing 300 mM sucrose, 50 M NaCl, and 50 mM K phosphate buffer (pH 6.9) at a chlorophyll concentration of 2–3 mg/mL. An aqueous solution of Triton X-100 (20% W/V) was added to the suspension with stirring until the ratio of Triton X-100 to chlorophyll became 25:1 (W/W). After incubation for 1 min, the suspension was centrifuged at 1,000×g for 2 min; the pellet was discarded, and the supernatant was centrifuged at 35,000×g for 10 min. The resultant pellet was collected and resuspended in 40 mM K phosphate buffer (pH 6.9), and the suspension was again centrifuged at 1,000×g for 2 min. The pellet was discarded, and the supernatant centrifuged at 35,000×g for 10 min. The final pellet constituted the preparation of PS II particles used in experiments. The ratio of chlorophylls a to b in the preparation was about 2.0:1. After incubating for 15 min in dim light, the PS II particles were collected by centrifugation at 144,000×g for 30 min. All of the above procedures were performed at 0–4°C. Chlorophyll of PS II was extracted in chilled 80% acetone and estimated spectrophotometrically (22).
Pb2+ Content of PS II
About 0.5 mg of PS II particles were digested and analyzed for Pb2+ content. Briefly, prior to elemental analysis, the PS II particles were digested overnight in nitric acid (ultrapure grade). After adding PS II particles were digested in nitric acid (ultrapure grade) overnight. After adding 0.5 mL of H2O2, the mixed solutions were heated to about 160°C using a high-pressure reaction container in an oven chamber until the samples were completely digested. The solutions were then heated to 120°C until the solutions were colorless and clear to remove remaining nitric acid. Finally, the solutions were diluted to 4 mL with 2% nitric acid. Inductively coupled plasma-mass spectrometry (ICP-MS, POEMS, Thermo Jarrel Ash, USA) was used to analyze the Pb2+ concentration in the samples. Data are expressed as nanograms per milligram PS II particles.
Circular Dichroism (CD) Spectrum of the Purified PS II
The CD spectra of the purified PSII were analyzed at room temperature on a JASCO-J-810 spectropolarimeter with a quartz sample cell with an optical path length of 1 cm. The concentration of PS II was 0.2 mg·mL−1. Molecular ellipticities [θ] in degrees per square centimeter per decimole were calculated using a mean residue weight of 115. The secondary structure indexes, α-helix, β-sheet, β-turn, and random coil of the PS II samples were determined by Perczel’s method (23). The method, called “convex constraint analysis,” is a general deconvolution method for a CD spectra set of any variety of conformational type. The algorithm, which is based on a set of three constraints, is able to deconvolute a set of CD curves to its common “pure”-component curves and conformational weights. To analyze a single CD spectrum with this method, the spectrum is appended to the data set used as a reference data set.
Absorption Spectrum and Fluorescence Spectrum of the Purified PS II
The absorption spectrum of the purified PS II particles was measured from 400–750 nm at room temperature with a dual-beam spectrophotometer (UV-3010, Hitachi, Japan). The fluorescence excitation spectra of the purified particles were recorded at 273 K from 200 to 300 nm when emission was at 304 nm, and from 200 to 400 nm when emission was at 683 nm, using a F-4500 fluorometer (Hitachi, Japan). The fluorescence emission was recorded at 273 K from 290 to 400 nm when excitation was at 280 nm and from 600 to 800 nm when excitation was at 436 nm.
Activities of the Oxygen Evolution of the Purified PSII
The PS II particles were suspended at 25°C in a medium containing 300 mM sucrose, 10 mM NaCl, 0.05% bovine serum albumin, and 25 mM MES-NaOH (pH 6.5). The concentration of the PS II particles was equivalent to about 15 μg of chlorophyll per milliliters. The oxygen evolution was measured with an Oxygraph oxygen electrode (Hansatech instruments, UK). All experiments were independently performed, and the presented data represent the average of the recordings from five independent experiments.
Statistical Analysis
Data were analyzed with analysis of variance, and the statistical significance of the differences between treatment means was determined using the Scheffe test (p < 0.05).
Results
Pb2+ Content in PS II
The Pb2+ contents of S. polyrrhiza PSII are presented in Table 1, which suggests that the Pb2+ content of PSII from Pb2+-treated S. polyrrhiza increased with increasing Pb2+ exposure concentration; no Pb2+ was detected in the control.
The Secondary Structure of PS II
The CD spectra of the PS II particles of S. polyrrhiza are shown in Fig. 1, which indicates that the CD spectra of the PSII particles from Pb2+-treated S. polyrrhiza are the same as that of the control, which are negative Cotton peaks in 208 and 220 nm, the α-helix and β-sheet contents of PS II were decreased, and the β-turn and random coil contents were increased in the PSII preparations from various Pb2+-treated S. polyrrhiza (Table 2).
Light Absorption of PS II
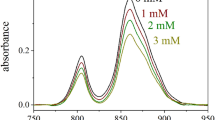
The visible absorption spectrum of the PS II particles purified from S. polyrrhiza is shown in Fig. 2. Chlorophyll-a (chla) absorption peaks in the red region and blue region were observed at 660–670 nm and at 430 nm (curve 1), respectively; there was a distinct shoulder at 640–650 nm, which can be ascribed to chlorophyll-b (chlb) absorption. A wide shoulder at around 450–470 nm indicates the presence of chlb and carotenoid. The absorption peak at about 415 nm suggests the presence cytochrome b-559 hem and pheophytin in the complex. These results are in agreement with those of previous reports (23–27). Lines 2–4 in Fig. 2 show that the visible absorption spectra of the PS II particles from various PbCl2-treated S. polyrhiza were nearly the same as that of the control; however, the absorption intensities were lower than that of the control.
Energy Transfer Among Amino Acids Within PS II
The fluorescence emission was recorded from 290 to 400 nm at 273 K. The emission peak of PS II at 304 nm was observed when excitation was at 280 nm, but there was no emission peak from 325 to 350 nm (Fig. 3a). In excitation spectra of PS II, the excitation peaks of PS II at 230 and 277 nm were observed when emission was at 304 nm, respectively (Fig. 3b). The excitation peak at 277 nm corresponds to absorption peak of tyrosine residues, suggesting that the emission peak at 304 nm was attributed to tyrosine residues of PS II. The carrier protein of pigment–protein complex of PS II displayed a fluorescence characteristic of tyrosine and was attributed to A type of protein (28). In addition, Fig. 3b shows the excitation peak of PS II at 230 nm, showing that excited energy from amino acids at 230 nm could be transferred to tyrosine group, which resulted in fluorescence emission.
Figure 3a,b shows that PbCl2 treatments at various concentrations had great influence on fluorescence spectra of PSII particles, i.e., the emission peak intensity at 304 nm was lower than that of the control; the excitation peaks of amino acids at 230 nm and tyrosine residues reduced, respectively, and the excitation peak at 230 nm was red-shifted by 2 nm and the ratio of F278/F230 decreased.
Energy Transfer Between Protein and Chlorophyll-a of PS II
The fluorescence excitation spectra of chla of PS II complex when emission was at 683 nm are shown in Fig. 4. The excitation peak at 343 nm is attributed to chla, the peaks at 230 and 277 nm are consistent with excitation peaks of intrinsic fluorescence of protein when emission was at 304 nm. It shows that there was an energy transfer between protein and chla within the pigment–protein complex, i.e., the excitation energy from amino acids at 230 nm and tyrosine residues at 278 nm could be transferred to chla. Upon PbCl2 treatment, the obvious reduction of excitation peaks at 230, 278, and 343 nm can be seen in Fig. 4; the reduction at 343 nm was the most significant.
Fluorescence Quantum Yield of PS II
In Fig. 5, the fluorescence emission spectrum at 273 K exhibits a main peak at 683 nm, which is a characteristic of the PSII particles when the excitation is 436 nm. The major part of the emission observed at 683 nm originated from the charge recombination of P680+ and the reduced pheophytin (29, 30). However, the emission peaks treated with various concentration of PbCl2 (lines 2–4) were blue-shifted by 2 nm, and the peak intensities (fluorescence quantum yield) were decreased by 35.61%, 40.44%, and 45.80%, respectively.
The Oxygen Evolution of the PS II
The effects of Pb2+ on oxygen evolution of PS II particles are shown in Fig. 6. The O2-evolving rates treated with various concentration of PbCl2 were lower by 41.04%, 53.64%, and 62.77%, respectively, compared with the control (p < 5%).
Discussions
In order to determine whether Pb2+ had entered chloroplasts and bound to PS II, we measured the Pb2+ content of PS II from the four PbCl2-treated groups of S. polyrrhiza. We found that Pb2+ accumulated in the PSII of S. polyrrhiza and also that Pb2+ increased with increasing Pb2+ exposure; the control was not detected. CD spectra suggested that the secondary structure of PS II from various Pb2+-treated S. polyrrhiza was damaged, thus establishing that Pb2+ was indeed bound to the PS II.
Experimental research has shown that the visible light absorption of PS II is obviously decreased in PbCl2-treated S. polyrrhiza. This is because PbCl2 destroyed the conformation of PS II, and the total chlorophyll content and relative contents proportion of chla and chlb were reduced through the inhibition of chlorophyll biosynthesis (11–15). The substitution of the central atom of chlorophyll, magnesium, by lead in vivo prevents photosynthetic light-harvesting in the affected chlorophyll molecules, resulting in a reduction of light absorbance (16, 18, 19).
We demonstrate that PS II exhibited a fluorescence characteristics of tyrosine; there is an energy transfer between amino acids at 230 nm and tyrosine at 278 nm and between protein and chlorophyll-a of intrinsic protein complex of PS II. The absorption of PSII at 230 nm might be attributable to polypeptide, or to cysteine residues of protein, which have an absorption peak at 235 nm (28, 31). The cysteine residue for the intrinsic carrier protein of PS II could absorb light and be excited. Its excitation energy was transferred to a tyrosine group and caused the enhancement of excitation energy at 277 nm, whereas it leads to an increase of excitation energy of chla at 343 nm. The ratio of F278/F230 of PS II from PbCl2-treated S. polyrrhiza decreased, suggesting that PbCl2 inhibited the excitation energy transfer from cysteine residue to tyrosine residue within PS II complex. In contrast, the ratio of F278/F343 was gradually increased by the PbCl2 treatments, indicating that PbCl2 could inhibit excitation energy transfer from cysteine residue to tyrosine residue, and from tyrosine residue to chla within PS II complex; this is because the Pb2+ bond to PSII and the protein skeleton was impaired, and a rotation of protein and pigments on the thylakoid membrane was produced by Pb2+. Therefore, the excitation energy from amino acids was barely transported to chla, resulting in a reduction of the excitation intensity at 343 nm.
The reduction of fluorescence quantum yield of PS II reaction center pigment—chla (P680)—was because the excitation energy from LHC II, CP43 and CP47 of the core antenna was inefficiently transferred to P680 upon Pb2+ treatment. We speculate that Pb2+ might impair the bound state of chla (P680) on the PS II reaction center. The reduction of the fluorescence quantum yield of PS II suggests that Pb2+ decreased the utilization and conversion efficiency of light energy within PS II.
Treatment with various concentrations of PbCl2 treatment resulted in a decrease in oxygen evolution, indicating that PbCl2 impaired the energy transfer among various compositions of PS II, slowed the transformation from light energy to electron energy, and inhibited electron transport, leading to the inhibition of water splitting and oxygen evolution. In addition, PbCl2 destroyed the structure of tyrosine residues within PS II and impaired the function of secondary electron donors, which are thought to be attributable to tyrosine residues of D1 protein in PS II (32). Harvesting electrons and protons for tyrosine residues from water was thereby reduced.
Taken together, Pb2+ bound to the PS II, which might replace Mg2+ in chlorophyll or Ca2+ in the oxygen-evolving center, resulted in alteration of PS II structure and inhibited both energy transfer within PS II and oxygen evolution.
References
Foy CD, Chaney RL, White MC (1978) The physiology of metal toxicity in plants. Annu Rev Plant Physiol 29:511–566
Reed RH, Gadd GM (1990) Metal tolerance in eukaryotic and prokaryotic algae. In: Shaw AJ (ed) Heavy metal tolerance in plants: evolutionary aspects. CRC, Boca Raton
De Filippis LF, Pallaghy CK (1992) Heavy metals: sources and biological effects. In: Rai LC, Gaur JP (eds) Phycological perspectivies of water pollution. E. Schweizerbart Verlag, Stuttgart
Luo LX, Sun TY (1998) Effect of cadmium-surfactant combined pollution on physiological characteristic of wheat leaf. Chin J Appl Ecol 9(1):95–100, (in Chinese)
Qin TC, Wu YS, Wang HX, Li QR (1998) Effect of cadmium, lead and their interactions on the physiological and ecological characteristics of root system of Brassica chinensis. Acta Ecol Sin 18(3):320–325, (in Chinese)
Luo LX, Sun TY (1998) Effect of cadmium stress on lipid peroxidation in wheat leaves. China Environ Sci 18(1):72–75, (in Chinese)
Zhou CF, Wu GR, Shi GX, Lu CM (2001) The role of antioxidant systems in Cu2+ stress resistance in Spirrodela polyrhiza. Acta Bot Sin 43:389–394
Yan SZ (1983) The illustrated handbook of high aquatic plant in China. Science, Beijing, p 294
Miles CD, Brandle JR, Daniel DJ, Chu-Der O, Schnare PD, Uhlik DJ (1972) Inhibition of photosystem II in isolated chloroplasts by lead. Plant Physiol 49:820–825
Parys E, Romanowska E, Siedlecka M, Poskuta JW (1998) The effect of lead on photosynthesis and respiration in detached leaves and in mesophyll protoplasts of Pisum sativum. Acta Physiol Plant 20:313–322
Shearan IS, Singh R (1993) Effect of heavy metals on photosynthesis in higher plants. In: Abrol YP, Mohanty P, Govindjee (eds) Photosynthesis: photoreactions to plant productivity. Kluwer, Dordrecht, pp 451–468
Ernst WHO, Nielssen HGM, Ten Bookum WM (2000) Combination toxicology of metal- enriched soils: physiological responses of a Zn- and Cd-resistant ecotypes of Silene vulgarison polymetallic soils. Environ Exp Bot 43:55–71
Sinha SK, Srivastava HS, Tripathi RD (1993) Influence of some growth regulators and cations on inhibition of chlorophyll biosynthesis by lead in maize. Bull Environ Contam Toxicol 51:241–246
Van Assche JA, Clijsters H (1990) Effects of heavy metals on enzyme activity in plants. Plant Cell Environ 13:195–206
Wu X, Liu C, Qu CX, Huang H, Liu XQ, Chen L, Su MY, H, ong FS (2008) Influences of PbCl2 on the nitrogen metabolism of spinach. Biol Trace Element Res 121:258–265
Wu X, Huang H, Liu XQ, Chen L, Liu C, Su MY, Hong FS (2008) Oxidative stress induced by lead in chloroplast of spinach. Biol Trace Elem Res. doi:10.1007/s12011-008-8195-7
Kupper H, Kupper F, Spiller M (1996) Environmental relevance of heavy metal substituted chlorophylls using the example of water plants. J Exp Bot 47:259–266
Wu X, Liu C, Qu CX, Huang H, Liu XQ, Chen L, Hong FS (2008) Effects of lead on activities of photochemical reaction and key enzymes of carbon assimilation in spinach chloroplast. Biol Trace Elem Res. doi:10.1007/s12011-008-8196-6
Wu X, Hong FS, Liu C, Su MY, Zheng L, Gao FQ, Yang F (2008) Effects of Pb2+ on energy distribution and photochemical activity of spinach chloroplast. Spectrochim Acta Part A 69:738–742
Meider H (1984) Class experiments in plant physiology. George Allen and Unwin, London, pp 72–74
Kuwabara T, Murata N (1982) Inactivation of photosynthetic oxygen evolution and concomitant release of three polypeptides in the photosystem II particles of spinach chloroplasts. Plant Cell Physiol 23:533–539
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Perczel A, Park K, Fasman GD (1992) Analysis of the circular dichroism spectrum of proteins using the convex constraint algorithm: A practical guide. Anal Biochem 203:83–93
Du LF, Tang XS, Liang HG, Kuang TY (1991) The Comparison of water-splitting activity, DCIP photoreduction activity and some property of photosystem II particles from different plants. Acta Phytophysiol Sin 17(4):342–348, (in Chinese)
Du LF, Tang XS (1992) Isolation and properties of oxygen-evolving photosystem II reaction center complex. Acta Phytophysiol Sin 18(1):17–23, (in Chinese)
Hong FS (2004) Effect of Eu3+ on phtosystem II spectral characteristics from spinach. Biol Trace Elem Res 100:186–196
Su MY, Wu X, Liu C, Qu CX, Liu XQ, Chen L, Huang H, Hong FS (2007) Promotion of energy transfer and oxygen evolution in spinach photosystem II by nano-anatase TiO2. Biol Trace Elem Res 119(2):183–192
Zhao NM, Zhou HM (2000) Biophysics. China Higher Education Press and Springer, Beijing, pp 299–309, (in Chinese)
Barber J, Chapman DJ, Telfer A (1987) Characterization of a PS II reaction center isolated from the chloroplasts of Pisum sativum. FEBS Lett 220:67–73
Seibert M, Picorel R, Rubin AB, Connoly JS (1988) Spectral, photophysical, and stability properties of isolated photosystem II reaction center. Plant Physiol 87:303–306
Guo YJ (1983) In fluorescence technique and its application to molecular biology. Science, Beijing, p 188, (in Chinese)
Buchanan BB, Gruissem W, Jones RL (2002) Biochemistry and molecular biology of plants. Science Press, American Society of Plant Physiologists, Beijing, pp 610–616
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 30470150, 30800068).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qufei, L., Fashui, H. Effects of Pb2+ on the Structure and Function of Photosystem II of Spirodela polyrrhiza . Biol Trace Elem Res 129, 251–260 (2009). https://doi.org/10.1007/s12011-008-8283-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-008-8283-8