Abstract
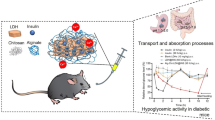
Effective delivery system for oral insulin administration is a promising way for diabetes therapy. Herein, we prepared alginate microbeads containing chitosan nanoparticles (CNP) for controlled release of insulin. CNP was developed by reaction between tripolyphosphate (TPP) and chitosan. The ratio of TPP to chitosan was optimized aiming with smaller and more unified distributed CNP. TEM and DLS analysis confirmed that CNP has size around 150 nm with low PDI value and strong surface charge. Encapsulate ability for bovine serum albumin, working as model protein, was 11.45%, and the encapsulate efficiency was 23.70%. To modify the release profile of protein suitable for oral insulin delivery, sodium alginate was applied to coat on the surface of CNP by electrostatic interaction. After that, CaCl2 was added to reinforce the alginate coating layer. FTIR analysis confirmed the interaction of alginate with chitosan and reaction with calcium ion. After reaction with Ca2+ ion, size measurement revealed that CNP was incorporated into alginate microbeads with mean diameter about 3.197 μm. Alginate microbeads presented irregular shape with small particles inside as revealed by optical microscope. Meanwhile, the release test demonstrated that protein release was pH-dependent. Acidic pH value retards protein release and neutral pH value promotes protein release. At last, insulin-loaded alginate microbeads were administrated to hyperglycemia model mice and blood glucose profile was monitored afterward. Insulin-loaded microbeads significantly lowered blood glucose level compared with mice treated with alginate microbeads without insulin. It is noted that insulin-loaded alginate microbeads could lower blood glucose level in much prolonged period of 96 h, indicating that insulin was released in controlled manner.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insulin administration is the most effective therapy for diabetes patients to control hyperglycemia and other complications [1]. However, usage of insulin is still quite problematic due to its administration method. By far, subcutaneous injection is the commonest way which also generates problems like allergic reaction, possible infection, skin bulges, needle phobia, and low patient compliance [2]. Compared with injection, oral insulin delivery is a more convenient and comfortable administration route exerting a long-term glycemic lowering effect and gains better patient compliance. Moreover, when delivered orally, insulin is transported to liver and exerts its function which works similar with endogenous pathway of insulin in the body [3]. But oral insulin administration also faces problems like low stability and bio-availability [4]. Insulin is not stable when pass through the stomach and intestinal tract due to severe pH variation and several proteases degradation in the digestive juice. Additionally, when reach to intestinal tract, insulin also needs to bypass the mucous layer adhered to the intestinal track in order to be absorbed by the intestinal epithelium. To circumvent the problems associated with oral insulin administration, development of effective oral insulin formulation has been put forward in recent decades.
Among the many proposed methods, polymer-based micro- and nanoparticle carriers gain much attention due to easy fabrication procedures and absorption promotion ability. Ideal polymer-based carriers should encapsulate insulin and protect it from harsh environments in the gastrointestinal (GI) system [1]. Both natural and synthetic polymers have been employed to fabricate polymer-based delivery tool for oral insulin administration. For this end, chitosan and alginate acid are two most investigated polymers.
Chitosan is a cationic polysaccharide derived from partial deacetylation of chitin which is the second largest natural polysaccharide only after cellulose. Chitosan is composed of glucosamine and N-acetyl-d-glucosamine linked by β(1, 4) glycosidic bonds. It is the only biopolymer that bears positive charge when dissolved in solution. Moreover, chitosan is biodegradable and biocompatible that guarantees its wide application in pharmaceutical and medical areas. Glucosamine group in chitosan could interact with linking agents such as sodium tripolyphosphate (TPP) and glutaraldehyde, forming stable and spherical carriers in optimized conditions [5]. The positive charge of chitosan also facilitates interaction with functional proteins like insulin which usually bears negative charge in natural pH solution. Chitosan and its derivatives formulated carriers that have strong mucoadhesive ability and could promote permeation and absorption of payload in the gut [6]. Juan Wang et al. prepared chitosan based nanogels with positive and negative surface charge for oral insulin delivery [7]. They reported that nanogels with negative charge could delivery insulin with more efficiency. However, as pointed out in previous research, chitosan based carrier tends to dissolve in stomach which lead to early release of payload and low efficiency due to protein denaturation [8]. Further modification of insulin loaded chitosan carriers is needed to adjust its release profile and increase the bioavailability of insulin.
Alginate acid is a natural linear polysaccharide isolated from brown algae [9]. It is composed of β-D-mannuronic acid (M) and α-L-guluronic acid (G) residues linked by β(1, 4) glycosidic bonds. Alginate acid forms gel when linked by divalent cations, especially Ca2+, making it one of the most commonly used materials for gel beads preparation [10]. Moreover, alginate acid beads are stable in lower pH value but swell and dissolve in neutral and higher pH value which imparts pH responsive release feature[11]. Alginate acid beads are also widely used for oral insulin delivery. Insulin-loaded alginate microbeads were prepared by emulsification and internal gelation induced by Ca2+, but the microbeads could not control the release of insulin in acidic medium which guarantees further investigations [12]. As pointed out by previous studies, although alginate microbeads are stable in gastric juice, it could not modulate the release speed of insulin which leads to the abovementioned problems [13]. Both chitosan and alginate acid can be employed to fabricate delivery tools which better protect and control the release profile of insulin. Chitosan-coated alginate nanoparticles and microbeads reduce the release of insulin in simulated gastric buffer but accelerate the release when in simulated intestinal condition [13, 14].
In the present study, we prepared alginate acid microbeads containing chitosan nanoparticles which could be used to encapsulate insulin for oral administration. Chitosan nanoparticles were prepared and added with alginate acid to form alginate microbeads due to electrostatic attraction. Then, alginate microbeads were reinforced by reaction with Ca2+. It shows controlled release feature in simulated gastric and small intestine solution. Animal study proved that it is effective to control the blood glucose concentration in diabetic mice in prolonged period.
Materials and Methods
Materials
Chitosan with low viscosity (<200 mPa.S) and sodium alginate (200 ± 20 mPa.S) were provided by Macklin Inc (Shanghai, China). Bovine serum albumin (BSA), streptozotocin, and insulin (27 IU/mg) were purchased from Aladin Inc (Shanghai, China). BCA protein assay kit was from Beyotime Biotechnology (Shanghai, China). All other reagents were provided by Sinopharm Chemical Reagent Co. Ltd (Shanghai, China).
Hyperglycemia Animal Model Development
C57BL/6 mice, 6 weeks old, were purchased from Laboratory Animal Center of Anhui Medical University, Hefei, China. Mice were housed in an animal experiment room with standard conditions (23 ± 2 °C, 55 ± 5% relative humidity, 12-h light/dark cycle) and fed standard diet for 1 week to acclimatize. After that, all mice were fed with high-fat diet which was formulated according to our previous research [15]. And during the experiment, low dosage of streptozocin (0.1 M sodium citrate and 0.1 M citric acid, pH 4.2–4.5, dosage of 30 mg kg−1 BW) was intraperitoneally injected to mice on day 20, day 27, and day 34 to develop diabetes according to our previous study [16]. After 8 weeks of high-fat diet treatment, fast blood glucose concentration higher than 7.5 mmol/L was adopted as the standard to select hyperglycemia mice (a total of 21 mice). The animal experiment was approved by the animal ethical committee, Hefei University of Technology.
Chitosan Nanoparticles (CNP) Preparation and Optimization
Chitosan was dissolved in 1% acetic acid solution to prepare chitosan solution at the concentration of 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, and 4.0 mg/mL. Sodium tripolyphosphate (TPP) was prepared in distilled water at concentration of 0.5 mg/mL. TPP solution was dripped at rate of 2 mL/min into chitosan solution magnetic stirred at the speed of 800 rpm. The mixture was stirred for further 20 min and centrifuged at 10,000 g for 15 min. Supernate fluid was removed to separate CNP. Protein was entrapped into CNP by dissolving in chitosan solution in advance. CNP was washed with distilled water for twice to remove adhered surface protein and free chitosan. During the preparation, appearance of solution with obvious aggregation and agglomeration was discarded, and only solution with transparent or semi-transparent appearance was processed for the following steps [17].
Alginate Microbeads Preparation
CNP was dissolved in distilled water with the aid of ultrasonication treatment for 2 min. Alginate sodium was fully dissolved in water at the concentration of 6 mg/mL. Then, dissolved CNP was dripped via a syringe into alginate sodium solution under stirring. Alginate microbeads were separated by centrifugation at 10,000 g for 15 min. The precipitate was removed and washed with distilled water and again centrifuged at the same conditions. Precipitate was dissolved in water with the aid of ultrasonication treatment. Then, CaCl2 solution at the concentration of 1% was dripped into alginate microbeads solution to initiate crosslink reaction. The reaction was processed for 30 min, and microbeads were separated by centrifugation at 3000 g for 15 min.
Hydrodynamic Parameters Examination
Size, PDI value, and zeta potential of CNP were measured by dynamic light scattering (DSL) on a ZS nanosizer analyzer (Malvern, Worcestershire, UK). Size and PDI was analyzed in a polystyrene cell. Zeta potential was measured in a zeta cell, and the measurement was conducted at 25 °C with a detector angle of 90° and wavelength of 633 nm. Size of alginate microbeads was analyzed on a Master Sizer 2000 (Malvern, Worcestershire, UK). Microbeads were added into a sample holder which contained about 500 mL distilled water, and size was measured by static light scattering [18].
Transmission Electron microscopy (TEM) Analysis of Chitosan Nanoparticle (CNP)
Morphology properties of CNP were observed via TEM. Samples were prepared according to our previous method [19]. Phosphotungstic acid (2%) was applied to stain the sample on a TEM grid. Morphological features were observed on a transmission electron microscopy (JEM, 1200EX, Japan) at an accelerating voltage of 120 kV.
Microscope Observation of Alginate Microbeads
Alginate microbeads contain CNP were prepared after sodium alginate coating and CaCl2 cross-linking. The appearance of alginate microbeads was observed through a common optical microscope (E100, Nikon, Japan) mounted with a digital camera (J1, Nikon, Japan). Images of microbeads magnified by 100 and 400 times were recorded.
Attenuated Total Reflecting-Fourier-Transform Infrared (ATR-FTIR) Investigation
After lyophilization, chitosan, alginate, CNP, alginate microbead containing CNP, and Ca2+ cross-linked alginate microbead powder was analyzed on a PerkinElmer Spectrum 100 spectrophotometer (Waltham, MA, USA). The sample powder was sandwiched between the ATR accessary and the diamond crystal. Spectra between 600 and 4000 cm-1 were recorded.
Differential Scanning Calorimetry (DSC)
After lyophilization, thermal properties of insulin, chitosan, alginate, insulin-loaded CNP, alginate microbeads containing CNP, and Ca2+ cross-linked alginate microbeads powder were analyzed by a different scanning calorimeter (DSC Q200, TA instruments, USA). Briefly, under constant nitrogen flow rate (50 mL/min), samples were heated from 20 to 300 °C at a rate of 10 °C/min.
Loading Ability and Efficiency Measurement
For the pilot study, BSA protein, as a model protein, was encapsulated into microbeads [20]. BSA protein was first dissolved in chitosan solution for preparing CNP. The other procedures are conducted according to above description. Protein concentration in the supernatant after centrifugation was determined by the BCA protein assay kit according to the instruction. Protein encapsulated into CNP was calculated as total protein minus protein in supernatant [21]. Loading ability and efficiency was calculated using the following formulas.
Release Profile of Alginate Microbeads Containing CNP
Protein release from alginate microbeads containing CNP was monitored by the BCA protein assay kit. Protein concentration was calculated using the following standard curve:
where X means protein concentration (mg/mL) and Y means the absorption value at 562 nm.
Alginate microbeads containing CNP were dissolved in 5 mL of simulated digestive juice (dilute hydrochloric acid with pH 1.2 or PBS pH 7.4, working as simulated gastric juice and simulated small intestinal juice respectively) and transferred to a dialysis tube with cutting molecular weight of 100 KDa. The dialysis tube was submerged into 20 mL of dilute hydrochloric acid with pH 1.2 or PBS pH 7.4 under stirring at the temperature of 37 °C. At 0, 1, 2, 4, 12, and 24 h, 30 μL of dialysate was collected for measurement of protein concentration [22].
Accelerated Stability Test
Insulin-loaded Ca2+ cross-linked alginate microbead powder was dissolved in 210 mL PBS solution and incubated at 50 °C. Sample solutions were taken out at 0, 1, 2, 3, 4, 5 and 6 h respectively. After centrifugation, the supernatant was used to measure the concentration of released insulin by HPLC (E2695, Waters, USA). The column was Symmetry C18 with 5 μm particle size, 4.6 mm id × 250 mm length. The mobile phase consists of 0.1% trifluoroacetic acid (TFA) and acetonitrile (ACT) aqueous solution. The flow rate is 1 mL/min and UV detection was used to identify insulin at 214 nm [23].
The remaining amount of insulin was calculated to indicate stability according to the following formulation:
Hyperglycemic Ability
Alginate microbeads containing CNP loaded with insulin (insulin loaded beads) and without insulin (blank beads) were dispersed in saline. The concentration of microbeads was adjusted so that 3 U insulin/kg BW was achieved when 0.2 mL of the solution was delivered to mice. The blood glucose level was monitored after microbeads treatment at 0, 1, 2, 3, 4, 5, 7, 10, 24, 48, 72, 96, 120, and 144 h respectively.
Statistical Analysis
All data were presented as mean ± standard deviation after being statistically processed. Software SPSS 13.0 was used for t test analysis of the inter group deviation. p < 0.05 means significant difference.
Results and Discussion
Chitosan Nanoparticles (CNP) Preparation and Optimization
Chitosan nanoparticles (CNP) were prepared using ionic gelation method. Weight ratio of chitosan to TPP was adjusted to 3:1 to 7:1 by varying concentration of chitosan from 1.5 to 3.5 mg/mL and fixing the concentration of TPP to 0.5 mg/mL. Size of CNP ranges from 245.5 to 143.0 nm (Fig. 1). When weight ratio of chitosan to TPP was 5:1, the size was smallest. Also, PDI value of CNP prepared by the same condition was smaller than other groups, indicating that CNP distributes in a unified pattern. Zeta potential is impacted by weight ratio of chitosan to TPP either. When the ratio was low, zeta potential value was smaller than the counterparts with higher ratio. TPP reacted with amino groups of chitosan and reduced the positive charge deployed on the surface of CNP, lowering the zeta potential value. As is well known, lower PDI and higher zeta potential value are important indicators of stable nanoparticles when dissolved in solution. Hence, the ratio of chitosan to TPP was determined as 5:1 in the following experiments. Fabrication conditions including chitosan/TPP ratio has been investigated which found that weight ratio of 3:1 was appropriate using flush method [24]. Lower concentration of chitosan could decrease the size of chitosan nanoparticles. It was reported that CNP formed with higher concentration of TPP was opalescent or even aggregated, meaning much larger particle size [17]. Other factors, such as molecular weight of chitosan, preparation temperature, solution pH value, and the way solutions mixed, all impact on the hydrodynamic properties of chitosan nanoparticles [25, 26].
Hydrodynamic properties of chitosan-sodium tripolyphosphate nanoparticles (CNP) at different preparation conditions. (a) size of CNP, Chit 3, Chit 4, Chit 5, Chit 6, Chit 7 indicates the concentration of chitosan solution are 1.5, 2.0, 2.5, 3.0, 3.5 mg/mL when fix the concentration of sodium tripolyphosphate at 0.5 mg/mL; (b) PDI of CNP; (c) zeta potential value of CNP
CNP Morphology Observation
Morphology properties of blank CNP and protein-loaded CNP were observed via TEM (Fig. 2). Size of CNP without protein was slightly lower than 100 nm which was also lower than that revealed in DLS. After preparation, wet CNP without protein should contain much hiatus or pores occupied by water. During TEM sample preparation, CNP was dried which may lead to shrinking of the particles. Moreover, as revealed in TEM images, blank CNP is only approximate spherical with much small irregular structures on the surface which could be ascribed to drying process when preparing TEM sample. On the other hand, CNP loaded with protein is larger than that without protein. The size of CNP revealed by TEM was about 150 nm which was similar with that obtained by DLS. Size of most CNP was similar, meaning that they distributed in a narrow range as also indicated by the low PDI value. CNP shows prefect spherical feature which was also presented by previous researches [27]. Protein loaded into CNP may replace water and occupy hiatus or pores in the CNP, such maintains its size and shape as well during TEM sample drying process.
Preparation of Alginate Calcium Microbeads Containing CNP
After the condition for CNP fabrication was optimized, CNP was dripped into sodium alginate solution to prepare alginate-coated CNP (Chit-TPP-Alg). Positive charges on the surface of CNP would interact with negative charge of alginate acid and form aggregation of CNP linked and coated by alginate. The alginate microbeads were further reinforced by adding CaCl2 solution (Chit-TPP-Alg-CaCl2). Alginate could form microbeads with divalent cation, especially Ca2+, by linking the guluronic acid block in the alginate acid polymer [28]. Frist of all, the size of Chit-TPP-Alg and Chit-TPP-Alg-CaCl2 was assessed which showed that after Ca2+ crosslinking size of alginate microbeads increased by about 30%. The mean size of Chit-TPP-Alg was 3.50 ± 0.24 μm, suggesting alginate interact with CNPs and form microbeads much larger than CNP. In the present experiment conditions, alginate was not coated on the surface of single CNP but may linked with several CNPs and form microbeads with CNPs inside. Chit-TPP-Alg was further treated with CaCl2 to strength the gel structure of microbeads. The size of Chit-TPP-Alg-CaCl2 was about 4.77 ± 0.40 μm. Ca2+ may linked alginate acid existed in the solution with that on the surface of microbeads, such increased size of the microbeads. Size distribution pattern of microbeads was depicted in Fig. 3. There are three main distribution peaks, around 0.2–1.0, 1.0–10, and 10–100 μm, respectively. The size distribution profile of Chit-TPP-Alg-CaCl2 and Chit-TPP-Alg was quite similar, showing Ca2+-alginate interaction exerted little impact on the size distribution pattern.
Microscope images indicate that apart from large microbeads, there exist microbeads in smaller size (Fig. 4). Most microbeads have irregular shape and contain very small little particles inside which should be CNPs. One typical microbead contains hundreds of CNPs inside, suggesting microbeads containing CNPs were formed as predicted (Fig.4 c).
Images of alginate microbeads contains CNP. (a) Images of alginate microbeads contain CNP without insulin loading at 100 X magnification. (b) Images of alginate microbeads contain CNP loaded with insulin loading at 100 X magnification. (c) Images of alginate microbeads contain CNP without insulin loading at 400 X magnification. (d) Images of alginate microbeads contain CNP without insulin loading at 400 X magnification
FTIR Spectrum Analysis
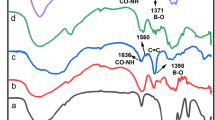
FTIR spectrum of chitosan, sodium alginate, chitosan TPP nanoparticles (Chit-TPP), alginate microbeads contain CNPs (Chit-TPP-Alg), and calcium alginate microbeads contain CNPs (Chit-TPP-Alg-CaCl2) were recorded and presented in Fig.5. Peak at 3360 cm-1 which is concluded as OH stretching, become more flat and wider in spectrum of Chit-TPP compared with spectrum of chitosan, indicating stronger hydrogen bonding [29]. Peaks at 1646 cm-1 and 1597 cm-1 of chitosan which are attributed to C=O stretching from amide I and NH2 bending vibration shifted to 1635 cm-1 and 1537 cm-1 in spectrum of Chit-TPP, respectively. This has been reported in previous publication as the result of linkage between phosphoric groups of TPP and the amino groups in chitosan [21]. A new peak at 1220 cm-1 of Chit-TPP is ascribed to P=O from TPP. Alginate bears negative charge in solution which could interact with positively charged Chit-TPP nanoparticles. FTIR spectrum of Chit-TPP-Alg showed significant peak shift compared with Chit-TPP and sodium alginate. C=O stretching from amide I at 1635 cm-1 was shifted to 1590 cm-1 and becomes stronger than that of Chit-TPP due to strong antisymmetric CO2- stretching of alginate acid [25]. While NH2 bending vibration at 1537 cm-1 was much weaker in spectrum of Chit-TPP-Alg, another peak at 1406 cm-1 becomes prominent which is contributed from symmetric CO2- stretch of alginate acid. The changes should be caused by interaction between amino group from chitosan and carboxyl group from alginate when forming the Chit-TPP-Alg microbeads. It should be noted that in the Chi-TPP-Alg microbeads, only electrostatic interaction is the main force holding the microbead gel structure which was further fortified with adding of Ca2+ ions. The FTIR spectrum of Chit-TPP-Alg-CaCl2 has strong peak at 3368 cm-1 which is correlated to OH stretching, indicating that Chit-TPP-Alg-CaCl2 may hold more bonding water than the Chit-TPP-Alg. The antisymmetric CO2- stretching signal shifted to 1629 cm-1 and becomes more prominent than that of Chit-TPP-Alg which is also revealed by our former report [11]. The peak of symmetric CO2- stretch also shifted to 1425 cm-1 in Chit-TPP-Alg-CaCl2. The above result indicates Ca2+ ions react with carboxyl groups of Chit-TPP-Alg microbeads.
Differential Scanning Calorimetry (DSC)
The thermal transition curve of insulin, chitosan, alginate, and insulin-loaded NPs were studied by DSC, which examine structural interactions between insulin and the wall material. The presence of the drug molecule can slightly affect the thermal behavior of the nanoparticles which depend on the interaction between drugs and wall materials [30]. In Fig. 6, the DSC thermogram of insulin presented a strong endothermic peak at 116.49 °C and a weak endothermic peak at 159.16 °C, which are attributed to the denaturation of insulin and water loss [31]. Compared with insulin, the endothermic peak of insulin loaded NPs became indistinct, which is due to the low loading ability (11.45%) and efficiency (23.70%) as mentioned in the following section. On the other hand, insulin-loaded NPs reached the endothermic peak at a lower temperature. As for exothermic peaks, insulin-loaded NPs started at a higher temperature, while the decomposition of insulin happened after 190 °C. Our result indicated that there is an interaction between insulin and chitosan, but the specific kind of interaction is unknown, which need further investigations.
In vitro Release Profile
Alginate microbeads are stable in acidic solution but swell and dissolve in solution with neutral and alkaline pH value. On the contrary, chitosan NPs tend to swell and debacle in acidic solution but stable in neutral and alkaline solution. The present study investigates the alginate microbeads containing chitosan NPs which adjust the release properties of payload (Fig. 7). Bovine serum albumin (BSA) was used as a model protein dissolved along with chitosan to prepare chitosan NPs. Loading ability and efficiency measurement of BSA in prepared microbeads were 11.45 and 23.70%, respectively. During the preparing process, protein could release into the solution which may limit the loading efficiency. Release profile of Chit-TPP-Alg-CaCl2 microbeads in simulated gastric juice (pH 1.2) and small intestinal juice (pH 7.4) showed that protein release was significantly impacted by the pH value. When in gastric juice, the gel structure of Chit-TPP-Alg-CaCl2 microbeads is stable which could inhibit free release of protein. At the end of the release test (24 h), only about 4% of protein was released. On the other hand, when simulated in small intestine juice, the gel structure of Chit-TPP-Alg-CaCl2 microbeads was destroyed and increases the release of protein. About 80% of protein was released at the end of the test. The release pattern verified our hypothesis that alginate microbeads contain chitosan NPs could control the release of payload, in response to the pH value.
Protein in microbeads prepared only by alginate releases very quickly when dissolved in simulated gastric juice, showing no pH responsive release properties [12]. In one previous study, hybrid microbeads were developed by adding protein containing sodium alginate into chitosan-CaCl2 solution [20]. Alginate/chitosan nanoparticles were also prepared by ionotropic pre-gelation of alginate microbead core followed by chitosan coating [32]. The release profile was improved compared with the previous one that higher pH value stimulates the protein release but only with very small difference. The microbeads also showed burst release pattern in Tris-HCl solution pH 7.4. Alginate-chitosan microspheres prepared via membrane emulsification technique were also used as a delivery tool for protein [33]. Alginate-chitosan microspheres showed pH-responsive release that when in simulated gastric juice, very small amount of protein was released, but when in simulated small intestine juice, release speed was greatly enhanced.
Accelerated Stability Test
In the Fig.8, through 6 h of high-temperature experiment, insulin still had a high remaining amount in the first 2 h. The insulin concentration decreased faster after the third hour, which should be cause by the high temperature. Therefore, the electrostatic attraction between chitosan and alginate became weaker, thereby losing the protective effect on insulin [34].The high temperature experiment proved that our nanoparticles have good stability, compared with NPs prepared by other researchers [35]. Our results indicated that even if NPs are exposed to high temperature in a short period of time during the manufacturing and transportation process, NPs can also have a good protective effect on insulin, while under normal storage conditions, insulin in NPs can maintain a high biological activity for longer period.
In vivo Hyperglycemic Ability
Chit-TPP-Alg-CaCl2 microbeads loaded with and without insulin were administrated to diabetes mice to monitor its hyperglycemic ability (Fig. 9). After 3 h of administration, the mean blood glucose concentration of mice begun to decrease which was stabilized from 5 h afterward. The blood glucose level decreased from about 20 mmol/L to about 10 mmol/L in mice treated insulin-loaded Chit-TPP-Alg-CaCl2 microbeads. On the contrary, blood glucose level of mice treated with blank beads fluctuated around 20 mmol/L. It is noted that insulin loaded Chit-TPP-Alg-CaCl2 exert hyperglycemic ability in much long period to about 100 h, suggesting that insulin could release from microbeads in much controlled manner. In the past decades, several chitosan and alginate acid-based delivery tools have been reported with hyperglycemic ability. But in most cases, hyperglycemic time was quite short, meaning more frequent medication is required. Insulin-loaded chitosan nanogel lowered blood sugar level within about 15 h [7]. In another study, insulin-loaded alginate/chitosan NPs lowered the blood glucose level within about 20 h [32]. Our results indicate that alginate microbeads contain chitosan NPs which is an effective delivery tool for insulin to lower the blood glucose level which is promising for diabetes treatment.
Conclusion
Alginate microbeads containing chitosan nanoparticles (NPs) were fabricated by electrostatic interaction between sodium alginate and chitosan NPs and followed by Ca2+ ions crosslinking. Chitosan NPs retain its spherical morphology inside the alginate microbeads as revealed by microscope. Prepared alginate microbeads release its payload in controlled and pH-responsive manner. Lower pH value inhibits the release of payload due to the fact that alginate microbeads are stable in acidic solution. Higher pH value promotes the decompose of microbeads structure and increases the release speed. When tested in vivo, insulin-loaded microbeads could lower the blood glucose level in much prolonged manner. Within about 100 h, insulin-loaded alginate microbeads showed significant hyperglycemic ability which is promising for diabetes treatment.
References
Fonte, P., Araujo, F., Silva, C., Pereira, C., Reis, S., Santos, H. A., & Sarmento, B. (2015). Polymer-based nanoparticles for oral insulin delivery: revisited approaches. Biotechnology Advances, 33(6), 1342–1354.
Khafagy, E. S., Morishita, M., Onuki, Y., & Takayama, K. (2007). Current challenges in non-invasive insulin delivery systems: a comparative review. Advanced Drug Delivery Reviews, 59(15), 1521–1546.
Chaturvedi, K., Ganguly, K., Nadagouda, M. N., & Aminabhavi, T. M. (2013). Polymeric hydrogels for oral insulin delivery. Journal of Controlled Release, 165(2), 129–138.
Mukhopadhyay, P., Mishra, R., Rana, D., & Kundu, P. P. (2012). Strategies for effective oral insulin delivery with modified chitosan nanoparticles: a review. Progress in Polymer Science, 37(11), 1457–1475.
Zokaei, E., Badoei-dalfrad, A., Ansari, M., Karami, Z., Eslaminejad, T., & Nematollahi-Mahani, S. N. (2019). Therapeutic potential of DNAzyme Loaded on chitosan/cyclodextrin nanoparticle to recovery of chemosensitivity in the MCF-7 cell line. Applied Biochemistry and Biotechnology, 187(3), 708–723.
Luo, Y. C., Teng, Z., Li, Y., & Wang, Q. (2015). Solid lipid nanoparticles for oral drug delivery: Chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydrate Polymers, 122, 221–229.
Wang, J., Xu, M. X., Cheng, X. J., Kong, M., Liu, Y., Feng, C., & Chen, X. G. (2016). Positive/negative surface charge of chitosan based nanogels and its potential influence on oral insulin delivery. Carbohydrate Polymers, 136, 867–874.
George, M., & Abraham, T. E. (2006). Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan - a review. Journal of Controlled Release, 114(1), 1–14.
Lopez-Hortas, L., Dominguez, H., & Torres, M. D. (2019). Valorisation of edible brown seaweeds by the recovery of bioactive compounds from aqueous phase using MHG to develop innovative hydrogels. Process Biochemistry, 78, 100–107.
Alexakis, T., Boadi, D. K., Quong, D., Groboillot, A. F., & Neufeld, R. J. (1995). Microencapsulation of DNA within alginate microspheres and crosslinked chitosan membranes for in vivo application. Applied Biochemistry and Biotechnology, 50(1), 93–106.
Li, J., Kim, S. Y., Chen, X., & Park, H. J. (2016). Calcium-alginate beads loaded with gallic acid: Preparation and characterization. LWT- Food Science and Technology, 68, 667–673.
Reis, C. P., Ribeiro, A. J., Neufeld, R. J., & Veiga, F. (2007). Alginate microparticles as novel carrier for oral insulin delivery. Biotechnology and Bioengineering, 96(5), 977–989.
Silva, C. M., Ribeiro, A. J., Figueiredo, I. V., Goncalves, A. R., & Veiga, F. (2006). Alginate microspheres prepared by internal gelation: development and effect on insulin stability. International Journal of Pharmaceutics, 311(1-2), 1–10.
Mukhopadhyay, P., Chakraborty, S., Bhattacharya, S., Mishra, R., & Kundu, P. P. (2015). pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. International Journal of Biological Macromolecules, 72, 640–648.
Liu, Y., Zong, S., & Li, J. (2019). Attenuation Effects of Bulk and Nanosized ZnO on Glucose, Lipid Level, and Inflammation Profile in Obese Mice. Applied Biochemistry and Biotechnology, 190, 475–486.
Wang, Y., Su, N., Hou, G., Li, J., & Ye, M. (2017). Hypoglycemic and hypolipidemic effects of a polysaccharide from Lachnum YM240 and its derivatives in mice, induced by a high fat diet and low dose STZ. Medchemcomm, 8(5), 964–974.
Liu, H., & Gao, C. Y. (2009). Preparation and properties of ionically cross-linked chitosan nanoparticles. Polymers for Advanced Technologies, 20(7), 613–619.
Kalam, M. A. (2016). Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. International Journal Of Biological Macromolecules, 89, 127–136.
Li, J., Shin, G. H., Chen, X. G., & Park, H. J. (2015). Modified curcumin with hyaluronic acid: Combination of pro-drug and nano-micelle strategy to address the curcumin challenge. Food Research International, 69, 202–208.
Hari, P., Chandy, T., & Sharma, C. P. (1996). Chitosan/calcium–alginate beads for oral delivery of insulin. Journal of Applied Polymer Science, 59(11), 1795–1801.
Zhang, H.-L., Wu, S.-h., Tao, Y., Zang, L.-q., & Su, Z.-q. (2010). Preparation and characterization of water-soluble chitosan nanoparticles as protein delivery system. Journal of Nanomaterials, 2010(1), 1–5. https://doi.org/10.1155/2010/898910.
Dehkordi, S. S., Alemzadeh, I., Vaziri, A. S., & Vossoughi, A. (2019). Optimization of alginate-whey protein isolate microcapsules for survivability and release behavior of probiotic bacteria. Applied Biochemistry and Biotechnology, 190, 182–196.
Zhang, N., Li, J., Jiang, W., Ren, C., Li, J., Xin, J., & Li, K. (2010). Effective protection and controlled release of insulin by cationic β-cyclodextrin polymers from alginate/chitosan nanoparticles. International Journal of Pharmaceutics, 393(1-2), 213–219.
Gan, Q., & Wang, T. (2007). Chitosan nanoparticle as protein delivery carrier--systematic examination of fabrication conditions for efficient loading and release. Colloids and Surfaces. B, Biointerfaces, 59(1), 24–34.
Moeini, A., Cimmino, A., Dal Poggetto, G., Di Biase, M., Evidente, A., Masi, M., Lavermicocca, P., Valerio, F., Leone, A., Santagata, G., & Malinconico, M. (2018). Effect of pH and TPP concentration on chemico-physical properties, release kinetics and antifungal activity of Chitosan-TPP-Ungeremine microbeads. Carbohydrate Polymers, 195, 631–641.
Morris, G. A., Castile, J., Smith, A., Adams, G. G., & Harding, S. E. (2011). The effect of prolonged storage at different temperatures on the particle size distribution of tripolyphosphate (TPP) - chitosan nanoparticles. Carbohydrate Polymers, 84(4), 1430–1434.
Xu, Y. M., & Du, Y. M. (2003). Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. International Journal of Pharmaceutics, 250(1), 215–226.
Moya, M. L., Morley, M., Khanna, O., Opara, E. C., & Brey, E. M. (2012). Stability of alginate microbead properties in vitro. Journal Of Materials Science-Materials In Medicine, 23(4), 903–912.
Shah, S., Pal, A., Kaushik, V., & Devi, S. (2009). Preparation and characterization of venlafaxine hydrochloride-loaded chitosan nanoparticles and in vitro release of drug. Journal of Applied Polymer Science, 112(5), 2876–2887.
Mumuni, M. A., Kenechukwu, F. C., Ofokansi, K. C., Attama, A. A., & Díaz, D. D. (2020). Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydrate Polymers, 229. https://doi.org/10.1016/j.carbpol.2019.115506 .
Sarmento, B., Ferreira, D., Veiga, F., & Ribeiro, A. (2006). Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydrate Polymers, 66(1), 1–7.
Sarmento, B., Ribeiro, A., Veiga, F., Sampaio, P., Neufeld, R., & Ferreira, D. (2007). Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharmaceutical Research, 24(12), 2198–2206.
Zhang, Y., Wei, W., Lv, P., Wang, L., & Ma, G. (2011). Preparation and evaluation of alginate–chitosan microspheres for oral delivery of insulin. European Journal of Pharmaceutics and Biopharmaceutics, 77(1), 11–19.
Sarkar, S., Das, D., Dutta, P., Kalita, J., & Manna, P. (2020). Chitosan: A promising therapeutic agent and effective drug delivery system in managing diabetes mellitus. Carbohydrate Polymers, 247, 116594. https://doi.org/10.1016/j.carbpol.2020.116594.
Kevin, L., Huixia, W., Andrew S. N., & Julie A. C. (2019) Alginate/chitosan microparticles for gastric passage and intestinal release of therapeutic protein nanoparticles. Journal of Controlled Release, 295, 174–186.
Funding
This work is supported by National Natural Science Foundation of China (31700015), Fundamental Research Funds for the Central Universities (JZ2018HGTB0244) and Anhui Natural Science Foundation (1808085QC66).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Financial Interests
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J., Wu, H., Jiang, K. et al. Alginate Calcium Microbeads Containing Chitosan Nanoparticles for Controlled Insulin Release. Appl Biochem Biotechnol 193, 463–478 (2021). https://doi.org/10.1007/s12010-020-03420-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03420-9