Abstract
Climate changes (droughts and floods) and anthropogenic activities (industrialization, urbanization, and growth of population) have significantly increased concerns about detrimental effects of pollutants on health and environment. Among the heavy metal ions, lead (II) ions are especially toxic and hazardous. Here, we report the application of purple photosynthetic bacteria in biomonitoring of lead pollution in aqueous habitats. The monitoring method is based on (steady state and flash-induced) light absorption and (induction and relaxation of) bacteriochlorophyll fluorescence of living microorganism to prompt appearance of lead ions in the solution. The Pb(II) ions penetrate the cell membrane immediately, attack and (in few mM external concentration) destroy the light harvesting system together with the reaction center protein. The purple bacterium Rubrivivax gelatinosus is about 1.000-times less sensitive to lead(II) than to mercury(II) ions. As these bacteria may function as bioaccumulators of lead, they can be also used for bioremediation of contaminated cultures. The advantages using photosynthetic bacteria for monitoring and accumulating Pb(II) pollution in aqueous environmental compartments are presented in the paper.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last two decades the world has experienced an abrupt change in climate including extreme weather-related events like heat waves, droughts, forest fires, changes of the pattern of precipitation resulting in a rise of the sea levels or in more frequent occurrence of floods. Flood events can disperse heavy metal ions (lead, mercury, cadmium, etc.) from mines and industrial areas to agricultural and residential land. The release of highly toxic and hazardous heavy metal ions into the biosphere has increased today to alarming levels (Tchounwou et al., 2012) and poses a significant threat to agricultural production. Several cases were reported. As a result of widespread flooding in west Wales in June 2012, the overbank sediment was contaminated up to 1900 mg/kg of sediment by Pb, which caused cattle poisoning and mortality. Because of climate related increases in flooding this problem is likely to continue and intensify (Foulds et al., 2014; Valencia-Avellan et al., 2017). Toxic sludge that spilled out of open pit coal mines during 10 days of heavy rains in 2015 contaminated seriously farmland, rivers, and coastal areas in northern Vietnam. The emanated hazardous arsenic, cadmium and lead caused immediate and ongoing health and environmental hazards (Ngoc et al., 2020). The discharge of high flood water into reservoirs located in the Upper Vistula River (Poland) catchment and into the overloaded Vistula River channel strongly contaminated the flooded area in 2010 (Strzebońska et al., 2015). The metal concentrations varied in wide ranges (mg/kg sediment) for small size fraction < 20 μm: Pb 17–263, Zn 59-1013, and Cd 2.6–23.
Out of the non-biodegradable heavy metal pollutants, lead(II) ions have special significance because even small amounts of lead can cause serious health problems like anaemia, weakness, and kidney and brain damage. The lead exposure can originate not only from floods caused by climate change but from wide range of human activities including the glass and metal industries, batteries, paints, pigments and ammunition, cables, alloys and steels, plastics, and petrol with anti-knocking agent. Water from industrial effluents, vehicular traffic and mixing of roadside run-offs is heavily contaminated by lead and its compounds. Due to different physiological disorders and toxicological effects caused to humans, the permissible level of lead contamination in drinking water (World Health Organization limit) is as low as 10 µg/l ≈ 50 nM.
Large efforts have been undertaken to investigate the behaviour of lead in different ecosystems (particularly water, due to pollution by floods or industrial wastewater) and to work out strategies for its control, abatement, and removal. Several analytical techniques have been applied to the assay of lead (Rose et al., 2001). Voltammetry provided a reliable and cost-effective technique for its monitoring, especially in drinking water (Mouhamed et al., 2018). Different chemical methods including reduction and precipitation, ion-exchange, electrolysis, and adsorption have been used for the removal of lead ions from water. In addition to the chemical methods, biological techniques are also available to detect and to remove the toxic heavy metal pollutants (Giotta et al., 2006). The induction of fluorescence of bacteriochlorophyll from photosynthetic purple bacteria (PPB) was successfully used to monitor the level of mercury(II) ions in aqueous habitats (Kocsis et al., 2010; Kis et al. 2017; Sipka et al., 2018a).
The present study was carried out to demonstrate the possibility of detection (biomonitoring) of Pb(II) ions by PPB in aqueous solutions and to assess the potential of these microorganisms to concentrate and to remove (bioremediate) lead contamination from the aqueous environment.
Experimental
Bacterial strains and growth conditions
The photosynthetic purple bacterium Rubrivivax (Rvx.) gelatinosus were grown in Siström’s medium in filled screw top vessels without oxygen (photoheterotrophic and anaerobic growth). The medium was inoculated from a dense batch culture (1:100) and was illuminated by tungsten lamps that assured 13 W m−2 irradiances on the surface of the vessel as described earlier (Maróti & Wraight, 1988).
Chemicals
The cells were harvested at the exponential phase of the growth and the bacterial culture (10 mM Tris, pH 8.0) was bubbled by nitrogen for 15 min before measurements. Variable amounts of Pb(CH3COO)2*3H2O (Pb(II)-acetate) were added to the bacterial culture for heavy metal ion treatment (Giotta et al., 2006). These chemicals are highly soluble in aqueous solution under physiological conditions. 100 mM Pb(CH3COO)2*3H2O stock solution was prepared freshly before the experiment. The durations of the Pb(II)-acetate treatments were prompt. Due to Tris buffer, addition of Pb(II)-acetate did not cause significant drop the pH of the solution.
Optical assays
Steady-state absorption spectrum
The absorption spectra of intact cells were measured by dual beam spectrophotometer with reference to scattering suspension of sand of similar size (~ 5 μm) and concentration (~ 1×108 particles/mL) as those of the bacteria.
Flash-induced absorption change kinetics
The kinetics of absorption changes of the whole cells induced by Xe flash were detected by a home-constructed spectrophotometer (Maróti & Wraight, 1988). The electrochromic shift of the carotenoids in the photosynthetic membrane were detected at 530 nm wavelength with reference to 510 nm wavelength.
Induction and relaxation of bacteriochlorophyll (BChl) fluorescence
The induction and subsequent decay of the BChl a fluorescence of intact cells was measured by a home built fluorometer (Kocsis et al., 2010). The light source was a laser diode (808-nm wavelength and 2 W light power) that produced rectangular shape of illumination and matched the 800 nm absorption band of the LH2 peripheral antenna of the cells. The BChl a fluorescence (centred at 900 nm in mature cells) was detected in the direction perpendicular to the actinic light beam, with a near infrared sensitive, large area (diameter 10 mm) and high gain Si-avalanche photodiode (APD; model 394-70-72-581; Advanced Photonix, Inc., USA) protected with an 850-nm high-pass filter (RG-850) from the scattered light of the laser. The usually very small deviation of the kinetics of the excitation from the rectangular shape was corrected by detection of the kinetics of extracted BChl a in organic solvent. The induction of fluorescence rise was measured during the actinic laser light and the subsequent dark relaxation was tested by attenuated short (halfwidth 3 µs) laser pulses distributed according to geometrical series in time.
Results and discussion
The novelty of this study is the use of purple phototrophic bacteria (PPB) for early and sensitive detection of heavy metal (here lead) pollution in aqueous systems including floods and waste streams. These microorganisms are widespread across the phylum of Proteobacteria. The mechanisms of free energy conversion from light to other forms of free energies attributed to redox species, ion-gradient across the membrane and phosphorylation have been principally revealed (Maróti & Govindjee, 2016). They can show up diversified metabolic capability as the bacteria are able to grow photo/chemoorganoheterotrophically or photo/chemolithoautotrophically, or in a combination of both. They use various electron donors, carbon and nitrogen sources, and light as primary energy source but can also involve chemical redox reactions like fermentations and respiration to generate ATP, e.g., in the dark. When growing photoorganoheterotrophically, PPB use light as an energy source allowing to power their metabolic bioprocesses. In addition, most organic compounds found in these streams, from amino acids to volatile fatty acid and organic acids, can be used by PPB as electron donors and carbon sources. This high metabolic flexibility makes PPB potentially suitable not only for biomonitoring and bioremediation of floods but more generally, for the development of biotechnological solutions in the context of creating a circular, eco-friendly, and biobased economy (Winans et al., 2017).
We utilized here part of the widespread benefits of PPB by following the changes of the physiological properties of Rubrivivax (Rvx.) gelatinosus upon addition of Pb(II) acetate pollutant to the aqueous culture. The variations (mainly disintegration) of the structure and function of the bacteria can be nicely tracked by optical methods including the absorption and fluorescence characteristics of the main pigments (bacteriochlorophyll (BChl) and carotenoids) and reaction center (RC) protein of the microorganism. Additional advantage of the investigations is that they can be carried out in whole cells, and there is no need to prepare membrane fragments (chromatophores). The importance of use of Rvx. gelatinosus in these experiments is further enhanced by the fact that, in response to environmental changes, the bacteria can switch from a planktonic lifestyle to a phototrophic biofilm. Like in critical phenomena, the colonization and sedimentation of the cells is abrupt and hard to predict causally but some essential features to understanding the microbial turnover of aggregates have been revealed (Kis et al., 2018). The possibility of biofilm formation of the bacteria opens the way to collect the pollutants attached to and accumulated by the PPB.
Figure 1 shows the changes of the steady state red absorption spectrum of Rvx. gelatinosus upon lead treatment of different concentrations. The bacteria responded to the poisoning by prompt (relative to the time of addition and mixing) and proportional (to the lead concentration) changes of the absorption spectrum. The red absorption bands characteristic of the peripheral (LH2, 800 and 860 nm) and core (LH1/RC, shown as shoulder at 875 nm) antenna complexes demonstrated prompt decomposition after treatment with lead acetate in the few mM concentration range. The loss of the pigments was not specific as the disintegration affected uniformly the pigment systems. The bacterium showed less sensitivity of damage to lead than that to mercury. While the I50 values (half lethal dose) of PPB exposed to prolonged heavy metal ion contamination are low (e.g., I50 = 2 µM for Hg2+, Giotta et al., 2006; Kocsis et al., 2010; Kis et al. 2017), prompt addition of lead to the culture evoked much (about 103 times) less changes probably due to small equilibrium binding constant of lead to the cell wall. The equilibration is fast, a small fraction of lead(II) ions can pass the cell wall of the bacteria in the short time range of exposure (prompt effect), and attack immediately the essential protein-pigment complexes including the peripheral and core light harvesting complexes. There is no preferential damage of the macromolecules as the observed rates of BChl degradation due to Pb(II) contamination are the same for the two antenna complexes.
The electrochromic signals due to absorption change of carotenoid pigments evoked by the membrane potential describe similar changes upon exposure to lead(II) ions (Fig. 2). The pigment carotenoids in the membrane serve as molecular voltmeter of the membrane potential: the electric field causes shift of the absorption spectra of the carotenoids therefore absorption change of the pigment can be detected. The magnitude and kinetics (rise and decay) of the optical signal is characteristic of the amount and generation/disappearance of the electric charges induced by light excitation. Figure 2 indicates that the amplitude and not the kinetics of the flash-induced absorption change is sensitive to the Pb(II) treatment. Therefore the magnitude of the membrane potential and not the pathways of appearance and disappearance of the initial charge pairs (leaks) is primarily influenced by lead(II) ions. The photogeneration of primary charges in the reaction center becomes less and less efficient upon increase of lead concentration. The analysis of the flash-induced electrochromic kinetics offers the same half lethal concentration (~ 2 mM) as that of the destruction of the steady-state absorption spectrum (Fig. 1).
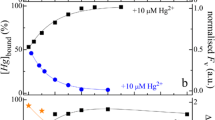
Beside the absorption, the BChl fluorescence is also sensitive to lead(II) contamination and the tools of fluorescence induction developed in the last decades can be routinely utilized (Zhu et al., 2005; Sipka et al., 2018b; Maróti et al., 2020). The magnitude of fluorescence induction (Fmax) shows major changes upon Pb(II) treatment (Fig. 3). The severe drops of Fmax and F0 (the initial fluorescence) indicate substantial loss of BChl pigments together with decoupling of the light harvesting antenna systems from the reaction center (see the drop of the variable fluorescence, Fmax─F0). The half-rise time of the fluorescence induction (~ 30 µs), which indicates the effectiveness of the primary photochemistry (light-induced charge separation), did not perform major changes upon Pb(II)-treatment. This is indicative of an “all-or-nothing” response of the photosynthetic apparatus to poisoning by lead(II)-ions: the cells remained either active or were demolished.
Lead-dependent changes of the maximum fluorescence (Fmax) and kinetics of BChl fluorescence (F) induction (inset) in intact cells of photosynthetic bacteria Rvx. gelatinosus grown in the light upon addition of Pb(II) acetate to the culture. The fluorescence (detected at 900 nm) was excited by laser light (808 nm) of rectangular shape (step function in time). The kinetic traces were normalized to the initial F0 fluorescence level of the untreated cells
While the BChl fluorescence induction probes the intactness of the light harvesting system and primary photochemistry taking place in the reaction center protein, the relaxation of the BChl fluorescence gives information about the pathways of re-opening of the closed RCs in the dark (Fig. 4). This is a sophisticated method which monitors the redox state of the RC by a series of testing flashes fired after the exciting flash (Kocsis et al., 2010). High fluorescence level can be detected if the RC is closed (any combinations of P+ (oxidized BChl dimer) and QA─ (reduced primary quinone acceptor)) and the fluorescence yield becomes low in open state of the RC (PQA). The transition from high to low fluorescence levels gives information about the ways and kinetics of opening of the RC from the closed state. To full opening of the RC, the contribution of both the acceptor and the donor sides is required to re-oxidize QA─ and re-reduce P+, respectively (Asztalos et al., 2015). As Rvx. gelatinosus has a cytochrome subunit attached to the RC, the re-reduction of flash-induced P+ by the cytochromes is extremely fast, therefore the donor side will not be the bottle neck of opening of the closed RC (Kis et al., 2022). What we see on Fig. 4. is the effect of Pb(II) ions on the interquinone electron transfer (QA─ QB→ QA QB─). The normalized variable fluorescence (the magnitude of the trace) performed substantial change as it dropped from 3.0 to 1.5 upon addition of 4 mM lead(II) acetate. This is a clear indication of severe disintegration of the photosynthetic apparatus. The time constant of relaxation of the untreated bacteria (about 100 µs), however, did not show major alteration upon poisoning, as far as it can be deduced from the small signal of the treated bacteria. The fluorescence relaxation measurements also support the all-or-nothing response of the bacteria to the lead exposure: while the untreated bacteria can perform photosynthesis, the treated cells are knocked out.
The different measurements carried out in this study can track and support evidences for the diverse structural and functional changes in the photosynthetic apparatus of the intact cell caused by Pb(II) contamination. Based on this knowledge, practical procedures can be worked out to use PPB to biomonitor and to remediate floods and waste waters contaminated by heavy metals including lead(II)-ions.
Conclusion
There is a need to monitor flood events in detail to quantify accurately contaminant dynamics, and to allow resource managers to prioritise areas for remediation. The implication of such events for aquatic ecology and remediation effectiveness should be identified. The purple photosynthetic bacteria are adequate biological monitors to fulfil this task. Steady state absorption spectra and kinetics of flash-induced absorption changes and fluorescence of intact photosynthetic bacteria are sensitive bioindicators of lead(II) contamination of aqueous cultures. After fast penetration through the cell wall, the Pb(II) ions cause prompt and detectable physiological changes by disconnection and damage of the antenna pigments followed by graduate destruction of the photosynthetic machinery of the bacteria. The recovery of aqueous resources is a currently very active field of research (Puyol et al., 2017) and PPB should play a major role in this scientific and socioeconomic revolution (Capson-Tojo et al., 2020).
References
Asztalos, E., Sipka, G., & Maróti, P. (2015). Fluorescence relaxation in intact cells of photosynthetic bacteria: donor and acceptor side limitations of reopening of the reaction center. Photosynthesis Research, 124(1), 31–44.
Capson-Tojo, G., Batstone, D. J., Grassino, M., Vlaeminck, S. E., Puyol, D., Verstraete, W., & Lema, J. M. (2020). Purple phototrophic bacteria for resource recovery: Challenges and opportunities. Biotechnology Advances, 43, 107567.
Foulds, S. A., Brewer, P. A., Macklin, M. G., Haresig, W., Betson, R. E., & Rassner, S. M. E. (2014). Flood-related contamination in catchments affected by historical metal mining: An unexpected and emerging hazard of climate change. Science of The Total Environment, 476–477, 165–180
Giotta, L., Agostiano, A., Italiano, F., Milano, F., & Trotta, M. (2006). Heavy metal ion influence on the photosynthetic growth of Rhodobacter sphaeroides. Chemosphere, 62, 1490–1499
Kis, M., Sipka, G., & Maróti, P. (2017a). Stoichiometry and kinetics of mercury uptake by photosynthetic bacteria. Photosynthesis Research, 132(2), 197–209
Kis, M., Sipka, G., Ayaydin, F., & Maróti, P. (2018). The biophysics of a critical phenomenon: colonization and sedimentation of the photosynthetic bacteria Rubrivivax gelatinosus. European Biophysics Journal, 47(8), 139–149. https://doi.org/10.1007/s00249-017-1236-4
Kis, M., Smart, J. L., & Maróti, P. (2022). Capacity and kinetics of light-induced cytochrome oxidation in intact cells of photosynthetic bacteria. Nature Scientific Reports, 12, 14298
Kocsis, P., Asztalos, E., Gingl, Z., & Maróti, P. (2010). Kinetic bacteriochlorophyll fluorometer. Photosynthesis Research, 105, 73–82
Maróti, P., & Wraight, C. A. (1988). Flash-induced H+ binding by bacterial photosynthetic reaction centers: comparison of spectrometric and conductometric methods. Biochimica Et Biophysica Acta, 934, 314–328
Maróti, P. & Govindjee (2016). Energy conversion in photosynthetic bacteria. Photosynthesis Research, 127(2), 257–271.
Maróti, P., Kovács, I. A., Kis, M., Smart, J. L., & Iglói, F. (2020). Correlated clusters of closed reaction centers during induction of intact cells of photosynthetic bacteria. Nature Scientific Reports, 10, 14012. https://doi.org/10.1038/s41598-020-70966-3
Mouhamed, N., Cheikhou, K., Rokhy, G. E. M., Bagha, D. M., Guèye, M. D. C., & Tzedakis, T. (2018). Determination of lead in water by linear sweep anodic stripping voltammetry (LSASV) at unmodified carbon paste electrode: Optimization of operating parameters. American Journal of Analytical Chemistry, 9, 171–186.
Ngoc, N. T. M., et al. (2020). Chromium, cadmium, lead, and arsenic concentrations in water, vegetables, and seafood consumed in a coastal area in Northern Vietnam. Environmental Health Insights, 14, 1–9.
Puyol, D., Batstone, D. J., Hülsen, T., Astals, S., Peces, M., & Krömer, J. O. (2017). Resource recovery from wastewater by biological technologies: opportunities, challenges, and prospects. Frontiers in Microbiology, 7, 2106. https://doi.org/10.3389/fmicb.2016.02106
Rose, M., Knaggs, M., Owen, L., & Baxter, M. (2001). A review of analytical methods for lead, cadmium, mercury, arsenic, and tin determination used in proficiency testing. Journal Of Analytical Atomic Spectrometry, 16, 1101–1106
Sipka, G., Kis, M., & Maróti, P. (2018a). Characterization of mercury (II)-induced inhibition of photochemistry in the reaction center of photosynthetic bacteria. Photosynthesis Research, 136(3), 379–392
Sipka, G., Kis, M., Smart, J. L., & Maróti, P. (2018b). Fluorescence induction of photosynthetic bacteria. Photosynthetica, 56(1), 125–131
Strzebońska, M., Kostka, A., Helios-Rybicka, E., & Jarosz-Krzemińska, E. (2015). Original research, effect of flooding on heavy metals contamination of vistula floodplain sediments in cracow; historical mining and smelting as the most important source of pollution. Polish Journal of Environmental Studies, 24(3), 1317–1326.
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2012). Heavy metal toxicity and the environment. In A. Luch (Ed.), Molecular, clinical and environmental toxicology. Experientia supplementum (Vol. 101, pp. 133–164). Basel: Springer.
Valencia-Avellan, M., Slack, R., Stockdale, A., & Mortimer, R. J. G. (2017). Effect of episodic rainfall on aqueous metal mobility from historical mine sites. Environmental Chemistry, 14, 469–475
Winans, K., Kendall, A., & Deng, H. (2017). The history and current applications of the circular economy concept. Renewable and Sustainable Energy Reviews, 68, 825–833
Zhu, X. G., Baker, N. R., deSturler, E., Ort, D. O., & Long, S. P. (2005). Chlorophyll a fluorescence induction kinetics in leaves predicted from a model describing each discrete step of excitation energy and electron transfer associated with Photosystem II. Planta, 223(1), 114–133. https://doi.org/10.1007/s00425-005-0064-4
Acknowledgements
The authors are indebted to Prof. S. Szatmári (University of Szeged, Hungary) for his help to restart the lab. Thanks to COST CA21146.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose any financial or non-financial interests to the present work submitted for publication.
Additional information
Dedicated to Govindjee’s 90th birthday. Thanks for being a gentle and kind mentor to us.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maróti, P., Kis, M. Environmental protection via biomonitoring lead exposure by photosynthetic purple bacteria. Plant Physiol. Rep. 27, 590–595 (2022). https://doi.org/10.1007/s40502-022-00694-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-022-00694-5