Abstract
Poly-γ-glutamic acid (γ-PGA) is an anionic polymer with wide-ranging applications in the areas of medicine, light chemical industry, wastewater treatment, and agriculture. However, the production cost of γ-PGA is high for the requirement of adding the expensive precursor L-glutamic acid during fermentation, which hinders its widespread application. In this study, in order to improve γ-PGA yield, central carbon metabolism was engineered to enhance the carbon flux of tricarboxylic acid (TCA) cycle and glutamic acid synthesis in a γ-PGA production strain Bacillus licheniformis WX-02. Firstly, pyruvate dehydrogenase (PdhABCD) and citrate synthase (CitA) were overexpressed to strengthen the flux of pyruvate into TCA cycle, resulting in 34.93% and 11.14% increase of γ-PGA yield in B. licheniformis WX-02, respectively. Secondly, the carbon flux to glyoxylate shunt was rewired via varying the expression of isocitrate lyase (AceA), and a 23.24% increase of γ-PGA yield was obtained in AceA down-regulated strain WXPbacAaceBA. Thirdly, deletion of pyruvate formate-lyase gene pflB led to a 30.70% increase of γ-PGA yield. Finally, combinatorial metabolic engineering was applied, and γ-PGA titer was enhanced to 12.02 g/L via overexpressing pdhABCD and citA, repressing aceA, and deleting pflB, with a 69.30% improvement compared to WX-02. Collectively, metabolic engineering of central carbon metabolism is an effective strategy for enhanced γ-PGA production in B. licheniformis, and this research provided a promising strain for industrial production of γ-PGA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poly-γ-glutamic acid (γ-PGA) is a natural anionic polymer with numerous valuable properties, such as water solubility, water holding, biodegradability, edibility, non-immunogenicity, and non-toxicity, and it has a variety of applications in the areas of medicine, light chemical industry, wastewater treatment, and agriculture [1]. However, high cost of γ-PGA impedes its large-scale production, which further affects γ-PGA application promotion [2].
The requirement of adding large quantities of expensive L-glutamate in the fermentation medium is a main cause of high cost of γ-PGA production [3]. Thus, improving synthetic capability of glutamic acid has been suggested to be effective strategy for reducing production cost [4]. Recently, many approaches have been implemented to improve the supply of intracellular glutamic acid for γ-PGA production. For instance, deletion of the glutamate dehydrogenase-encoded genes rocR and gudB significantly improved γ-PGA biosynthesis, enabling the engineering of B. amyloliquefaciens LL3 to produce 4.55 g/L γ-PGA, increased by 38% compared with wild type [5]. Introducing an energy-saving NADPH-dependent glutamate dehydrogenase pathway in B. amyloliquefaciens led to a 9% improvement of γ-PGA production [6]. Overexpression of zwf (encodes glucose-6-phosphate dehydrogenase) improved NADPH generation and increased synthetic capability of glutamic acid from α-ketoglutaric acid and eventually enhanced γ-PGA production by 35% in B. licheniformis WX-02 [4].
Metabolic engineering refers to rewiring metabolism of cells for efficiently producing desired products [7]. It is crucial in the construction of strains for the production of platform chemicals, biomaterials, and pharmaceuticals from renewable resources [8]. The central carbon metabolism, including glycolysis (Embden-Meyerhof (EMP) pathway), the pentose phosphate pathway (PPP), and tricarboxylic acid cycle (TCA cycle), plays a critical role in precursor synthesis, energy metabolism, and cofactor balance [1]. Metabolic engineering of carbon metabolic pathways is of great significance for improving the production of many products. Double-deletion mutants of phosphofructokinase (∆pfkA1∆pfkA3) in the EM pathway markedly increased chloramphenicol production by increasing the carbon flux in PP pathway in Streptomyces avermitilis [9]. Directing more carbon flux through oxPP pathway efficiently enhanced provision of acetyl-CoA and NADPH and improved 3-hydroxypropionic acid production by more than 24-fold in Saccharomyces cerevisiae [7]. The fumaric acid yield was effectively improved by engineering glucose uptake system and manipulation of precursor and by-product pathways in Escherichia coli [10]. Systematically engineering of TCA cycle and glyoxylate cycle resulted in high production of 4-hydroxybutyric acid in E. coli [11]. Moreover, GlcNAc titer was increased by 1.59-fold through rewiring central carbon metabolism in B. subtilis [12]. In the previous research of our group, strengthening TCA cycle efficiently improved the supplies of intracellular amino acids for bacitracin synthesis in B. licheniformis DW2 [13]. Glutamic acid involved in γ-PGA synthesis is mainly generated from α-ketoglutaric acid from TCA cycle [14]. Addition of citric acid accelerated the conversion of α-ketoglutaric acid to glutamic acid and eventually improved γ-PGA production [15]. Therefore, metabolic engineering of central carbon metabolic pathways to strengthen the supply of citric acid and glutamic acid is suggested as an efficient strategy to improve γ-PGA production.
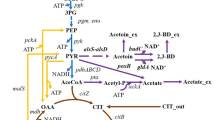
B. licheniformis WX-02 is a well-established producer that can synthesize γ-PGA from glucose [16]. However, the γ-PGA productivity was much lower in the medium without glutamic acid addition [4, 17,18,19], which impedes its production and application. In this study, central carbon metabolism was optimized for efficient production of γ-PGA in B. licheniformis WX-02. Specifically, TCA cycle, glyoxylate shunt and competing pathway were genetically modified to improve γ-PGA yield (Fig. 1). The results of this research suggest that metabolic engineering of central carbon metabolism is an effective strategy for improving the production of glutamate-relevant metabolites.
The schematic diagram of metabolic engineering of central carbon metabolic pathways for efficient production of γ-PGA in B. licheniformis. Abbreviations: EMP, glycolysis; HMP, pentose phosphate pathway; pdh, encoding pyruvate dehydrogenase; citZ and citA, encoding citrate synthase; aceA, encoding isocitrate lyase; aceB, encoding malate synthase; pflB, encoding pyruvate formate-lyase
Materials and Methods
Strains, Plasmids, and Culture Conditions
The strains and plasmids used in this study were listed in Supplementary Table 1. B. licheniformis and E. coli were cultivated at 37 °C in Lysogeny-broth (LB) broth (1% tryptone, 0.5% yeast extract, 1% NaCl, and pH 7.2). The seed culture of B. licheniformis was prepared in a 250 mL flask containing 50 mL LB medium and incubated at 37 °C in a rotatory shaker (240 rpm), until OD600 reached 4.6 ~ 5.0. The seed culture (1.50 mL) was transferred into 250 mL flask containing 50 mL γ-PGA production medium (g/L: glucose 60, sodium nitrate 10, sodium citrate 10, NH4Cl 8, CaCl2 1, K2HPO4·3H2O 1, MgSO4·7H2O 1, ZnSO4·7H2O 1, and MnSO4·H2O 0.15) and shaken at 37 °C and 240 rpm for 36 h. All fermentation experiments were performed in three replicates. The antibiotic kanamycin was added into medium at the final concentration of 20 mg/L, when necessary.
Substitution of Promoter in B. licheniformis
Substitution of the original promoters of pdhABCD, citA, and aceBA were performed according to the previously described method [3]. The procedure for replacing the native promoter of pdhABCD operon by P43 promoter served as an example. Briefly, the P43 promoter was amplified from B. subtilis 168, and homology arms of Ppdh were amplified from chromosomal DNA of B. licheniformis WX-02. These fragments were purified and ligated by Splicing Overlapping Extension PCR (SOE-PCR). And the fused fragment was inserted into T2(2)-Ori vector, generating promoter replacement plasmid, named T2-P43pdh. Then, T2-P43pdh was transformed into WX-02 by high-osmolality electroporation. The positive colonies were cultured in LB medium containing kanamycin (20 mg/L) at 45°C for 8 h to obtain the single-crossover recombinants. The double-crossover recombinants were screened after serial subculture of single-cross recombinants in LB medium at 37°C. The kanamycin-sensitive colonies resulting from double-crossover event were selected and confirmed by DNA sequence.
Gene Deletion in B. licheniformis
To delete pyruvate formate-lyase gene pflB, homology arms of pflB were amplified from B. licheniformis WX-02 genome, respectively. The resulting fragments were ligated by SOE-PCR, and inserted into T2(2)-Ori, resulting in gene knockout plasmid T2-pflB. Furthermore, pflB gene knockout mutant was obtained via homologous double crossover, similar with that of promoter replacement [20].
Determination of Biomass, γ-PGA, and Glucose
The cell biomass was detected by measuring dry cell weight. The γ-PGA concentration was measured by high-performance liquid chromatography (HPLC) according to the method described in previous research [16]. The concentration of residual glucose was measured using a SBA-40C biosensor analyzer (Institute of Biology, Shandong Province Academy of Sciences, P.R. China).
Determination of Enzymatic Activities
Crude extracts of B. licheniformis for measuring the activities of pyruvate dehydrogenase, citrate synthase, and isocitrate lyase were prepared as follows: cells were harvested by centrifugation (4℃, 8000 × g, 5 min) at mid-exponential phase, and cell pellet was washed once with 20 mL buffer (50 mmol/L Tris–HCl, pH 7.4, containing 10 mmol/L β-mercaptoethanol) and resuspended with the same buffer. The suspensions were disrupted by sonication (150 W, 20 kHz, pulse: 1 s on; 2 s off; total: 15 min), and supernatant was collected for enzyme activity analysis. Activity of pyruvate dehydrogenase was determined spectrophotometrically through monitoring the reduction of 2, 6-dichlorophenolindophenol (2, 6-DCPIP) at 600 nm [21]. The citrate synthase (CS) activity was assayed via 5,5-dithiobios-(2-nitrobenzoic acid) (DTNB) method [22]. The isocitrate lyase activity was detected based on the formation of glyoxylic acid phenylhydrazone from glyoxylate and phenylhydrazine at 324 nm [23].
Statistical Analyses
All samples were analyzed in three replicates. The data were presented as the mean value ± the standard deviation for each sample.
Results
Channeling Carbon Flux into TCA Cycle to Enhance γ‑PGA Production
Acting as the precursor for γ-PGA synthesis, glutamic acid supply serves as the critical role in γ-PGA production. Glutamic acid is generated from α-ketoglutaric acid, an important intermediate metabolite in TCA cycle. However, TCA cycle is repressed in the presence of excess glucose, and a large proportion of by-products (such as lactate, acetoin, and 2, 3-butanediol) are converted from silted pyruvate [24], all of which hinder γ-PGA synthesis.
Pyruvate dehydrogenase (PDH), encoded by pdhABCD operon, is an important enzyme that mediates glycolysis pathway to TCA cycle. Previously, overexpression of PDH could strengthen acetyl-coenzyme A (CoA) supply and TCA cycle flux [25]. Here, in order to channel more glycolytic flux into TCA cycle, the origin promoter of pdhABCD operon was replaced by a stronger promoter (P43) in B. licheniformis WX-02, according to the procedure described in the section of “Substitution of promoter in B. licheniformis”, resulting in recombinant strain WXP43pdh. In addition, strains WXPbacApdh and WXPsrfpdh were constructed according to the same method, respectively. As showed in Fig. 2a, PDH activities of WXP43pdh, WXPbacApdh, and WXPsrfpdh were increased by 4.58%, 3.09%, and 8.04%, respectively, compared with that of control strain WX-02. Consistently, γ-PGA yields of WXP43pdh, WXPbacApdh, and WXPsrfpdh were enhanced to 7.44 g/L, 7.38 g/L, and 9.58 g/L, which were 4.79%, 3.94%, and 34.93% higher than that of WX-02 (7.10 g L−1) (Fig. 2b), respectively.
Channeling carbon flux into tricarboxylic acid (TCA) cycle to enhance γ‑PGA production. a Specific PDH activity in different B. licheniformis strains (WX-02, WXP43pdh, WXPbacApdh, and WXPsrfpdh). b The effect of overexpression of pdh on γ‑PGA production. c Specific CS activity in different B. licheniformis strains (WX-02, WXP43citA, WXPbacAcitA, and WXPsrfcitA). d The effect of overexpression of aceA on γ‑PGA production
CS catalyzes the first step of TCA cycle and is generally regarded as the rate-limiting enzyme in TCA cycle. Overexpression of CS redirected more carbon flux towards TCA cycle, which further benefited γ-PGA production [26]. In B. licheniformis WX-02, two genes citZ (encodes CS-II) and citA (encodes CS-I) encode CS [27]. Previous studies revealed that CS-II is inhibited by reduced nicotinamide adenine dinucleotide (NADH), and CS-I is insensitive to NADH [28]. In this study, the native promoter of citA in WX-02 was replaced with promoters P43, PbacA, and Psrf, resulting in the mutants WXP43citA, WXPbacAcitA, and WXPsrf citA, respectively. As shown in Fig. 2c, CS activities in WXPsrf citA and WXP43citA were increased by 87.78% and 22.49%, respectively. And CS activity in WXPbacAcitA was decreased by 26.54% (Fig. 2c). The mutants WXPsrf citA and WXP43citA produced 7.89 g/L and 7.76 g/L γ-PGA, which were 11.14% and 9.26% higher than that of WX-02, respectively (Fig. 2d), while the mutant WXPbacAcitA produced 6.91 g/L γ-PGA, decreased by 2.70% compared to that of WX-02 (Fig. 2d).
Effects of Glyoxylate Shunt on γ-PGA Production
The glyoxylate shunt is a bypass of TCA cycle. And it is indispensable for glutamate overproduction in Corynebacterium glutamicum, since it supplies the key metabolites as well as energy during cell growth phase [29]. However, high flux of glyoxylate shunt might lead to a shortage of ɑ-ketoglutarate, which is not conducive to glutamic acid production [30]. To optimize the carbon flux of glyoxylate cycle, the activities of enzymes involved in glyoxylate shunt were adjusted by replacing the native promoter of aceBA with promoter P43, PbacA, or Psrf, resulting in mutant strains WXP43aceBA, WXPbacAaceBA, and WXPsrfaceBA, respectively. As shown in Fig. 3a, the activities of isocitrate lyase in WXP43aceBA (2.05 U/mL) and WXPsrfaceBA (3.42 U/mL) were increased by 93.40% and 222.64% than that in WX-02 (1.06 U/mL), respectively. However, the activity of isocitrate lyase in WXPbacAaceBA (0.62 U/mL) was decreased by 41.51% (Fig. 3a). The productions of γ-PGA by mutants WXP43aceBA (6.94 g/L) and WXPsrfaceBA (7.10 g/L) exhibited no significant difference from WX-02 (Fig. 3b), while WXPbacAaceBA showed 23.24% increase in γ-PGA yield (8.65 g/L) (Fig. 3b). Collectively, these results indicated that reducing flux of glyoxylate pathway was beneficial for γ-PGA production.
Minimization of Carbon Loss by Deleting pflB
The formation of γ-PGA could increase the viscosity of fermentation broth and decrease oxygen transfer efficiency [17]. Under micro-aerobic condition, most pyruvate is resolved to formate and acetyl-CoA through pyruvate formate-lyase (PFL) [31]. In silico analysis of genome sequence showed that B. licheniformis WX-02 possesses gene pflB that encodes PFL [27]. In this research, the pflB gene was deleted to block the metabolic flux from pyruvate to formate and acetyl-CoA, obtaining mutant strain WXΔpflB. As shown in Fig. 4, WXΔpflB produced 9.28 g/L γ-PGA, 30.70% higher than that of WX-02.
Combinatorial Metabolic Engineering for Enhanced Production of γ-PGA
To further improve γ-PGA production, combinatorial metabolic engineering was applied to overlay the above priority strategies. Firstly, origin promoter of gene citA was replaced with promoter Psrf in WXPsrfpdh, generating strain WX-PA. The γ-PGA yield of WX-PA reached 9.63 g/L, increased by 35.63% compared with that of WX-02 (Fig. 5a). Then, strain WX-PAA was constructed by replacing origin promoter of aceBA operon with promoter PbacA based on strain WX-PA. The WX-PAA strain showed a 53.24% improvement in γ-PGA production (Fig. 5a). Finally, pflB gene was deleted, resulting in final strain WX-PAAB. As shown in Fig. 5b, the glucose consumption of WX-PAAB (36 h) was slower than that of WX-02 (30 h). The maximum cell biomass of WX-PAAB (6.44 g/L) was enhanced by 16.04% compared with that of WX-02 (Fig. 5c). The γ-PGA titer of WX-PAAB increased with the increasing fermentation time, and the maximum titer reached 12.02 g/L at 36 h, increased by 69% compared with that of WX-02 (Fig. 5d). The γ-PGA yield and productivity reached 1.71 g/g cell and 0.33 g/L/h, respectively, significantly higher than those of WX-02 (1.27 g/g cell and 0.20 g/L/h, respectively).
Discussion
γ-PGA is a natural polymer with wide-ranging applications. Currently, microbial synthesis of γ-PGA with Bacillus species relies on supplementation of expensive precursor L-glutamic acid, which sharply increases overall cost [2]. Central carbon metabolism is crucial for cell growth and product synthesis. In this study, we improved γ-PGA production via overexpression of pyruvate dehydrogenase and citrate synthase, weakening the glyoxylic acid cycle, and disrupting the conversion of pyruvate to formate and acetyl-CoA in B. licheniformis WX-02. Our results confirmed that metabolic engineering of central carbon metabolism is an effective strategy for enhanced production of γ-PGA in B. licheniformis.
Glucose is an efficient carbon source for γ-PGA production [1]. It is degraded to pyruvate via EMP pathway. Pyruvate is converted to citrate that is subsequently metabolized to α-ketoglutaric acid through TCA cycle. Then α-ketoglutaric acid is converted to glutamic acid, the precursor for the synthesis of γ-PGA (Fig. 1). Therefore, γ-PGA production can possibly be improved by enhancing the TCA cycle and glutamate synthesis. In general, Bacillus species have a strong overflow metabolic pathway from glucose, owing to the lower activity of TCA cycle and respiratory chain relative to the glucose uptake rate [32]. The TCA cycle is repressed by CcpA-dependent catabolite repression mechanism in the presence of excess glucose [24]. Moreover, the expression of enzymes encoded by alsSD involved in acetoin production is activated by CcpA [33]. Acetoin can be reduced to 2, 3-butanediol by 2, 3-BD dehydrogenase (BDH) with oxidation of NADH to NAD+ [34]. In addition, the high viscosity of γ-PGA hinders the oxygen supply, which prevents the oxidation of NADH via respiratory chain and increases the pool of NADH availability for 2, 3-butanediol generation [17, 35, 36]. Therefore, a large proportion of glucose is metabolized to pyruvate and subsequently converted to acetoin and 2, 3-butanediol that are excreted to the outside of cell as by-products during γ-PGA fermentation, when using glucose as carbon source in B. licheniformis [36, 37]. Reducing the production of acetoin and 2, 3-butanediol was suggested to be beneficial for γ-PGA production [3, 38, 39]. However, deletion of gene alsS (encoded acetolactate synthase) resulted in poor cell growth [40], which was unfavorable for γ-PGA production. Pyruvate is converted to acetyl-CoA under the catalysis of PDH. Overexpression of PDH improved acetyl-CoA supply for TCA cycle [25, 26, 41], which may be beneficial for γ-PGA synthesis. In this study, substitution of the native promoter of pdhABCD operon with stronger promoters (P43, PbacA, and Psrf) successfully enhanced PDH activity (Fig. 2a) and increased the γ-PGA yield by 4.79%, 3.94%, and 34.93%, respectively (Fig. 2b).
The TCA cycle is one of the important metabolic pathways involved in the production of energy. Also, TCA cycle provides precursors for anabolism, such as α-ketoglutaric acid for glutamic acid and oxaloacetate for aspartic acid families [13]. Thus, enhancing TCA cycle was confirmed to be an efficient strategy to improve the supply of ATP and precursors for metabolite production [13, 30, 42]. Overexpression of citA, citB, and icd shifted the carbon flux to α-ketoglutarate, resulting in a slight increase in γ-PGA production in B. subtilis [26]. In the present study, the substitution of stronger promoter Psrf for the native promoter of NADH-insensitive citrate synthase CitA effectively increased the CS activity and enhanced γ-PGA production (Fig. 2c and d). This result was consistent with the previous research [26].
The glyoxylate shunt is an important anaplerotic pathway of TCA cycle for replenishing key metabolites, such as succinate and oxaloacetate [29]. Engineering the glyoxylate bypass has been used to improve the production of various products. Enhancing glyoxylate shunt has been applied to produce succinate [43], acetyl-CoA-derived chemicals (phloroglucinol and 3-hydroxypropionate) [44], L-threonine [45, 46], and tyrosine [47]. The glyoxylate cycle is crucial for glutamate overproduction in C. glutamicum [29]. The relationship between glutamate production and glyoxylate cycle is complicated. High flux of glyoxylate shunt may lead to a shortage of α-ketoglutarate, the precursor of glutamic acid. Conversely, insufficient flux may result in the lack of other key metabolites, such as succinate and oxaloacetate, which impairs cell growth. Appropriate activity of glyoxylate cycle enhanced TCA cycle and provided more α-ketoglutarate for the syntheses of related metabolites in E. coli [45, 48]. In this study, the native promoter of aceBA was replaced with different strength promoters P43, PbacA, and Psrf. The activity of isocitrate lyase was decreased by 41.51% in WXPbacAaceBA, while that in WXP43aceBA and WXPsrfaceBA were increased by 93.40% and 222.64%, respectively. The WXPbacAaceBA showed a 23.24% increase in γ-PGA production compared to WX-02. These results indicated that the flux of glyoxylate cycle is high in B. licheniformis WX-02, which is detrimental to γ-PGA production. Therefore, glyoxylate shunt flux should be rewired at appropriate level for optimal γ-PGA production.
In conclusion, central carbon metabolism was engineering for efficient production of γ-PGA in B. licheniformis. Firstly, the flux of pyruvate to the TCA cycle was enhanced by replacing the native promoter of pdhABCD operon with stronger promoter Psrf. Secondly, the TCA cycle was strengthened by enhancing the activity of NADH-insensitive citrate synthase CitA through substitution of the origin promoter of citA. Subsequently, the carbon flux to glyoxylate shunt was rewired via varying the expression of isocitrate lyase AceA, and γ-PGA yield was increased in AceA down-regulated strain WXPbacAaceBA. Furthermore, the pyruvate formate-lyase gene pflB was deleted to reduce the carbon loss. Finally, through a combinatorial metabolic engineering approach, the γ-PGA yield of the final strain WX-PAAB reached 12.02 g/L, 69% higher than that of the starting strain WX-02. These results demonstrated that metabolic engineering of central carbon metabolic pathways is an effective strategy for enhanced production of γ-PGA, and this strategy may be widely applicable for the production of other important chemicals.
References
Sirisansaneeyakul, S., Cao, M., Kongklom, N., Chuensangjun, C., Shi, Z., & Chisti, Y. (2017). Microbial production of poly-gamma-glutamic acid. World Journal of Microbiology & Biotechnology, 33(9), 173.
Xu, G., Zha, J., Cheng, H., Ibrahim, M. H. A., Yang, F., Dalton, H., Cao, R., Zhu, Y., Fang, J., Chi, K., Zheng, P., Zhang, X., Shi, J., Xu, Z., Gross, R. A., & Koffas, M. A. G. (2019). Engineering Corynebacterium glutamicum for the de novo biosynthesis of tailored poly-gamma-glutamic acid. Metabolic Engineering, 56, 39–49.
Zhan, Y., Sheng, B., Wang, H., Shi, J., Cai, D., Yi, L., Yang, S., Wen, Z., Ma, X., & Chen, S. (2018). Rewiring glycerol metabolism for enhanced production of poly-gamma-glutamic acid in Bacillus licheniformis. Biotechnology for Biofuels, 11, 306.
Cai, D., He, P., Lu, X., Zhu, C., Zhu, J., Zhan, Y., Wang, Q., Wen, Z., & Chen, S. (2017). A novel approach to improve poly-gamma-glutamic acid production by NADPH regeneration in Bacillus licheniformis WX-02. Scientific Reports, 7, 43404.
Zhang, W., He, Y., Gao, W., Feng, J., Cao, M., Yang, C., Song, C., & Wang, S. (2015). Deletion of genes involved in glutamate metabolism to improve poly-gamma-glutamic acid production in B. amyloliquefaciens LL3. Journal of Industrial Microbiology & Biotechnology, 42(2), 297–305.
Feng, J., Quan, Y., Gu, Y., Liu, F., Huang, X., Shen, H., Dang, Y., Cao, M., Gao, W., Lu, X., Wang, Y., Song, C., & Wang, S. (2017). Enhancing poly-gamma-glutamic acid production in Bacillus amyloliquefaciens by introducing the glutamate synthesis features from Corynebacterium glutamicum. Microbial Cell Factories, 16(1), 88.
Qin, N., Li, L., Ji, X., Li, X., Zhang, Y., Larsson, C., Chen, Y., Nielsen, J., & Liu, Z. (2020). Rewiring central carbon metabolism ensures increased provision of Acetyl-CoA and NADPH required for 3-OH-propionic acid production. Acs Synthetic Biology, 9(12), 3236–3244.
Kopp, D., & Sunna, A. (2020). Alternative carbohydrate pathways - enzymes, functions and engineering. Critical Reviews in Biotechnology, 40(7), 895–912.
Doi, S., Komatsu, M., & Ikeda, H. (2020). Modifications to central carbon metabolism in an engineered Streptomyces host to enhance secondary metabolite production. Journal of Bioscience and Bioengineering, 130(6), 563–570.
Liu, H., Song, R., Liang, Y., Zhang, T., Deng, L., Wang, F., & Tan, T. (2018). Genetic manipulation of Escherichia coli central carbon metabolism for efficient production of fumaric acid. Bioresource Technology, 270, 96–102.
Choi, S., Kim, H. U., Kim, T. Y., & Lee, S. Y. (2016). Systematic engineering of TCA cycle for optimal production of a four-carbon platform chemical 4-hydroxybutyric acid in Escherichia coli. Metabolic Engineering, 38, 264–273.
Gu, Y., Lv, X., Liu, Y., Li, J., Du, G., Chen, J., Rodrigo, L. A., & Liu, L. (2019). Synthetic redesign of central carbon and redox metabolism for high yield production of N-acetylglucosamine in Bacillus subtilis. Metabolic Engineering, 51, 59–69.
Liu, Z., Yu, W., Nomura, C. T., Li, J., Chen, S., Yang, Y., & Wang, Q. (2018). Increased flux through the TCA cycle enhances bacitracin production by Bacillus licheniformis DW2. Applied Microbiology and Biotechnology, 102(16), 6935–6946.
Gao, W., He, Y., Zhang, F., Zhao, F., Huang, C., Zhang, Y., Zhao, Q., Wang, S., & Yang, C. (2019). Metabolic engineering of Bacillus amyloliquefaciens LL3 for enhanced poly-gamma-glutamic acid synthesis. Microbial Biotechnology, 12(5), 932–945.
Zhang, H., Zhu, J., Zhu, X., Cai, J., Zhang, A., Hong, Y., Huang, J., Huang, L., & Xu, Z. (2012). High-level exogenous glutamic acid-independent production of poly-(gamma-glutamic acid) with organic acid addition in a new isolated Bacillus subtilis C10. Bioresource Technology, 116, 241–246.
Li, B. C., Cai, D. B., Hu, S. Y., Zhu, A. T., He, Z. L., & Chen, S. W. (2018). Enhanced synthesis of poly gamma glutamic acid by increasing the intracellular reactive oxygen species in the Bacillus licheniformis 1-pyrroline-5-carboxylate dehydrogenase gene ycgN-deficient strain. Applied Microbiology and Biotechnology, 102(23), 10127–10137.
Cai, D., Chen, Y., He, P., Wang, S., Mo, F., Li, X., Wang, Q., Nomura, C. T., Wen, Z., Ma, X., & Chen, S. (2018). Enhanced production of poly-gamma-glutamic acid by improving ATP supply in metabolically engineered Bacillus licheniformis. Biotechnology and Bioengineering, 115(10), 2541–2553.
Wang, J., Yuan, H., Wei, X., Chen, J., & Chen, S. (2016). Enhancement of poly-γ-glutamic acid production by alkaline pH stress treatment in Bacillus licheniformis WX-02. Journal of Chemical Technology & Biotechnology, 91(9), 2399–2403.
Wei, X., Ji, Z., & Chen, S. (2010). Isolation of halotolerant Bacillus licheniformis WX-02 and regulatory effects of sodium chloride on yield and molecular sizes of poly-gamma-glutamic acid. Applied Biochemistry and Biotechnology, 160(5), 1332–1340.
Cai, D., Hu, S., Chen, Y., Liu, L., Yang, S., Ma, X., & Chen, S. (2018). Enhanced production of poly-gamma-glutamic acid by overexpression of the global anaerobic regulator Fnr in Bacillus licheniformis WX-02. Applied Biochemistry and Biotechnology, 185(4), 958–970.
Nemeria, N., Yan, Y., Zhang, Z., Brown, A. M., Arjunan, P., Furey, W., Guest, J. R., & Jordan, F. (2001). Inhibition of the Escherichia coli pyruvate dehydrogenase complex E1 subunit and its tyrosine 177 variants by thiamin 2-thiazolone and thiamin 2-thiothiazolone diphosphates. Evidence for reversible tight-binding inhibition. The Journal of Biological Chemistry, 276(49), 45969–45978.
Schendel, F. J., August, P. R., Anderson, C. R., Hanson, R. S., & Flickinger, M. C. (1992). Cloning and nucleotide sequence of the gene coding for citrate synthase from a thermotolerant Bacillus sp. Applied and Environmental Microbiology, 58(1), 335–345.
Li, N., Zhang, B., Chen, T., Wang, Z., Tang, Y. J., & Zhao, X. (2013). Directed pathway evolution of the glyoxylate shunt in Escherichia coli for improved aerobic succinate production from glycerol. Journal of Industrial Microbiology & Biotechnology, 40(12), 1461–1475.
Sonenshein, A. L. (2007). Control of key metabolic intersections in Bacillus subtilis. Nature Reviews Microbiology, 5(12), 917–927.
Guo, H. W., Madzak, C., Du, G. C., Zhou, J. W., & Chen, J. (2014). Effects of pyruvate dehydrogenase subunits overexpression on the alpha-ketoglutarate production in Yarrowia lipolytica WSH-Z06. Applied Microbiology and Biotechnology, 98(16), 7003–7012.
Sha, Y., Sun, T., Qiu, Y., Zhu, Y., Zhan, Y., Zhang, Y., Xu, Z., Li, S., Feng, X., & Xu, H. (2019). Investigation of glutamate dependence mechanism for poly-gamma-glutamic acid production in Bacillus subtilis on the basis of transcriptome analysis. Journal of Agricultural and Food Chemistry, 67(22), 6263–6274.
Yangtse, W., Zhou, Y., Lei, Y., Qiu, Y., Wei, X., Ji, Z., Qi, G., Yong, Y., Chen, L., & Chen, S. (2012). Genome sequence of Bacillus licheniformis WX-02. Journal of Bacteriology., 194(13), 3561–3562.
Stokell, D. J., Donald, L. J., Maurus, R., Nguyen, N. T., Sadler, G., Choudhary, K., Hultin, P. G., Brayer, G. D., & Duckworth, H. W. (2003). Probing the roles of key residues in the unique regulatory NADH binding site of type II citrate synthase of Escherichia coli. The Journal of Biological Chemistry, 278(37), 35435–35443.
Yu, B. Q., Shen, W., Wang, Z. X., & Zhuge, J. (2005). Glyoxylate cycle is required for the overproduction of glutamate but is not essential for Corynebacterium glutamicum growth on glucose. Sheng Wu Gong Cheng Xue Bao, 21(2), 270–274.
Deng, Y., Ma, N., Zhu, K., Mao, Y., Wei, X., & Zhao, Y. (2018). Balancing the carbon flux distributions between the TCA cycle and glyoxylate shunt to produce glycolate at high yield and titer in Escherichia coli. Metabolic Engineering, 46, 28–34.
Alexeeva, S., de Kort, B., Sawers, G., Hellingwerf, K. J., & de Mattos, M. J. (2000). Effects of limited aeration and of the ArcAB system on intermediary pyruvate catabolism in Escherichia coli. Journal of Bacteriology, 182(17), 4934–4940.
Qi, G., Kang, Y., Li, L., Xiao, A., Zhang, S., Wen, Z., Xu, D., & Chen, S. (2014). Deletion of meso-2,3-butanediol dehydrogenase gene budC for enhanced D-2,3-butanediol production in Bacillus licheniformis. Biotechnology for Biofuels, 7(1), 16.
Mitsunaga, H., Meissner, L., Palmen, T., Bamba, T., Buchs, J., & Fukusaki, E. (2016). Metabolome analysis reveals the effect of carbon catabolite control on the poly(gamma-glutamic acid) biosynthesis of Bacillus licheniformis ATCC 9945. Journal of Bioscience and Bioengineering, 121(4), 413–419.
Fu, J., Huo, G., Feng, L., Mao, Y., Wang, Z., Ma, H., Chen, T., & Zhao, X. (2016). Metabolic engineering of Bacillus subtilis for chiral pure meso-2,3-butanediol production. Biotechnology for Biofuels, 9, 90.
Fu, J., Wang, Z., Chen, T., Liu, W., Shi, T., Wang, G., Tang, Y. J., & Zhao, X. (2014). NADH plays the vital role for chiral pure D-(-)-2,3-butanediol production in Bacillus subtilis under limited oxygen conditions. Biotechnology and Bioengineering, 111(10), 2126–2131.
Rebecchi, S., Pinelli, D., Zanaroli, G., Fava, F., & Frascari, D. (2018). Effect of oxygen mass transfer rate on the production of 2,3-butanediol from glucose and agro-industrial byproducts by Bacillus licheniformis ATCC9789. Biotechnology for Biofuels, 11, 145.
Cruz Ramos, H., Hoffmann, T., Marino, M., Nedjari, H., Presecan-Siedel, E., Dreesen, O., Glaser, P., & Jahn, D. (2000). Fermentative metabolism of Bacillus subtilis: Physiology and regulation of gene expression. Journal of Bacteriology, 182(11), 3072–3080.
Guo, J., Zhang, H., Wang, C., Chang, J. W., & Chen, L. L. (2016). Construction and analysis of a genome-scale metabolic network for Bacillus licheniformis WX-02. Research in Microbiology, 167(4), 282–289.
Guo, J., Cheng, G., Gou, X. Y., Xing, F., Li, S., Han, Y. C., Wang, L., Song, J. M., Shu, C. C., Chen, S. W., & Chen, L. L. (2015). Comprehensive transcriptome and improved genome annotation of Bacillus licheniformis WX-02. FEBS Letters, 589(18), 2372–2381.
Huo, Y., Zhan, Y., Wang, Q., Li, S., Yang, S., Nomura, C. T., Wang, C., & Chen, S. (2018). Acetolactate synthase (AlsS) in Bacillus licheniformis WX-02: Enzymatic properties and efficient functions for acetoin/butanediol and L-valine biosynthesis. Bioprocess and Biosystems Engineering, 41(1), 87–96.
Kozak, B. U., van Rossum, H. M., Luttik, M. A., Akeroyd, M., Benjamin, K. R., Wu, L., de Vries, S., Daran, J. M., Pronk, J. T., & van Maris, A. J. (2014). Engineering acetyl coenzyme A supply: Functional expression of a bacterial pyruvate dehydrogenase complex in the cytosol of Saccharomyces cerevisiae. MBio, 5(5), e01696-e1614.
Chiang, C. J., Ho, Y. J., Hu, M. C., & Chao, Y. P. (2020). Rewiring of glycerol metabolism in Escherichia coli for effective production of recombinant proteins. Biotechnology for Biofuels, 13(1), 205.
Li, Y., Huang, B., Wu, H., Li, Z., Ye, Q., & Zhang, Y. P. (2016). Production of succinate from acetate by metabolically engineered Escherichia coli. Acs Synthetic Biology., 5(11), 1299–1307.
Liu, M., Ding, Y., Chen, H., Zhao, Z., Liu, H., Xian, M., & Zhao, G. (2017). Improving the production of acetyl-CoA-derived chemicals in Escherichia coli BL21(DE3) through iclR and arcA deletion. Bmc Microbiology, 17(1), 10.
Zhao, H., Fang, Y., Wang, X., Zhao, L., Wang, J., & Li, Y. (2018). Increasing L-threonine production in Escherichia coli by engineering the glyoxylate shunt and the L-threonine biosynthesis pathway. Applied Microbiology and Biotechnology, 102(13), 5505–5518.
Zhu, L., Fang, Y., Ding, Z., Zhang, S., & Wang, X. (2019). Developing an l-threonine-producing strain from wild-type Escherichia coli by modifying the glucose uptake, glyoxylate shunt, and l-threonine biosynthetic pathway. Biotechnology and Applied Biochemistry, 66(6), 962–976.
Jo, M., Noh, M. H., Lim, H. G., Kang, C. W., Im, D. K., Oh, M. K., & Jung, G. Y. (2019). Precise tuning of the glyoxylate cycle in Escherichia coli for efficient tyrosine production from acetate. Microbial Cell Factories, 18(1), 57.
Noh, M. H., Lim, H. G., Park, S., Seo, S. W., & Jung, G. Y. (2017). Precise flux redistribution to glyoxylate cycle for 5-aminolevulinic acid production in Escherichia coli. Metabolic Engineering, 43(Pt A), 1–8.
Funding
This work was supported by the Educational Research Projects for Young and Middle-Aged Teachers in Fujian Province (No. JAT190788) and Fujian Provincial Key Laboratory of Eco-Industrial Green Technology (No. WYKF-GCT2020-3).
Author information
Authors and Affiliations
Contributions
B Li, D Cai, and S Chen designed the study. B Li carried out the molecular biology studies, construction of engineering strains, and the fermentation studies. B Li, D Cai, and S Chen analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, B., Cai, D. & Chen, S. Metabolic Engineering of Central Carbon Metabolism of Bacillus licheniformis for Enhanced Production of Poly-γ-glutamic Acid. Appl Biochem Biotechnol 193, 3540–3552 (2021). https://doi.org/10.1007/s12010-021-03619-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03619-4