Abstract
Scytonemin acts as a natural photoprotectant against high-intensity solar photosynthetically active radiation and harmful ultraviolet radiation, which has been reported in several cyanobacteria, and gets accumulated in their extracellular polysaccharide sheath. UV-B radiation (280–315 nm) has detrimental effect on physiological and biochemical processes of living organisms. In this study, scytonemin was extracted from dried cyanobacterial mats collected from the bark of mango trees and was partially characterized using high-performance liquid chromatography and electrospray ionization–mass spectrometry (retention time: 1.7 min; UVλmax: 386 nm; [M + H]+m/z: 545.1). Thereafter, photoprotective capabilities of scytonemin against UV-B radiation in a non-sheathed cyanobacterium Nostoc sp. strain HKAR-2 were assessed followed by studying its role in the recovery process. We found that UV-B radiation inhibited growth, survival, Chl a content and total protein concentration and caused an increase in total carotenoid content. The activities of nitrogen-assimilating enzymes, glutamine synthetase and nitrate reductase, were also affected. In vivo nitrate reductase activity exhibited a stimulatory response, while in vivo glutamine synthetase activity was adversely inhibited after UV-B exposure. Scytonemin with different concentrations exhibited efficient photoprotective ability by nullifying the deleterious effects of UV-B and also enhanced the recovery process. UV-screening effects of scytonemin in terms of growth, pigmentation, survival and nitrogen metabolism enzymes in the cyanobacterial strain have been reported for the first time in the present study. Our results suggest that scytonemin aids in the better survival and adaptability of cyanobacteria in stressed habitats facing harsh environmental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recent global climate models predict that continuous accumulation of anthropogenically and naturally released ozone-depleting substances, such as organobromides, chlorocarbons, chlorofluorocarbons and reactive nitrogen species, namely peroxynitrite (ONOO), nitrous oxide (N2O) and nitric oxide (NO), in the stratosphere has resulted in an increased incidence of harmful solar UVR (100–400 nm) (Williamson et al. 2014). The necessity to carry out life-driven phenomena, photosynthesis and nitrogen fixation exposes cyanobacteria to lethal doses of solar UV-B (280–315 nm) and UV-A (315–400 nm) radiation in their habitats. The highly energetic UV-B radiation has great potential to affect the metabolism of terrestrial and aquatic organisms, directly by effecting their key cellular machinery such as lipids, proteins and DNA, or induces indirect effects by aiding the generation of ROS and other free radicals as well (Vincent and Neale 2000; Rastogi et al. 2010; Rajneesh et al. 2017, 2019). ROS-mediated protein modification, lipid peroxidation and DNA damages, such as the formation of pyrimidine/purine dimers and strand breaks, may result in the ceasing of the vital functions of the cell. Moreover, ROS also inhibits photosynthesis by damaging the photosynthetic apparatus of cyanobacteria (He and Häder 2002).
Extraordinary tolerance of cyanobacteria toward several abiotic stresses and their physiological adaptation makes them model organisms for extra-terrestrial research apart from a novel role in biotechnology (Cockell et al. 2011; Olsson-Francis et al. 2013). In the process of nutrient cycling, cyanobacteria occupy a key position owing to their capacity of atmospheric N2 fixation through the enzyme “nitrogenase” into NH4+, the reduced form of N which enters the food chain. Although the Earth’s atmosphere contains large quantities (~ 78% by volume) of N2, it gets incorporated into the biosphere through assimilation by the enzymes NR and GS only via microorganisms and plants. Different biological processes such as orientation and motility, growth and development, enzyme activity, pigmentation, CO2 assimilation, photosynthesis, N2 fixation and assimilation are severely affected in organisms which are exposed to UV-B radiation (Newton et al. 1979; Döhler 1990; Tyagi et al. 1992; Sinha and Häder 1998; Rajneesh et al. 2019). Synthesis and accumulation of certain UV-protective compounds such as scytonemin aid in alleviating the cells from harmful effects of UVR (Sinha et al. 1998; Rastogi et al. 2013; Pathak et al. 2017, 2019a, b). Scytonemin is a brownish yellow, lipophilic, non-fluorescent pigment, and its biosynthesis is induced upon UV exposure and accumulates in the extracellular sheath as a stable photoprotective layer which absorbs 90% of incident radiation (Fig. 1) (Garcia-Pichel and Castenholz 1991; Garcia-Pichel et al. 1992; Rastogi et al. 2013; Pathak et al. 2017). Purified scytonemin shows an absorption maximum at 386 nm; additionally, it also shows significant absorbance of 300, 278 and 252 nm.
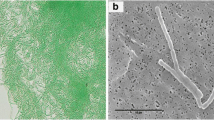
Bark of a mango tree harboring dry Lyngbya mats (shown by arrow) (a), light microscopic image of Lyngbya sp. showing the filament migrating out of its sheath opened at the ends (b), empty sheath containing orange-brown-colored scytonemin (shown by arrow) (c), scanning electron microscopic image of Lyngbya sp. (d), cell morphology of Nostoc sp. strain HKAR-2 observed under a light (e) and scanning electron microscope (f)
The cyanobacterium Nostoc sp. strain HKAR-2 was originally isolated from a hot spring and cultured under laboratory conditions to test its tolerance against UV-B radiation. Lyngbya sp. is the dominant cyanobacterium growing on the bark of mango trees throughout the year irrespective of fluctuation and variation in the UVR and heat regime. Thus, Lyngbya sp. are intermittently exposed to high solar radiation, UVR and desiccation. The Lyngbya sp. possess scytonemin in their well-defined sheath. Therefore, the aim of the present study was to evaluate the photoprotective role of scytonemin extracted from Lyngbya sp. against damaging effects of UV-B radiation in terms of survival, growth, total protein content, photosynthetic pigments content, photosynthetic activity of PS II and N metabolism enzymes in scytonemin-less unsheathed cyanobacterium Nostoc sp. strain HKAR-2. In addition, we have also emphasized the study of free radical production in the absence and presence of scytonemin.
2 Materials and methods
Experimental organisms and growth conditions –
The cyanobacterium Lyngbya sp. was collected from the bark of Mangifera indica Linnaeus (Fig. 1a), growing in the campus of Banaras Hindu University, Varanasi (25°15′51.89″N, 82°59′42.05″E). A laboratory-grown culture of cyanobacterium Nostoc sp. strain HKAR-2, originally isolated from the hot springs of Rajgir, Bihar, India (25°2′0″N, 85°25′0″E) (Rastogi et al. 2012), was taken as a test organism. Identification of both cyanobacteria was done with the help of standard monographs and taxonomic keys (Desikachary 1959; Komárek et al. 2014), and Nostoc sp. strain HKAR-2 (accession number: FJ939126) was previously identified by 16S rRNA gene sequencing (Rastogi et al. 2012). Lyngbya sp. is a member of Oscillatoriales characterized by filamentous, non-spore and non-heterocyst-forming organism. Nostoc sp. strain HKAR-2 is a member of Nostocales characterized by a frothy thallus, circinate, gelatinous, non-sheathed trichomes with ellipsoidal cells and is a heterocyst-forming cyanobacterium. The morphology of both cyanobacteria is shown in Fig. 1. Nostoc sp. strain HKAR-2 was cultured utilizing standard microbiological techniques as done previously (Sinha et al. 1995). Axenic cultures of the cyanobacteria were grown in BG-11 medium (Rippka et al. 1979) without nitrogen supplement at 20 ± 2 °C with a 14/10-h light/dark cycle illuminated with cool white fluorescent tubes (Philips TL 40 W/54, India) having an irradiance of 12 ± 2 Wm−2 at the surface of the vessels. For avoiding contamination, the culture was subcultured at regular intervals and was hand-shaken five times daily. Exponentially growing cultures were used for all experiments having an initial optical density of 0.6 ± 2 at 750 nm. The initial experiments for the analysis of scytonemin were performed with naturally growing mats of Lyngbya sp. growing on mango trees, and laboratory-grown cultures of Nostoc sp. strain HKAR-2 were used to see the changes in cell biochemistry and physiology after exposure to UV-B radiation with or without scytonemin.
Extraction and partial purification of scytonemin –
Extraction of scytonemin was done by incubating oven-dried (60 °C) cyanobacteria mats (dry weight = 1 g) in 10 mL methanol: ethyl acetate (1:1 v/v) at 4 °C overnight followed by sonication (2011-Sonic, cycle 30%, power 40%) for 4 min. Centrifugation of the resulting extract was done at 11,200 g for 5 min, and a vacuum evaporator was used for evaporating the supernatant at 38 °C which was re-dissolved in methanol/ethyl acetate (1:1 v/v). 500 μL filtration of samples was done through 0.22-μm pore-sized microcentrifuge syringe-driven filter (Axiva Sichem Biotech., New Delhi) before performing the UV/Vis and HPLC analyses (Rastogi et al. 2013).
Characterization of scytonemin – UV–Vis spectroscopy –
Spectroscopic analysis between 200 and 700 nm was performed using a UV–Vis double-beam spectrophotometer (U-2910, UV/VIS, Hitachi, Tokyo, Japan). The raw data were transferred to a microcomputer, and peaks were analyzed with the software provided by the manufacturer.
High-Performance Liquid Chromatography (HPLC) –
Scytonemin was purified using a reverse-phase HPLC system (Waters 2998 with photodiode array (PDA) detector, auto-injector 717 plus, 515 PUMP, Milford, USA) which was equipped with Empower-2 software. The HPLC system had an ODS-2 (RP 18) column (Water, Spherisorb analytical column, 5 μm, 4.6 × 250 mm diameter, Ireland) along with a guard (4.6 × 10 mm inside diameter). An auto-injector (Waters 717 plus autosampler) was used for injecting samples (10 μL) into the HPLC column. Elution was maintained at a flow rate of 1.0 mL min−1 utilizing the mobile phase (solvent A: ultra-pure water; solvent B: acetonitrile–methanol–tetrahydrofuran, 75:15:10, v/v). A gradient elution program of 30 min was set which increases linearly with 0–15 min from 15% solvent A to 100% solvent B and 15–30 min at 100% solvent B. The detection wavelength was set at 386 nm, and a PDA scan wavelength was set in the range of 250–750 nm (Rastogi and Incharoensakdi 2014). Identification of scytonemin was done through its characteristic absorption maximum (386 nm) in the solvent corresponding to the appropriate retention time (RT), and scytonemin concentration was determined using an extinction coefficient of 112.6 L g−1 cm−1 at 384 nm (Garcia-Pichel et al. 1992, Rath et al. 2012). Thereafter, we collected the pure scytonemin containing fraction, evaporated it by using a Rotovap and further dried in a vacuum manifold for 30 min to form a powder. The pure scytonemin was further characterized by electrospray ionization–mass spectrometry (ESI–MS) and Fourier transform infrared (FTIR) spectroscopy.
Electrospray Ionization–Mass Spectrometry (ESI–MS) –
HPLC-purified scytonemin was collected for the production of protonated molecules by ESI. An Amazon SL mass spectrometer (Bruker Daltonics Inc., Bremen, Germany) was utilized for recording mass spectra. A cone voltage of 30 V was found to induce the formation of (M + H)1+ with a scan range from 100 to 600 m/z. Precursor ions were specifically selected for MS/MS based on previously determined retention times and m/z values. Other MS settings were: capillary voltage (5500 V) and temperature (300 °C). The manual mode was selected for carrying out MS/MS with fragmentation via collision-induced dissociation (CID) of the precursor ion using He as the collision gas at 40 V. Precursor ions were selected within an isolation width of 2 u (mass-to-charge ratio), and an accumulation of scans was done with varying RF signal amplitudes. Calibration of the m/z scale of MS was done utilizing the external calibration standard electrospray “tuning mix”(Agilent Technologies, Santa Rosa, USA). The data were analyzed using the software Data Analysis 4.0 (Bruker Daltonics Inc., Bremen, Germany).
Fourier Transform Infrared (FTIR) –
Infrared (IR) transmission measurements were taken between 4000 and 650 cm−1 with an FTIR/FIR spectrophotometer version 10 (Perkin Elmer, Waltham, MA, USA) and a KBr beam splitter. The scytonemin was fused with oven-dried potassium bromide (stored in a desiccator) in a 1:100 ratio. The samples were presented in the form of a transparent KBr disk, and the spectra were recorded at a resolution of 4 cm−1 with 64 scans.
UV-blocking function of scytonemin against high-energy UV-B radiation – Treatment with UV-B radiation and scytonemin –
Cyanobacterial cultures (growing exponentially, under the irradiance of 12 ± 2 Wm−2) were transferred to sterile Petri dishes (150 mm in diameter) and were UV-B irradiated (1 Wm−2) by radiation passing through various concentrations (0, 0.4 and 0.8 mM) of a thin layer of HPLC-purified scytonemin. The source of UV-B radiation was a UV-B tube (Philips TL 40 W/12, Holland) having its main output at 312 nm. Along with the UV-B treatment, cultures were also exposed to PAR (12 ± 2 Wm−2) (OSRAM L 36 W: 32 Lumilux de luxe warm white and Radium NL 36 W: 26 Universal white, Germany) and also gently agitated by a magnetic stirrer to ensure uniform exposure. The Petri dishes were covered with 295-nm and 395-nm cut-off filters (Ultraphan; Digefra, Munich, Germany) for getting desired irradiation regimes of UV-B and PAR, respectively. To determine the UV-protective function of scytonemin against UV-induced damages, the cultures treated with various concentrations of HPLC-purified scytonemin were exposed to UV-B radiation up to 8 h. At regular time intervals, i.e., (0 (control) 2, 4, 6 and 8 h), a fixed amount of cyanobacterial cultures were withdrawn from the Petri dishes following continuous UV-B exposure. During all experiments, a constant temperature of 20 ± 2 °C was maintained and samples were shaken regularly during exposure for avoiding heating and self-shading of the cyanobacterial cells. After irradiation, samples were taken for the analysis of survival, growth, protein content, pigmentation, photosynthetic activity of PS II, nitrogen-assimilating enzymes and free radical production. For further recovery/repair following exposure to UV-B radiation and scytonemin, the cultures were re-suspended in fresh medium and allowed to recover for 10 days in normal culture condition (under PAR). Recovery in growth, protein content and pigmentation was observed at regular intervals for 10 days.
Percent survival determination –
For determining percent survival, 50 µL aliquots from each culture treated with scytonemin and UV-B at an irradiance of 1 Wm−2 were withdrawn at 0, 2, 4, 6 and 8 h and the cyanobacterial samples were plated on agar plates, which were incubated in the dark for 48 h, and thereafter, these were exposed to light and were kept in the culture room. After 15 days of incubation, cyanobacterial colonies started appearing which were counted by a colony counter, and the percentage survival of cyanobacteria was determined.
Determination of growth and protein content –
Exponentially growing cultures treated with scytonemin were exposed to UV-B radiation at irradiance of 1 Wm−2 for 0, 2, 4, 6 and 8 h, thereafter incubated in the culture room at 20 ± 2 °C and illuminated with cool white fluorescent light (12 ± 2 Wm−2). Equal volume samples were removed at desired time intervals, and optical density was estimated at 750 nm in a double-beam spectrophotometer (U-2910, UV–Vis, Hitachi, Tokyo, Japan). Cell density was determined at regular intervals for 10 days. The method of Lowry et al. (1951) was utilized for measuring the concentration of total soluble protein in the extracts taking bovine serum albumin (BSA) as standard.
Determination of pigment contents –
Methanol (90% v/v) was used for extraction of pigments, and the absorption spectra of all samples were measured in a double-beam spectrophotometer in the wavelength range of 200–800 nm using quartz cuvettes. Chl a and carotenoids were determined as per the methods of Tandeau de Marsac and Houmard (1988) and Myers and Kratz (1955), respectively.
Determination of photosystem II efficiency –
The photosynthetic efficiency of the cyanobacterial samples (treated and control) was measured with a pulse amplitude-modulated fluorometer (PAM-2500, Heinz Walz GmbH, 2008, Effeltrich, Germany) after 0-, 2-, 4-, 6- and 8-h exposure. Complete oxidation of PSII reaction centers was accomplished by incubating the cultures in the dark for 30 min, and the variable-to-maximal-fluorescence ratio (Fv/Fm) for Chl a of photosystem II (PSII) was measured in those dark-adapted samples.
Determination of nitrate reductase (NR) and glutamine synthetase (GS) activity –
In vivo NR activity was estimated as described by Camm and Stein (1974). Nitrite produced was determined by the method of Lowe and Evans (1964) based on diazo coupling. Prior to irradiation, cultures were supplemented with 5 mM KNO3 maintaining a pH of 7.5 with overnight incubation. During UV-B exposure, at desired intervals, 1 mL aliquots were withdrawn to be mixed with sulfanilamide. After an interval of 10 min, N-(1-naphthyl) ethylenediamine (NED) was added to the mixture and the absorbance of the developed color (pink) was detected at 540 nm spectrophotometrically. The enzyme activity was expressed as µM NO2- mg−1 protein min−1. The method of Sampaio et al. (1979) was followed for in vivo measurement of GS activity, the calculation of which was based on the formation of γ-glutamyl hydroxamate from a known amount of cyanobacterial cultures after a fixed time interval. The enzyme activity was expressed as µM γ-glutamyl hydroxamate mg−1 protein min−1.
Antioxidant function of scytonemin – In vivo ROS scavenging assay –
A molecular probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) dye (Sigma-Aldrich, Merck, USA) was used for the detection of in vivo ROS generation. The ethanol-solubilized DCFH-DA (5 μM final concentration) was added to both treated and untreated cultures. These samples were incubated for 1 h at room temperature on a shaker in the dark (He and Häder 2002; Rastogi et al. 2010; Rajneesh et al. 2017, 2019). DCFH has an excitation wavelength of 485 nm and an emission band between 500 and 600 nm. Samples were subjected to fluorescence microscopy and fluorescence spectrophotometric analysis after 1-h incubation.
Fluorescence microscopy and fluorescence spectrophotometry –
Fluorescence images of Nostoc sp. strain HKAR-2 were taken with a fluorescence microscope (Nikon Eclipse 90I, Japan). The microscope was equipped with the following filters: UV (DAPI; EX/EM 340/488 nm), blue (FITC; EX/EM 495/510 nm) and green (PI 550; EX/EM 550/650 nm). Cells were imaged in the epifluorescence mode with a 40 × objective lens. The data were analyzed by NIS Element AR analyzer software provided by the manufacturer. Green (G) to red (R) fluorescence intensities were observed for randomly selected regions in the filaments. In addition, the fluorescence of the samples was measured by a spectrofluorophotometer (G9800AA, Agilent Technologies, Cary Eclipse, USA) with an excitation wavelength of 485 nm and an emission band between 500 and 600 nm. All fluorescence measurements were performed at room temperature.
In vitro antioxidant assay –
The radical scavenging capacity of extracted scytonemin was measured by using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay (Kulisic et al. 2004). The samples (100 µL) of varying amounts of scytonemin (0.33 and 0.66 mg in methanol) were mixed with 1400 µL of 0.1 mM DPPH (in 80% methanol) solution. The mixtures in test tubes were cautiously shaken and incubated for an hour in the dark at room temperature. The decolorization of DPPH radical was visually observed, and a decrease in absorbance of the reaction mixture was measured at 517 nm. Ascorbic acid was taken as a positive control, while a mixture of DPPH solution and methanol was taken as a negative control. Antioxidant capacity for each concentration of scytonemin was expressed as percentage activity in terms of radical scavenging as described earlier by Kulisic et al. (2004). The capability to scavenge DPPH radical was calculated using the following equation: DPPH scavenging activity (%) = [(A1 − A2)/A1] × 100, where A1 is the absorbance of the control and A2 is the absorbance of the test sample.
Statistical analyses –
All the experiments were performed in triplicates (mean ± SD). A one-way analysis of variance was applied to evaluate the significance of the data. Multiple comparisons were made by using the Tukey HSD post hoc test at P ≤ 0.05 to assess the differences among treatments. For statistical analyses, SPSS 16 and SigmaPlot 11 software was used.
3 Results
Analysis and characterization of scytonemin –
In the present investigation, the HPLC chromatogram as well as the absorption spectrum of Lyngbya sp., extracted with 1:1 (v/v) methanol/ethyl acetate, revealed the presence of a single prominent peak of scytonemin having an RT of 1.7 min (Fig. 2a) and a UV absorption maximum (UVλmax) at 386 nm (Fig. 2b). The concentration of total scytonemin was 1.8 mg g−1 dry wt of cyanobacterial mat, as calculated by using an extinction coefficient of 112.5 L g−1 cm−1 at 384 nm. Eluted scytonemin was collected for further characterization. ESI–MS data showed a prominent ion peak of the protonated molecule ([M + H] +) at m/z 545.1 (Fig. 3). The FTIR spectrum of scytonemin (Fig. 4) exhibited a broad absorption at 3431 cm−1 which corresponds to the phenol group. Absorbance at 2940 cm−1 and 2920 cm−1 is typical of C–H stretching vibrations of alkanes. C–O stretching is generally observed at 1000–1320 cm−1. The spectral bands at 1585, 1513, 1385 and 1173 cm−1 are tentatively assigned for (CCH) p-disubstituted aromatic ring, (N=C–C=C) ring mode, (CCN) indole ring and (CC) ring breathing pyrrole, respectively.
UV-blocking function of scytonemin against high-energy UV-B radiation –
The changes in survival (%) of Nostoc sp. strain HKAR-2 observed under different treatments of UV-B, UV-B + 0.4 mM and 0.8 mM scytonemin are shown in Fig. 5. It was evident from our results that survival (%) was inhibited by UV-B exposure and inhibition level was UV-B exposure time dependent. The number of colonies appearing in the control (untreated) was taken as 100%. The cell survival (%) considerably declined after 8 h of exposure to UV-B radiation. Moreover, UV-B reduced cell viability by approximately 55% after 8 h of exposure. It was observed that after 4–8 h of UV-B exposure, cell viability (%) declined significantly (P < 0.05) as compared to the control. However, up to 2-h exposure of UV-B radiation, there was no significant difference (P > 0.05) in cell viability. In our experiment, a significant increase (P < 0.05) of 11% and 33% (higher than that of 8-h treatment of UV-B radiation alone) in survival was observed in the cells treated with 0.4 mM and 0.8 mM concentration of scytonemin, respectively. The effect of scytonemin was found to be concentration dependent as its protective ability enhanced with increasing concentrations.
In addition to survival, growth (absorbance at 750 nm) of the test strain in liquid medium was found to be adversely affected by UV-B radiation. Figure 6a shows the growth of Nostoc sp. strain HKAR-2 cells inhibited by exposure to UV-B radiation. The growth remained constant up to 2 h of UV-B exposure followed by a significant decline (P < 0.05) in the subsequent 8 h of UV-B exposure. Contrary to the control, a significant inhibition (P < 0.05) in growth (30%) was found in the cyanobacterium following 8 h of continuous UV-B exposure, which was partially restored (160% higher than that of 8-h treatment with UV-B radiation alone) following 10-day recovery and thus exhibited a strong tolerance in response to UV-B radiation. However, the treatment with scytonemin significantly (P < 0.05) alleviated UV-induced growth inhibition in the UV exposed cells in comparison with those which were exposed to UV-B radiation alone. In our experiment, a significant increase in growth (P < 0.05) of 19% and 34% (higher than that of 8 h of treatment with UV-B radiation alone) was observed in the cyanobacterial cells treated with 0.4 mM and 0.8 mM concentrations of scytonemin, respectively. However, recovery was slow in 8-h treated cells in comparison with 2-h and 4-h treated cells. The total protein content of Nostoc sp. strain HKAR-2 cells decreased on the exposure of UV-B radiation which varied with duration of exposure (Fig. 6b). In comparison with the control, the protein content was declined to approximately 12% and 50% following UV-B exposure for 2 h and 8 h, respectively. The protein content of cyanobacterial cells increased significantly, following exposure to UV-B radiation (P < 0.05) in cells treated with scytonemin, which showed an increase of 11% and 27% in protein content compared to the UV-B treated cells.
As shown in Fig. 7a, Chl a showed a decreasing trend with increasing duration of exposure to UV-B radiation as compared to the control. However, carotenoids were less affected than Chl a. UV-B exposure enhanced the carotenoid production up to 4 h of UV-B exposure followed by a decrease in the subsequent treatment of UV-B exposure (Fig. 7b). The significant increase (P < 0.05) in carotenoids was 9% and 27%, as compared to the control after 2 h and 4 h of exposure. Compared with the control, Chl a and carotenoids declined significantly (P < 0.05) to approximately 47% and 30%, respectively, after 8 h of continuous UV-B exposure and were partially restored (200% and 90% higher than that of 8-h treatment with UV-B radiation alone) following 10-day recovery. The treatment with 0.4 mM and 0.8 mM concentrations of scytonemin partially increased Chl a and carotenoids to 21% and 43% (higher than that of 8 h of treatment with UV-B radiation alone), respectively, and they were restored significantly (P < 0.05) during the recovery process. The changes in growth, total protein content, Chl a and carotenoids in response to exposure to 1 Wm−2 UV-B and UV-B plus scytonemin and following 10-day recovery are shown in Table 1.
Fv/Fm measurements under different treatments are shown in Fig. 8a. As compared to the control, a decrease of 40% and 70% in Fv/Fm value was observed after UV-B exposure for 6 h and 8 h, respectively. Cells treated with 0.4 mM and 0.8 mM scytonemin showed a significant increase in Fv/Fm value in comparison with UV-B exposed samples, which showed an increase of 6% and 16%, respectively, as compared to 8-h UV-B-treated cells.
Changes in photosynthetic efficiency (Fv/Fm) (a) NR (b) and GS (c) activity of Nostoc sp. strain HKAR-2 in response to exposure time under UV-B radiation and UV-B radiation plus scytonemin. Horizontal line over bars indicates no significant difference (P > 0.05) among treatments. The error bars represent the standard deviation of mean (mean ± SD, n = 3). Similar letters over bars represent homogeneous mean group (P > 0.05)
On exposure of the cells to UV-B radiation, stimulation of NR activity of Nostoc sp. strain HKAR-2 was observed. From the data in Fig. 8b, it is clear that the activity of NR shows stimulation up to 6-h following UV-B exposure and later on it became constant. Compared with the control, NR activity was increased significantly (P < 0.05) to 55% and 145% following UV-B exposure for 2 h and 8 h, respectively. The stimulation of NR activity of cyanobacterial cells following UV-B radiation treatment decreased significantly in the cells treated with scytonemin, which showed a decrease of 10% and 24% in NR activity compared to the 8-h UV-B-treated cells. GS is the main enzyme of ammonia assimilation and plays an important role in the regulation of NR activity, which was also tested to study the effect of UV-B radiation on as its activity. Inhibition of GS activity was observed in response to UV-B exposure (Fig. 8c). There was an about 58% significant (P < 0.05) loss in its activity after 8-h treatment. Cells treated with scytonemin showed a significant increase in GS activity in comparison with UV-B-exposed samples.
Antioxidant function of scytonemin – In vivo detection of free radicals in the filaments of Nostoc sp. strain HKAR-2 was done by using fluorescence microscopy and spectrophotometry after different durations of exposure to UV-B radiation and plus scytonemin. The production of free radicals was enhanced significantly following UV-B radiation (4–8 h); however, the scytonemin-treated cultures showed a significant decrease in the production of ROS as compared to the cultures treated with UV-B alone (Fig. 9). DCF fluorescence was more prominent after 4–8 h of UV-B-treated samples. Moreover, a decreased production of intracellular ROS in scytonemin-treated samples signified the photoprotective role of scytonemin against the oxidative stress induced by UV irradiation. The DCF to chlorophyll auto-fluorescence, green-to-red ratio (G/R) of Nostoc sp. strain HKAR-2 after various combinations of treatments is shown in Fig. 10a. A significant increase in the G/R ratio was observed in the samples treated with UV-B; however, the increase was lower in the samples treated with both concentrations of scytonemin. Similar to the G/R ratio, the DCF fluorescence was also significantly (P < 0.05) higher in the 8 h of UV-B-treated samples in comparison with 0–6-h samples (Fig. 10b). The emission spectra of samples incubated with DCFH-DA after 0–8 h of UV-B exposure showing the emission peak at 523 nm after excitation at 485 nm are illustrated in Fig. 10c, d. The emission spectra also suggested the maximum generation of ROS in 8 h of UV-B-treated samples.
DCF to chlorophyll fluorescence-based green/red (G/R) ratio (a) fluorescence intensities (b) and emission spectra (c, d) of DCF in cells of Nostoc sp. strain HKAR-2 retrieved from fluorescence microscopic analysis and fluorescence spectrophotometer in response to exposure time under UV-B radiation. Scytonemin is used as a photoprotectant. Horizontal line over bars indicates no significant difference (P > 0.05) among treatments. The error bars represent the standard deviation of mean (mean ± SD, n = 3). Similar letters over bars represent homogeneous mean group (P > 0.05)
The DPPH scavenging potential of extracted scytonemin with respect to their doses is given in Fig. 11. The dose-dependent antioxidant activity of scytonemin was 22% and 52% at concentrations of 0.4 and 0.8 mM, respectively, as compared to that of 0.5 mM ascorbic acid used as a positive control. DPPH scavenging activity was observed in both doses of scytonemin as compared to the negative control, which indicates that the scytonemin acts as the potent radical scavenger.
4 Discussion
The aim of present study was to isolate and partially characterize scytonemin, the UV-screening cyanobacterial pigment from Lyngbya sp., and to assess its UV-protective role against UV-B-induced damages. The photoprotective role of scytonemin was studied using various concentrations in terms of survival, growth, photosynthetic pigment contents—Chl a and carotenoids, total protein content, NR and GS activity as well as free radical production in Nostoc sp. strain HKAR-2, a native cyanobacterium of hot springs of Rajgir, India. The HPLC chromatogram of scytonemin extracted from Lyngbya sp. showed the presence of a prominent peak at RT of 1.7 min with a similar absorption maximum (UVλmax) at 386 nm, which was consistent with previous reports (Rath et al. 2012). In the mass spectrum of the extract from Lyngbya sp., the presence of prominent ion at m/z 545.1 was found to be consistent with the data of scytonemin given in online MS spectra of extracts from cyanobacteria (Squier et al. 2004; Rastogi et al. 2013). The long-term treatment with UV-B (4–8 h) causes significant lethal effects on the growth and survival of the cyanobacterium. ROS, which causes a disturbance in photosynthesis, protein synthesis and membrane permeability, is one of the reasons for reduced growth and survival of cyanobacteria under UVR (Rastogi et al. 2011; Singh et al. 2014; Rajneesh et al. 2019). In photosynthetic organisms, their density or cell number, Chl a content, biomass and total protein content are indicators or parameters for measurements of their growth. In our study, the growth of Nostoc sp. strain HKAR-2 decreased significantly under UV-B treatment in comparison with the control. The growth response of Nostoc sp. strain HKAR-2 to UV-B radiation showed significant differences possibly due to a varying degree of damage inflicted to the cyanobacterial cells either indirectly or directly on proteins, DNA and the photosynthetic apparatus (Melis et al. 1992; Friso et al. 1994; Callaghan et al. 2004; Rajneesh et al. 2019). The presence of the UV-protecting pigment scytonemin and its UV-screening/absorbing function have been demonstrated in several cyanobacterial strains, and varying levels of such UV-absorbing compounds in different species of cyanobacteria may be responsible for their different levels of sensitivity to UVR (Garcia-Pichel et al. 1992). The presence of a sheath, antioxidative machinery and DNA repairing systems also determines such responses in cyanobacteria (He and Häder 2002; Pathak et al. 2019b). However, the combination of UV-B and scytonemin had less deleterious effects in comparison with UV-B radiation alone, which indicates that scytonemin acted as a UV-B absorber decreasing the irradiance.
In Nostoc sp. strain HKAR-2, exposure to UV-B radiation attenuates photosynthetic pigments in order to conserve metabolic energy for carrying out other vital cellular functions. Chlorophylls and carotenoids are negatively affected by elevated UVR, where carotenoids are generally being less affected. The intensive UV-B irradiation shows rapid bleaching of photosynthetic pigments and simultaneously inhibits photosynthesis processes in cyanobacteria (Kannaujiya and Sinha 2017). The damaging effect of UV-B radiation on photosynthetic pigments occurs either due to bleaching or via peroxidation mediated by active oxygen (Nultsch and Agel 1986). UVR stimulates the biosynthesis of UV-absorbing compounds and carotenoids, both of which perform a photoprotective function. Photosynthesis inhibition by UV-A radiation can be effectively reduced by scytonemin (indicated by O2 evolution), and it also reduces photobleaching of Chl a (Cockell and Knowland 1999). The present study showed that increasing duration of UV-B exposure resulted in a decrease in Chl a content and an increase in total carotenoids content of the selected cyanobacterium, namely Nostoc sp. strain HKAR-2. Carotenoids protect chlorophylls and photosynthetic membranes from photooxidative damage. Hence, the decrease in carotenoids exhibits adverse effects on Chl a, and the thylakoid membranes, resulting in a decrease in photosynthetic efficiency of cyanobacteria. UVR damages enzymes and proteins, especially which are rich in aromatic amino acids such as tyrosine, tryptophan, histidine and phenylalanine and show strong absorption in the UV range from 270 to 290 nm (Vass 1997). Exposure of UVR and other stresses results in the production of ROS, which may oxidize proteins that disrupt their structural entity, finally leading to the inactivation of proteins (Kulkarni and Golden 1994).
One of the remarkable findings of our study was that after exposure of the cyanobacterium to UV-B, stimulation of the NR activity was observed. However, the exact mechanisms of stimulation of NR under UV-B exposed conditions are not known. NR activity in response to UV-B exposure has been studied in the filamentous heterocystous cyanobacterium Nostoc calcicola Brebisson, and it was found that its activity was stimulated twofold in comparison with the cells exposed to PAR (Kumar et al. 1996). Similar to UV-B, in Oscillatoria princeps Vaucher ex. Gomont stimulation of NR activity was found by exposure of blue light to the cells (Kumar et al. 1986). Increased NR activity in vivo was found in a hot-spring cyanobacteria Scytonema sp. strain HKAR-3 and Nostoc sp. strain HKAR-2 following UV-B exposure (Richa et al. 2013a, b).
UV-B irradiation also affects the GS enzyme, which incorporates N into C skeletons for the production of amino acids (Döhler 1992). Similarly, UV-B causes a significant reduction in GS activity as observed in Nostoc calcicola (Kumar et al. 1996). Inhibition of GS activity by UV-B supports the finding of Döhler (1986) in Thalassiosira rotula Meunier who demonstrated that reduced GS activity by UV-B irradiation resulted in a tremendous decrease in the glutamine pool of the cells. In Anabaena doliolum Bharadwaja, exposure of UV-B radiation resulted in decreased GS activity, which may be related to a direct action of stress factors on the enzyme complexes (Rai et al. 1998). It is well known that exposure of UV-B radiation generates free radicals in cyanobacteria. Our experiment revealed that maximum production of free radicals was found in the samples treated with UV-B exposure, whereas significant inhibition of free radicals was observed in the samples treated with scytonemin. The photoprotective role of scytonemin was very well evidenced in our study as the addition of scytonemin decreased cell damage significantly. Scytonemin acts as an antioxidant by preventing cellular damage occurring from ROS produced upon UVR exposure (Takamatsu et al. 2003; Matsui et al. 2012). Scytonemin exhibited photoprotective ability by scavenging the ROS generated in vivo upon UVR exposure by reducing the thymine dimer formation (Rastogi et al. 2013). Owing to its efficient UV-absorbing ability as well as its antiproliferative and non-toxic properties, scytonemin finds immense importance in cosmetic and pharmaceutical industries (Stevenson et al. 2002a, b). In the present investigation, the cyanobacterium Lyngbya sp. was found to be a potent producer of the high value and small biomolecule anti-UV pigment, scytonemin. Scytonemin has UV-screening as well as antioxidant properties. Moreover, scytonemin being a highly stable and non-toxic pigment having significant efficacy against UV-B induced harmful effects and associated damages as indicated in the present investigation and thus can serve as an active ingredient in cosmetics and other biomedical industries.
References
Callaghan TV, Björn LO, Chemov Y, Chapin T, Christensen TR, Huntley B, Ims RA, Johansson M, Jolly D, Jonasson S, Matveyeva N, Panikov N, Oechel W, Shaver G, Elster J, Jonsdóttir IS, Laine K, Taulavuori K, Taulavuori E, Zockler C (2004) Responses to projected changes in climate and UV-B at the species level. Ambio 33:418–435. https://doi.org/10.1579/0044-7447-33.7.418
Camm EL, Stein JR (1974) Some aspects of the nitrogen metabolism of Nadularia spumigena (Cyanophyceae). Can J Bot 52:719–726. https://doi.org/10.1139/b74-093
Cockell CS, Knowland J (1999) Ultraviolet radiation screening compounds. Biol Rev 74:311–345. https://doi.org/10.1017/S0006323199005356
Cockell CS, Rettberg P, Rabbow E, Olsson-Francis K (2011) Exposure of phototrophs to 548 days in low Earth orbit: microbial selection pressures in outer space and on early earth. ISME J 5:1671–1682. https://doi.org/10.1038/ismej.2011.46
Desikachary TY (1959) Cyanophyta. Indian Council of Agricultural Research, New Delhi
Döhler G (1986) Impact of UV-B radiation on [15N] ammonia and [15N] nitrate uptake of Dictylum brightweilli. Photobiochem Photobiophys 11:115–121
Döhler G (1990) Effect of UV-B (290–320 nm) radiation on uptake of 15N-nitrate by marine diatoms. In: Ullrich WR, Rigano C, Fuggi A, Aparicio PJ (eds) Inorganic nitrogen in plants and microorganisms: uptake and metabolism. Springer, Berlin, pp 349–354. https://doi.org/10.1007/978-3-642-75812-6_52
Döhler G (1992) Impact of UV-B radiation on uptake of 15N-ammonia and 15N nitrate by phytoplankton of the Wadden sea. Mar Biol 112:485–489. https://doi.org/10.1007/BF00356294
Friso G, Spetea C, Giacometti GM, Vass I, Barbato R (1994) Degradation of photosystem II reaction center D1 protein induced by UV-B radiation in isolated thylakoids: identification and characterization of C and N terminal break down products. Biochim Biophys Acta 1184:78–84. https://doi.org/10.1016/0005-2728(94)90156-2
Garcia-Pichel F, Castenholz RW (1991) Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J Phycol 27:395–409. https://doi.org/10.1111/j.0022-3646.1991.00395.x
Garcia-Pichel F, Sherry ND, Castenholz RW (1992) Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chlorogloeopsis sp. Photochem Photobiol 56:17–23. https://doi.org/10.1111/j.1751-1097.1992.tb09596.x
He YY, Häder D-P (2002) Reactive oxygen species and UV-B: effect on cyanobacteria. Photochem Photobiol Sci 1:729–736. https://doi.org/10.1039/B110365M
Kannaujiya VK, Sinha RP (2017) Impacts of diurnal variation of ultraviolet-B and photosynthetically active radiation on phycobiliproteins of the hot-spring cyanobacterium Nostoc sp. strain HKAR-2. Protoplasma 254:423–433. https://doi.org/10.1007/s00709-016-0964-0
Komárek J, Kastovsky J, Mares J, Johansen JR (2014) Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 86:295–335
Kulisic T, Radonic A, Katalinic V, Milos M (2004) Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem 85:633–640
Kulkarni RD, Golden SS (1994) Adaptation to high light intensity in Synechococcus sp. strain PCC 7942: regulation of three psbA genes and two forms of the D1 protein. J Bacteriol 176:959–965. https://doi.org/10.1128/jb.176.4.959-965.1994
Kumar HD, Jha M, Kumar A (1986) Stimulation of nitrate reductase activity by blue light in a thermophilic cyanobacterium Oscillatoria princeps. Br Phycol J 21:165–168. https://doi.org/10.1080/00071618600650191
Kumar A, Sinha RP, Häder D-P (1996) Effect of UV-B on enzymes of nitrogen metabolism in the cyanobacterium Nostoc calcicola. J Plant Physiol 148:86–91. https://doi.org/10.1016/S0176-1617(96)80298-7
Lowe RH, Evans HJ (1964) Preparation and some properties of a soluble nitrate reductase from Rhizobium japonicum. Biochem Biophys Acta 85:377–389
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Matsui K, Nazifi E, Hirai Y, Wada N, Matsugo S, Sakamoto T (2012) The cyanobacterial UV-absorbing pigment scytonemin displays radicals scavenging activity. J Gen Appl Microbiol 58:137–144. https://doi.org/10.2323/jgam.58.137
Melis A, Nemson JA, Harrison MA (1992) Damage to functional components and partial degradation of photosystem II reaction center proteins upon chloroplast exposure to ultraviolet-B radiation. Biochim Biophys Acta 1100:312–320. https://doi.org/10.1016/0167-4838(92)90487-X
Myers J, Kratz WA (1955) Relationship between pigment content and photosynthetic characteristics in blue-green algae. J Gen Physiol 39:11–21. https://doi.org/10.1085/jgp.39.1.11
Newton JW, Tyler DD, Slodki ME (1979) Effect of ultraviolet-B (280–320 nm) radiation on blue-green algae (cyanobacteria), possible biological indicators of stratospheric ozone depletion. Appl Environ Microbiol 37:1134–1141
Nultsch W, Agel G (1986) Fluence rate and wavelength dependence of photobleaching in the cyanobacterium Anabaena variabilis. Arch Microbiol 144:268–271. https://doi.org/10.1007/BF00410961
Olsson-Francis K, Watson JS, Cockell CS (2013) Cyanobacteria isolated from the high-intertidal zone: a model for studying the physiological prerequisites for survival in low Earth orbit. Int J Astrobiol 12:292–303. https://doi.org/10.1017/S1473550413000104
Pathak J, Sonker AS, Richa Rajneesh, Kannaujiya VK, Singh V, Ahmed H, Sinha RP (2017) Screening and partial purification of photoprotective pigment scytonemin from cyanobacterial crusts dwelling on the historical monuments in and around Varanasi, India. Microbiol Res (Pavia) 8:6559. https://doi.org/10.4081/mr.2017.6559
Pathak J, Pandey A, Maurya PK, Rajneesh Sinha RP, Singh SP (2019a) Cyanobacterial secondary metabolite scytonemin: a potential photoprotective and pharmaceutical compound. Proc Natl Acad Sci India Sect B Biol Sci. https://doi.org/10.1007/s40011-019-01134-5
Pathak J, Ahmed H, Singh PR, Singh SP, Häder DP, Sinha RP (2019b) Mechanisms of photoprotection in cyanobacteria. In: Mishra AK, Tiwari DN, Rai AN (eds) cyanobacteria. Academic Press, New York, pp 145–171. https://doi.org/10.1016/b978-0-12-814667-5.00007-6
Rai LC, Tyagi B, Rai PK, Mallick N (1998) Interactive effects of UV-B and heavy metals (Cu and Pb) on nitrogen and phosphorus metabolism of a N2-fixing cyanobacterium, Anabaena doliolum. Environ Exp Bot 39:221–231. https://doi.org/10.1016/S0098-8472(98)00011-2
Rajneesh Pathak J, Chatterjee A, Singh SP, Sinha RP (2017) Detection of reactive oxygen species (ROS) in cyanobacteria using the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). Bio-protocol 7:e2545. https://doi.org/10.21769/BioProtoc.2545
Rajneesh Pathak J, Richa Häder D-P, Sinha RP (2019) Impacts of ultraviolet radiation on certain physiological and biochemical processes in cyanobacteria inhabiting diverse habitats. Environ Exp Bot 161:375–387. https://doi.org/10.1016/j.envexpbot.2018.10.037
Rastogi RP, Incharoensakdi A (2014) Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol Ecol 87:244–256. https://doi.org/10.1111/1574-6941.12220
Rastogi RP, Singh SP, Häder D-P, Sinha RP (2010) Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem Biophys Res Commun 397:603–607. https://doi.org/10.1016/j.bbrc.2010.06.006
Rastogi RP, Singh SP, Häder D-P, Sinha RP (2011) Ultraviolet-B-induced DNA damage and photorepair in the cyanobacterium Anabaena variabilis PCC 7937. Environ Exp Bot 74:280–288. https://doi.org/10.1016/j.envexpbot.2011.06.010
Rastogi RP, Kumari S, Richa Han T, Sinha RP (2012) Molecular characterization of hot spring cyanobacteria and evaluation of their photoprotective compound. Can J Microbiol 58:719–727. https://doi.org/10.1139/w2012-044
Rastogi RP, Sinha RP, Incharoensakdi A (2013) Partial characterization, UV-induction and photoprotective function of sunscreen pigment, scytonemin from Rivularia sp. HKAR-4. Chemosphere 93:1874–1878. https://doi.org/10.1016/j.chemosphere.2013.06.057
Rath J, Mandal S, Adhikary SP (2012) Salinity induced synthesis of UV-screening compound scytonemin in the cyanobacterium Lyngbya aestuarii. J Photochem Photobiol B 115:5–8. https://doi.org/10.1016/j.jphotobiol.2012.06.002
Richa Kumari S, Kannaujiya VK, Mishra S, Sinha RP (2013a) Response of a hot-spring cyanobacterium Scytonema sp. strain HKAR-3 to ultraviolet-B radiation. Int J Curr Biotechnol 1:32–36
Richa Kannaujiya VK, Kumari S, Mishra S, Sinha RP (2013b) Effects of ultraviolet-B radiation on a hot-spring cyanobacterium Nostoc sp. strain HKAR-2. Acta Biol Ind 2:265–276
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure culture of cyanobacteria. J Gen Microbiol 111:1–61
Sampaio MJ, Rowell AMP, Stewart WDP (1979) Purification and some properties of glutamine synthetase from the nitrogen-fixing cyanobacteria Anabaena cylindrica and a Nostoc sp. J Gen Microbiol 111:181–191. https://doi.org/10.1099/00221287-111-1-181
Singh SP, Rastogi RP, Häder D-P, Sinha RP (2014) Temporal dynamics of ROS biogenesis under simulated solar radiation in the cyanobacterium Anabaena variabilis PCC 7937. Protoplasma 251:1223–1230. https://doi.org/10.1007/s00709-014-0630-3
Sinha RP, Häder D-P (1998) Effects of ultraviolet-B radiation in three rice field cyanobacteria. J Plant Physiol 153:763–769. https://doi.org/10.1016/S0176-1617(98)80232-0
Sinha RP, Kumar HD, Kumar A, Häder D-P (1995) Effects of UV-B irradiation on growth, survival, pigmentation and nitrogen metabolism enzymes in cyanobacteria. Acta Protozool 34:187–192
Sinha RP, Klisch M, Gröniger A, Häder D-P (1998) Ultraviolet absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J Photochem Photobiol B 47:83–94. https://doi.org/10.1016/S1011-1344(98)00198-5
Squier AH, Airs RL, Hodgson DA, Keely BJ (2004) Atmospheric pressure chemical ionization liquid chromatography/mass spectrometry of the ultraviolet screening pigment scytonemin: characteristic fragmentations. Rapid Commun Mass Spectrom 18:2934–2938. https://doi.org/10.1002/rcm.1714
Stevenson CS, Capper EA, Roshak AK, Marquez B, Eichman C, Jackson JR, Mattern M, Gerwick WH, Jacobs RS, Marshall LA (2002a) The identification and characterization of the marine natural product scytonemin as a novel antiproliferative pharmacophore. J Pharmacol Exp Ther 303:858–866. https://doi.org/10.1124/jpet.102.036350
Stevenson CS, Capper EA, Roshak AK, Marquez B, Grace K, Gerwick WH, Jacobs RS, Marshall LA (2002b) Scytonemin-a marine natural product inhibitor of kinases key in hyperproliferative inflammatory diseases. Inflamm Res 51:112–114. https://doi.org/10.1007/BF02684014
Takamatsu S, Hodges TW, Rajbhandari I, Gerwick WH, Hamann MT, Nagle DG (2003) Marine natural products as novel antioxidant prototypes. J Nat Prod 66:605–608. https://doi.org/10.1021/np0204038
Tandeau de Marsac N, Houmard J (1988) Complementary chromatic adaptation: physiological conditions and action spectra. Methods Enzymol 167:318–328. https://doi.org/10.1016/0076-6879(88)67037-6
Tyagi R, Srinivas G, Vyas D, Kumar A, Kumar HD (1992) Differential effect of ultraviolet-B radiation on certain metabolic processes in a chromatically adapting Nostoc. Photochem Photobiol 55:401–407. https://doi.org/10.1111/j.1751-1097.1992.tb04254.x
Vass I (1997) Adverse effects of UV-B light on the structure function of photosynthetic apparatus. In: Pessarakli M (ed) Handbook of photosynthesis. Dekker, New York, pp 931–949
Vincent WF, Neale PJ (2000) Mechanisms of UV damage to aquatic organisms. In: de Mora SJ, Demers S, Vernet M (eds) The effects of UV radiation on marine ecosystems. Cambridge University Press, Cambridge, pp 149–176. https://doi.org/10.1017/cbo9780511535444.007
Williamson CE, Zepp RG, Lucas RM, Madronich S, Austin AT, Ballaré CL, Norval M, Sulzberger B, Bais AF, McKenzie RL, Robinson SA, Häder D-P, Paul ND, Bornman JF (2014) Solar ultraviolet radiation in a changing climate. Nat Clim Change 4:434–441. https://doi.org/10.1038/nclimate2225
Acknowledgements
Abha Pandey (09/013(0619)/2016-EMR-I), Jainendra Pathak (09/013/0515/2013-EMR-I), Deepak K. Singh (09/013(0612)/2015-EMR-I) and Vidya Singh (09/013(0568)/2014-EMR-I) are thankful to Council of Scientific and Industrial Research (CSIR), New Delhi, India, for the financial support in the form of fellowships. University Grant Commission, New Delhi, India, is thankfully acknowledged for providing funds in the form of fellowship to Haseen Ahmed (UGC-JRF-21/12/2014 (ii) EU-V). Deepak Kumar (DST/INSPIRE Fellowship/2015/IF150191) is thankful to the Department of Science and Technology, Inspire Programme, New Delhi. We are thankful to the Interdisciplinary School of Life Sciences (ISLS), BHU, Varanasi, India, for providing access to the fluorescence microscopy facility.
Author information
Authors and Affiliations
Contributions
AP performed the experiments, analyzed experimental results and wrote the manuscript; JP helped in writing the paper; DKS performed the statistical analysis; HA, VS and DK helped in the experimental work; RPS designed the experiments, provided laboratory facilities and reviewed the manuscript. The authors accepted the final version of our manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pandey, A., Pathak, J., Singh, D.K. et al. Photoprotective role of UV-screening pigment scytonemin against UV-B-induced damages in the heterocyst-forming cyanobacterium Nostoc sp. strain HKAR-2. Braz. J. Bot 43, 67–80 (2020). https://doi.org/10.1007/s40415-020-00589-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-020-00589-5