Abstract
ZnO and ZnO nanoparticles (ZnO NPs) are widely used in food packaging, food preservation, cosmetic preparation, and animal feed. ZnO is alleged showing multiple bioactivities including antimicrobial and anti-inflammation. It is hypothesized in this study that bulk ZnO and ZnO NPs could attenuate symptoms associated with high-fat-diet–induced obesity. Bulk ZnO and ZnO NPs with diameters of 30 and 90 nm were administered to high-fat-diet (HFD)–induced obese mice. Body weight, liver and fat tissue indices of ZnO-treated mice were decreased compared with those of obese mice (MOD). Blood glucose levels in oral glucose tolerant test and insulin tolerant test of ZnO-treated mice were lower than those of MOD. Serum lipid profile of ZnO-treated mice was ameliorated with lower total cholesterol, total triglyceride, and low-density lipoprotein cholesterol levels compared with that of MOD. In addition, the levels of serum IL-1β and LPS-binding protein were also decreased by ZnO treatment. Both bulk and nanosized ZnO could attenuate HFD-induced phenotypes related with obesity, but ZnO NP is more efficient to lower the fat index and bulk ZnO is better to restore the disturbed serum lipid profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc is one essential microelement for many biological functions. Zinc oxide (ZnO) is a widely used food additive for zinc fortification. Due to its safety profile, ZnO is considered by the FDA as a generally recognized safe substance. ZnO nanoparticle (ZnO NP) is one ZnO form with a diameter smaller than 1 μm which presents several unique biological properties including anticancer and antimicrobial activities [1]. Recent studies proved that ZnO NPs could significantly inhibit the growth of several important food-borne pathogens including Escherichia coli, Salmonella typhimurium, and Staphylococcus aureus [2, 3]. Hence, ZnO was also added to prepare food package film, endowing higher mechanical strength, lower water evaporation, lower degree of UV-induced oxidation, etc. [4].
On the other hand, both bulk ZnO and ZnO NPs are widely used to prevent and treat diarrhea in weaning piglets [5]. A large amount (3000 mg/kg BW) of ZnO and ZnO NP supplements could decrease the abundance of Campylobacterales and increase the abundance of Enterobacteriales in weaning piglets. Additionally, ZnO NP decreases the mRNA expression level of several inflammation cytokines, such as IFN-γ, IL-1β, TNF-α, and NF-κB of weaning piglets, and promotes growth performance [6]. As a nutritional fortification and antibiotic alternative, ZnO NP is superior to bulk ZnO in terms of higher intestine absorption and higher bioavailability due to its nanoscale size and consequent physicochemical properties.
However, due to its wide usage in food, feed, and cosmetic industry, its toxic characteristic also attracts much attention. When in a biological environment, ZnO NP dissociates and releases Zn ions, which may produce reactive oxygen species and elevate cellular oxidative stress [7]. Casey et al. investigated the impact of three ZnO NPs with different diameters (26, 78, and 147 nm) on human lymphoblastoid cells and concluded that both particle internalization and cytotoxicity were size dependent [8]. Kim et al. proved that ZnO NP treatment suppressed the level of several inflammatory cytokine expression in C57BL/6 mice and concluded that ZnO NP may induce immunotoxicity [9]. On the other hand, many researchers assessed toxicity of nanomaterials like ZnO NP using novel analysis methods [10, 11]. Hence, the impact of ZnO NP supplement on health status is inconsistent and needs more researches to resolve this problem.
Obesity is one ubiquitous health problem both in developed and developing countries. It is evident that obesity is more prominent in young people with the popularity of fast food and a sedentary lifestyle. As released from a World Health Organization survey in 2016, about 39% of adults are overweight, 13% of whom are obese. In the global scale, more than 41 million children were overweight in 2016 [12]. It should be noted that obesity is not only an esthetic problem but also a serious health risk related with many chronic diseases like diabetes, low-degree inflammation, cardiovascular disease, and cancer [13].
To curb the obesity epidemic, several food supplements have been investigated. For instance, functional polysaccharides isolated from fermentation broth, vegetable, and animals show strong antihyperlipidemic and anti-obesity activities [14,15,16]. A cohort survey of Chinese people indicated that a low level of zinc consumption was highly associated with obesity-related biomarkers including blood pressure, fasting glucose, and triglycerides which could be ascribed to substantial roles played by zinc in immunity, enzyme activity, inhibition of oxidative stress, etc. [17]. Several other studies also proved that zinc supplements could be helpful to regulate obesity and related phenotypes [18, 19]. But information of how bulk and nanosized ZnO could impact obesity is still very scarce.
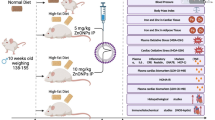
Hence, the present study was aimed to investigate effects of bulk ZnO and ZnO NPs on phenotypes of obesity, especially glucose and lipid metabolism, inflammation profile, and other important biomarkers. Mice were fed with a high-fat diet (HFD) to induce obesity which was followed by treatment of bulk ZnO and ZnO NPs (30 nm and 90 nm) for 4 weeks. Our result will provide valuable information to further researches about the biological functions of bulk ZnO and ZnO NPs.
Materials and Methods
Chemicals and Reagents
Bulk ZnO (pharmaceutical grade, purity of 99–100.5%) and ZnO NPs (30 ± 10 nm and 90 ± 10 nm, both purity of 99.9% on metal basis) were purchased from Aladdin Chemical Co., Ltd. (Shanghai, China). Test kits for low-density lipoprotein cholesterol (LDL-c) (reference number A113-1), high-density lipoprotein cholesterol (HDL-c) (reference number A112-1), total cholesterol (TC) (reference number A111-1), lipopolysaccharide (LPS) (reference number A054-2-1), lipopolysaccharide-binding protein (LBP) (reference number H253), and triglyceride (TG) (reference number A110-1) were all provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). ELISA test kits for IL-1β (reference number PI301), IL-6 (reference number PI326), IL-10 (reference number PI522), TNF-α (reference number PT512), and insulin (reference number PI-602) were purchased from Shanghai Meilian Co., Ltd. (Shanghai, China). All other chemicals used in this study were of analytical grade without further purification.
Animal Grouping and Treatment
A total of 35 Kunming mice, with an average body weight of 20 ± 2 g and age of 8 weeks, were purchased from Laboratory Animal Center of Anhui Medical University, Hefei, China. All mice were raised in standard conditions (23 ± 2 °C, 55 ± 5% relative humidity, 12 h light/dark cycle), and fed a standard diet for 1 week to acclimatize before the experiment started. After that, mice were randomly allocated into five groups (n = 7): normal control group (CON), obesity model group (MOD), bulk ZnO treatment group (ZnOb), ZnO NP 30 nm treatment group (ZnO NP 30), and ZnO NP 90 nm treatment group (ZnO NP 90). Mice in CON were fed with a standard diet and all other groups were fed with HFD for 8 weeks successively to induce obesity. The formula of HFD is displayed in Table S1 which was determined according to a previous publication [20]. From the 9th week, mice in CON and MOD were gavaged with 0.2 mL distilled water, while mice in ZnOb, ZnO NP 30, and ZnO NP 90 were gavaged with 0.2 mL of suspension solution containing bulk ZnO, ZnO NP 30 nm, and ZnO NP 90, respectively, at the concentration of 0.18 g/mL [5]. Gavage treatment was performed for 4 weeks. Afterward, all the mice were fasted overnight before sacrifice by dislocation of the neck. Serum was separated and stored in a fridge at − 80 °C for subsequent analyses. Liver and fat tissues were harvested and weighted. Histological changes were observed on a light microscope after tissue fixation and paraffin sections. Organ index was calculated as organ weight (g)/body weight (g) × 100%.
Liver, Fat, and Colon Histological Observation
Tissue was fixed in 10% formalin, imbedded in paraffin, dehydrated in graded alcohol, and cut into 5-μm-thick slices. Tissue slices were stained with hematoxylin-eosin (H&E), followed by observation on a light microscope.
Oral Glucose Tolerance Test and Insulin Tolerance Test
Three days before sacrifice of mice, oral glucose tolerance test (OGTT) was performed according to the reported method [21]. After fasting for 6 h, all the mice were gavaged with 2.0 g/kg BW of the glucose solution. Blood glucose levels were measured with a glucometer at 0 (basal glucose level), 30, 60, 90, and 120 min after glucose loading. One day before sacrifice of mice, insulin tolerance test (ITT) was undertaken in accordance with the reported method [22]. Mice were fasted for 6 h and injected intraperitoneally with insulin saline solution at the dosage of 1 U/kg BW. Blood glucose levels were recorded at 0 (basal blood glucose level), 30, 60, 90, and 120 min after insulin injection. Areas under the curve of OGTT and ITT were calculated as OGTT-AUC and ITT-AUC.
Serum Lipid Profiles and ELISA Biochemical Biomarkers
Concentrations of HDL-c, LDL-c, TG, and TC in serum were measured using commercial kits. Concentrations of LPS, LBP, and insulin in serum, IL-1β, IL-6, IL-10, and TNF-α in serum, adipose, and liver tissue were determined by commercial ELISA kits.
Statistical Analysis
All data were presented as mean ± standard deviation after being statistically processed. Software SPSS 13.0 was used for ANOVA analysis of the intergroup deviation and p < 0.05 was used to determine significant difference.
Results and Discussions
Body Weight, Organ Indices, and Organ Morphology
From the start of HFD treatment, the body weight (BW) of each group was monitored which was showed in Fig. 1a. BW of MOD was significantly higher than that of CON, indicating that HFD induced obesity in MOD. On the other hand, mean BW of ZnOb, ZnO NP 30, and ZnO NP 90 was lower than that of MOD. It is noted that BW of ZnO NP 30 was quite similar with that of CON. BW result suggests that both bulk ZnO and ZnO NP treatments are beneficial to reverse obesity induced by HFD while ZnO NP is superior than bulk ZnO. Impact of ZnO NP on body weight is related with the dosage as low levels of ZnO NP increase the BW but high levels of ZnO NP decrease the BW of mice fed with a normal diet [23]. Mice treated with ZnO NP at 5000 mg/kg BW showed decreased BW as well as other toxic symptoms. However, Feng et al. pointed out that ZnO NP has little impact on BW of hens [24]. They treated hens with a relatively low level of ZnO NP within the range of 25–100 mg/kg BW and suggested that ZnO NP impacts major blood metabolites through modulating the microbiota profile. However, when fed to broilers, a low dosage of ZnO NP increases the bird’s BW but a high dosage of ZnO NP decreases the bird’s BW [25]. Hence, the effect of ZnO on BW may relate with both the dosage and the ZnO NP size. It should be also noted that mice in each group presented high individual differences, which necessitates further studies to investigate the effect of ZnO on BW.
(a) Body weight change started from the 1st week after high-fat diet (HFD) was provided to mice, ending at 12th week afterward. (b) Liver index of control (CON), HFD-induced obesity model (MOD), bulk ZnO-treated group (ZnOb), ZnO NP 30 nm–treated group (ZnO NP 30), and ZnO NP 90 nm–treated group (ZnO NP 90). (c) Fat index of each group. (d) Kidney index of each group. A bar with different superscripts means significant difference (p < 0.05)
Liver, fat tissue, and kidney were harvested after all the mice were sacrificed. It was found that compared with that in CON, liver index in MOD was higher which resulted from HFD-induced obesity (Fig. 1b). Obese mice accumulated high levels of fat in their liver which increased its weight and pro-inflammation cytokine expression. Zinc supplement in the form of zinc sulfate in solution could ameliorate alcohol-induced liver damage through attenuation of oxidative stress, expression of inflammation cytokines, and major enzymes in the ethanol metabolic pathway [26]. However, a high dose of ZnO NP (mean diameter of 270 nm) treatment increased alanine aminotransferase and alkaline phosphatase concentration and induced lesions and oxidative stress to the liver [27]. It is speculated that the impact on liver function of ZnO is related with both the dosage and its particle size. In our study, HFD increases serum lipid level that induces fat accumulation in liver which is attenuated by ZnO treatment.
Fat tissue index in MOD was also higher than that of CON (Fig. 1c). ZnO and ZnO NP treatments decreased fat tissue index, which was lower than that of MOD but higher than that of CON. Furthermore, bulk ZnO decreased fat level but with no significant difference. Both ZnO NP 30 and 90 treatments significantly decreased fat tissue index. Hence, it was speculated that ZnO supplement could inhibit HDF-induced obesity. Kidney index showed that both bulk ZnO and ZnO NP treatments presented no renal toxicity (Fig. 1d) which was also confirmed by a previous report [23].
Histological images of liver, fat, and colon were displayed in Fig. 2. The liver cell of CON was neatly arranged with no obvious fat droplets. An oval white spot in the center of CON liver is a blood vessel. HFD accelerates fat accumulation in the liver as shown in the picture of a MOD liver which contains a significant amount of fat droplets (round white spots). Treatment by bulk ZnO significantly reduced the number of fat droplets which was similar with that of CON. The liver morphology of ZnO NP 90 also resembled that of CON while the liver of ZnO NP 90 contains a small amount of fat droplets. The morphology of fat tissue reveals the same trend. Fat cells in MOD were significantly enlarged compared with those of CON, indicating more fat was deposited in fat cells in MOD. However, both bulk and ZnO NP treatments reduced the size of individual fat cells and the number of fat cells in one field of microscope. Hence, both liver and fat tissue histological properties indicate that ZnO treatment could inhibit fat accumulation. There is no significant difference between the pictures of the colon of different groups. Besides, morphology of the small intestine and kidney was also examined (shown in Fig. S1). It is obvious that the ZnO supplement presented no obvious toxic effect on these two important organs. Miri Baek et al. reported that ZnO NP treatment exerted no significant impact on histological properties of liver and kidney [28]. They also proved that even administered at a very high level (2000 mg kg−1 BW), no ZnO NP was detected in major organs examined by TEM [29].
OGTT and ITT
Obesity is highly correlated with high blood glucose level and insulin resistance as proven in a previous publication [30]. OGTT was carried out to examine the response of mice to high levels of glucose loading (Fig. 3). It was obvious that MOD presented a higher blood glucose level than CON, suggesting that obesity interferes with glucose metabolism and enhances glucose level. The ZnO supplement decreased the peak value of glucose levels while ZnO NP was more efficient. OGTT-AUC area also indicates that MOD increased the AUC area compared with CON. ZnO NP treatment decreased the OGTT-AUC value which is comparable with that of CON. Hence, OGTT revealed that both bulk and ZnO NPs could revert the abnormal high blood glucose level induced by HFD, and ZnO NP has a stronger effect. Umrani and Paknikar reported that diabetic rats treated with ZnO NP at concentrations of 3 and 10 mg/kg BW showed lower peak value and AUC value in the OGTT which corroborated our result [19]. The improved OGTT profile may be related with increased antioxidant capacity, or inhibition of α-glucosidase and α-amylase activities in the intestinal track [31].
(a) Glucose level changes in the oral glucose tolerant test (OGTT) of control (CON), HFD-induced obesity model (MOD), bulk ZnO–treated group (ZnOb), ZnO NP 30 nm–treated group (ZnO NP 30), and ZnO NP 90 nm–treated group (ZnO NP 90). (b) The OGTT-area under curve (OGTT-AUC) value of each group. (c) Glucose level changes in the insulin tolerant test (ITT). (d) The ITT-AUC value of each group. A bar with different superscripts means significant difference (p < 0.05)
Insulin could decrease the blood glucose level. But obesity renders animals resistant to insulin functions, exhibiting a higher blood glucose level than normal. As shown in Fig. 3c, after insulin injection, the glucose level of CON decreased significantly. The ITT curve of MOD decreased at first and began to increase 30 min after insulin injection. After 120 min since insulin injection, the glucose level of MOD almost rebounded to the basal value while the level of CON was still very low. The curves of ZnO showed significant differences among each other. It was obvious that the ITT curve of ZnO NP 90 was similar with that of MOD, but that of bulk ZnO and ZnO NP 30 resembles that of CON. The ITT-AUC value verifies our observation of the ITT curve. The different responses of ITT and OGTT to different zinc sources may be attributed to the surface properties of ZnO NP as shown by Adersh Asok et al. that smaller ZnO quantum dots are more efficient to inhibit α-amylase and α-glucosidase activities which are highly related to serum glucose level [31]. The authors concluded that smaller ZnO quantum dots present a higher surface defect density that was responsible for their bioactivities. Moreover, as an essential microelement, zinc could reinforce the activities of insulin by enhancing its signal transduction and reducing the cytokine production [32].
Very little information is available about the impact of ZnO supplement on OGTT and ITT in obese mice. Short- and long-term treatments by ZnO NP reduced the OGTT and ITT values and their respective AUC value in diabetic rats [19]. Mohd Idreesh Khan et al. also pointed out that high-dose zinc sulfate supplement to type 2 diabetic nephropathy patients significantly decreased mean fasting blood glucose and post-prandial blood glucose levels [18]. Our result was in line with that of previous publications. However, Huang et al. reported that long-term treatment of high-dose zinc supplement increased the OGTT value while the ITT value decreased [33]. The inconsistent results may be ascribed to different zinc sources, dosages, or treatment periods which requires further researches.
Serum Lipid Profile and Insulin Level
HFD-treated mice present higher serum levels of TC, TG, and LDL-c with lower levels of HDL-c [34]. ZnO supplement significantly reverted the disturbed serum lipid profile as shown in Fig. 4. The elevated level of TC was decreased by both bulk and nanosized ZnO. But the values of ZnO-treated mice were higher than those of CON. On the other hand, TG values of ZnO-treated mice showed no significant difference from those of CON. Our observation agreed with published results as zinc supplement could significantly reduce the level of TG [17, 18]. Meanwhile, LDL-c was also decreased which was still higher than that of CON. The HDL-c value was enhanced by the treatment of ZnO. The insulin level of MOD is higher than that of CON. ZnO supplement showed no significant effect on the insulin level, indicating ZnO treatment exerts no impact on the secretion of insulin in HFD-induced obese mice (Fig. S2). It was reported that RIN-5F cells treated low levels of ZnO NP (1 μg/mL and 3 μg/mL) secreted a higher level of insulin which was decreased when treated with ZnO NP of higher concentrations [19]. Moreover, type 2 diabetic rats treated a higher dosage of ZnO NP (10 mg/kg) showed higher serum insulin levels compared with control and lower dosage treated ones. Different results about insulin secretion may be caused by the different animal models employed in our study.
(a) Serum level of total cholesterol (TC) of control (CON), HFD-induced obesity model (MOD), bulk ZnO–treated group (ZnOb), ZnO NP 30 nm–treated group (ZnO NP 30), and ZnO NP 90 nm–treated group (ZnO NP 90). (b) The serum level of total triglyceride (TG) of each group. (c) The serum level of low-density lipoprotein cholesterol (LDL-C) of each group. (d) The serum level of high-density lipoprotein cholesterol (HDL-C) of each group. A bar with different superscripts means significant difference (p < 0.05)
Inflammation Profile and LPS Level
Obesity usually disturbs the inflammation balance which increases the level of pro-inflammatory cytokines and decreases the level of anti-inflammatory cytokines. Our results showed that MOD presented higher IL-1β and slightly decreased level of IL-10, but showed little impact on IL-6 and TNF-α (Fig. 5). ZnO supplement significantly decreased the elevated IL-1β which was similar with that of CON. IL-6, TNF-α, and IL-10 were not altered by the ZnO treatment. Nagajyothi et al. also reported that ZnO NP could decrease the expression of iNOS, COX-2, IL-1β, IL-6, and TNF-α in the cell [35]. In the present study, both bulk and ZnO NPs decreased the level of IL-1β but bulk ZnO and ZnO NP 30 were more effective than ZnO NP 90. Size and surface properties may be responsible for the different anti-inflammation abilities.
(a) Serum IL-1β level of control (CON), HFD-induced obesity model (MOD), bulk ZnO–treated group (ZnOb), ZnO NP 30 nm–treated group (ZnO NP 30), and ZnO NP 90 nm–treated group (ZnO NP 90). (b) Serum IL-6 level of each group. (c) The IL-10 level of each group. (d) Serum TNF-α level of each group. (e) Serum lipopolysaccharide (LPS) level of each group. (f) serum LPS-binding protein (LBP) level of each group. A bar with different superscripts means significant difference (p < 0.05)
At the same time, inflammation profiles in the liver and fat tissues were also examined (Fig. S2). In line with the trend revealed in the serum, the IL-10 level in the liver and fat tissue of MOD was slightly decreased compared with that of CON, indicating lower anti-inflammation capacity caused by obesity. On the other hand, bulk ZnO– and ZnO NP–treated groups showed higher IL-10 concentration than MOD. The level of IL-6 in MOD was higher than that in CON in fat but not in the liver, suggesting a higher pro-inflammation level in fat tissue. ZnO reverted the IL-6 level which was similar with that of CON. The same trend was also showed in the concentration of TNF-α as both ZnO and ZnO NP treatments reduced the level of elevated TNF-α. ZnO NP with different sizes and surface properties induced immunosuppression both in cell and mice as serum levels of pro-/anti-inflammatory cytokines were suppressed [9]. However, little information about the impact of ZnO NP on obese mice is available. Hence, the size and surface properties of ZnO NP should be carefully manipulated to exert positive effects on the inflammation profile.
HFD also disturbs the permeability of colon and increases the serum level of LPS which also contributes to deterioration of inflammation status [36]. Hence, we analyzed serum LPS and LBP levels. As stated in previous publications, HFD tends to increase serum LPS and LBP levels due to the decreased colon permeability and disturbed microbiota profile [36]. In the present study, LPS concentration in MOD was slightly higher than that in CON. Treatment by both bulk and nanosized ZnO reduced the LPS level, indicating a lower endotoxin level. LBP is the binding protein for LPS, which reflects the level of LPS. Additionally, the level of LBP in MOD was significantly higher than that in CON, suggesting that LBP is more sensitive for detection than LPS. Moreover, the LBP level of both bulk- and nanosized ZnO-treated groups was significantly lower than that of MOD. There is no significant difference between CON and ZnO-treated groups. As LPS could activate the NF-κB pathway and induce expression of several pro-inflammation cytokines, reducing the level of LPS is of importance to ameliorate the inflammation state induced by obesity [37].
Collectively, the ZnO treatment could reduce the pro-inflammation cytokine level and enhance the anti-inflammation cytokine concentration both in serum, liver, and fat tissue of HFD-induced obese mice. Moreover, LPS and LBP levels were also restored by the ZnO treatment. It should be also noted that there is no significant difference between bulk ZnO and ZnO NPs, but further studies are needed to investigate their impact on other important biomarkers pertaining obesity.
Conclusion
In the present study, ameliorated effects of ZnO and ZnO NP on HFD-induced obesity were investigated. Body weight was decreased slightly by the ZnO treatment, and the liver index was also decreased compared with that of MOD. The ZnO NP treatment could significantly decrease the fat index as well as the size of fat cell compared with MOD. Meanwhile, both bulk ZnO and ZnO NPs could regulate the blood glucose level in OGTT and ITT. Profiles of serum lipids and pro-inflammation cytokines were improved by the ZnO treatment. A reduced level of pro-inflammation cytokines and LPS could restore the impaired insulin pathway in obese mice. Along with improved serum lipid profile, insulin sensitivity in fat and liver could also be improved. The present study proved that both bulk and ZnO NP supplements are beneficial to restore the phenotypes of HFD-induced obesity, especially the glucose and lipid levels, but the mechanism of which needs further investigations.
References
Priyadharshini, R. I., Prasannaraj, G., Geetha, N., & Venkatachalam, P. (2014). Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-algae (gracilaria edulis) extracts and its anticancer activity against human PC3 cell lines. Applied Biochemistry and Biotechnology, 174(8), 2777–2790.
Akbar, A., & Anal, A. K. (2014). Zinc oxide nanoparticles loaded active packaging, a challenge study against Salmonella typhimurium and Staphylococcus aureus in ready-to-eat poultry meat. Food Control, 38, 88–95.
Yazhini, K. B., & Prabu, H. G. (2015). Antibacterial activity of cotton coated with ZnO and ZnO-CNT composites. Applied Biochemistry and Biotechnology, 175(1), 85–92.
Nafchi, A. M., Alias, A. K., Mahmud, S., & Robal, M. (2012). Antimicrobial, rheological, and physicochemical properties of sago starch films filled with nanorod-rich zinc oxide. Journal of Food Engineering, 113(4), 511–519.
Pei, X., Xiao, Z., Liu, L., Wang, G., Tao, W., Wang, M., Zou, J., & Leng, D. (2019). Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. Journal of the Science of Food and Agriculture, 99(3), 1366–1374.
Xia, T., Lai, W. Q., Han, M. M., Han, M., Ma, X., & Zhang, L. Y. (2017). Dietary ZnO nanoparticles alters intestinal microbiota and inflammation response in weaned piglets. Oncotarget, 8(39), 64878–64891.
Donaldson, K., & Stone, V. (2003). Current hypotheses on the mechanisms of toxicity of ultrafine particles. Annali dell'Istituto Superiore di Sanità, 39(3), 405–410.
Yin, H., Casey, P. S., McCall, M. J., & Fenech, M. (2015). Size-dependent cytotoxicity and genotoxicity of ZnO particles to human lymphoblastoid (WIL2-NS) cells. Environmental and Molecular Mutagenesis, 56(9), 767–776.
Kim, C. S., Nguyen, H. D., Ignacio, R. M., Kim, J. H., Cho, H. C., Maeng, E. H., Kim, Y. R., Kim, M. K., Park, B. K., & Kim, S. K. (2014). Immunotoxicity of zinc oxide nanoparticles with different size and electrostatic charge. International Journal of Nanomedicine, 9(Suppl 2), 195–205.
Shah, P., Kaushik, A., Zhu, X. N., Zhang, C. X., & Li, C. Z. (2014). Chip based single cell analysis for nanotoxicity assessment. Analyst, 139(9), 2088–2098.
Hondroulis, E., Zhang, Z. Q., Chen, C. Y., & Li, C. Z. (2012). Impedance based nanotoxicity assessment of graphene nanomaterials at the cellular and tissue level. Analytical Letters, 45(2–3), 272–282.
Delgado, G. T. C., & Tamashiro, W. M. D. C. (2018). Role of prebiotics in regulation of microbiota and prevention of obesity. Food Research International, 113, 183–188.
Tomiyama, A. J., Carr, D., Granberg, E. M., Major, B., Robinson, E., Sutin, A. R., & Brewis, A. (2018). How and why weight stigma drives the obesity ‘epidemic’ and harms health. BMC Medicine, 16(1), 123–129.
Wang, Y., Su, N., Hou, G., Li, J., & Ye, M. (2017). Hypoglycemic and hypolipidemic effects of a polysaccharide from Lachnum YM240 and its derivatives in mice induced by high fat diet and low dose STZ. Medchemcomm, 8(38), 680–689.
Hu, S. W., Jiang, W., Li, S. J., Song, W. D., Ji, L. L., Cai, L., & Liu, X. Q. (2015). Fucosylated chondroitin sulphate from sea cucumber reduces hepatic endoplasmic reticulum stress-associated inflammation in obesity mice. Journal of Functional Foods, 16, 352–363.
Ning, C., Wang, X. Y., Gao, S., Mu, J. J., Wang, Y. H., Liu, S. W., Zhu, J. Y., & Meng, X. J. (2017). Chicory inulin ameliorates type 2 diabetes mellitus and suppresses JNK and MAPK pathways in vivo and in vitro. Molecular Nutrition & Food Research, 61(8), 1–9.
Wang, Y., Jia, X. F., Zhang, B., Wang, Z. H., Zhang, J. G., Huang, F. F., Su, C., Ouyang, Y. F., Zhao, J., Du, W. W., Li, L., Jiang, H. R., Zhang, J., & Wang, H. J. (2018). Dietary zinc intake and its association with metabolic syndrome indicators among chinese adults: an analysis of the China nutritional transition cohort survey 2015. Nutrients, 10(5), 1–15.
Khan, M. I., Siddique, K. U., Ashfaq, F., Ali, W., Reddy, H. D., & Mishra, A. (2013). Effect of high-dose zinc supplementation with oral hypoglycemic agents on glycemic control and inflammation in type-2 diabetic nephropathy patients. The Journal of Natural Science, Biology and Medicine, 4(2), 336–340.
Umrani, R. D., & Paknikar, K. M. (2014). Zinc oxide nanoparticles show antidiabetic activity in streptozotocin-induced type 1 and 2 diabetic rats. Nanomedicine, 9(1), 89–104.
Chen, L., Liu, L., Li, C. X., Hu, C. L., Su, F., Liu, R., Zeng, M. Q., Zhao, D. N., Liu, J. K., Guo, Y. R., & Long, J. G. (2017). A mix of apple pomace polysaccharide improves mitochondrial function and reduces oxidative stress in the liver of high-fat diet-induced obese mice. Molecular Nutrition & Food Research, 61(3), 1–12.
Hsu, C. Y., Shih, H. Y., Chia, Y. C., Lee, C. H., Ashida, H., Lai, Y. K., & Weng, C. F. (2014). Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Molecular Nutrition & Food Research, 58(6), 1168–1176.
Brunham, L. R., Kruit, J. K., Pape, T. D., Timmins, J. M., Reuwer, A. Q., Vasanji, Z., Marsh, B. J., Rodrigues, B., Johnson, J. D., Parks, J. S., Verchere, C. B., & Hayden, M. R. (2007). Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nature Medicine, 13(3), 340–347.
Wang, C., Lu, J. J., Zhou, L., Li, J., Xu, J. M., Li, W. J., Zhang, L. L., Zhong, X., & Wang, T. (2016). Effects of long-term exposure to zinc oxide nanoparticles on development, zinc metabolism and biodistribution of minerals (Zn, Fe, Cu, Mn) in mice. PLoS One, 11(10), 1–14.
Feng, Y., Min, L., Zhang, W., Liu, J., Hou, Z., Chu, M., Li, L., Shen, W., Zhao, Y., & Zhang, H. (2017). Zinc oxide nanoparticles influence microflora in ileal digesta and correlate well with blood metabolites. Frontiers in Microbiology, 8, 1–10.
Zhao, C. Y., Tan, S. X., Xiao, X. Y., Qiu, X. S., Pan, J. Q., & Tang, Z. X. (2014). Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biological Trace Element Research, 160(3), 361–367.
Zhou, Z. X., Wang, L. P., Song, Z. Y., Saari, J. T., McClain, C. J., & Kang, Y. J. (2005). Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. The American Journal of Pathology, 166(6), 1681–1690.
Sharma, V., Singh, P., Pandey, A. K., & Dhawan, A. (2012). Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles. Mutation Research, Genetic Toxicology and Environmental Mutagenesis, 745(1–2), 84–91.
Baek, M., Chung, H. E., Yu, J., Lee, J. A., Kim, T. H., Oh, J. M., Lee, W. J., Paek, S. M., Lee, J. K., Jeong, J., Choy, J. H., & Choi, S. J. (2012). Pharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticles. International Journal of Nanomedicine, 7, 3081–3097.
Paek, H. J., Lee, Y. J., Chung, H. E., Yoo, N. H., Lee, J. A., Kim, M. K., Lee, J. K., Jeong, J., & Choi, S. J. (2013). Modulation of the pharmacokinetics of zinc oxide nanoparticles and their fates in vivo. Nanoscale, 5(23), 11416–11427.
Wang, J., Tang, H., Zhang, C., Zhao, Y., Derrien, M., Rocher, E., van -Hylckama Vlieg, J. E., Strissel, K., Zhao, L., Obin, M., & Shen, J. (2015). Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. The ISME Journal, 9(1), 1–15.
Asok, A., Kulkarni, A. R., Ghosh, S., More, P. A., Chopade, B. A., & Gandhi, M. N. (2015). Surface defect rich ZnO quantum dots as antioxidant inhibiting α-amylase and α-glucosidase: a potential anti-diabetic nanomedicine. Journal of Materials Chemistry B, 3(22), 4597–4606.
Jansen, J., Karges, W., & Rink, L. (2009). Zinc and diabetes--clinical links and molecular mechanisms. Journal of Nutritional Biochemistry, 20(6), 399–417.
Huang, X. H., Jiang, D. D., Zhu, Y. G., Fang, Z. F., Che, L. Q., Lin, Y., Xu, S. Y., Li, J., Huang, C., Zou, Y. F., Li, L. X., Wu, D., & Feng, B. (2017). Chronic high dose zinc supplementation induces visceral adipose tissue hypertrophy without altering body weight in mice. Nutrients, 9(10), 1–14.
Yu, Q. H., Zhao, J. J., Xu, Z. K., Chen, Y. W., Shao, T. Y., Long, X. H., Liu, Z. P., Gao, X. M., Rengel, Z., Shi, J. F., & Zhou, J. (2018). Inulin from Jerusalem artichoke tubers alleviates hyperlipidemia and increases abundance of bifidobacteria in the intestines of hyperlipidemic mice. Journal of Functional Foods, 40, 187–196.
Nagajyothi, P. C., Cha, S. J., Yang, I. J., Sreekanth, T. V. M., Kim, K. J., & Shin, H. M. (2015). Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. Journal of Photochemistry and Photobiology, B: Biology, 146, 10–17.
Jiang, T. T., Gao, X. J., Wu, C., Tian, F., Lei, Q. C., Bi, J. C., Xie, B. X., Wang, H. Y., Chen, S., & Wang, X. Y. (2016). Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients, 8(3), 1–20.
Louvet, A., & Mathurin, P. (2015). Alcoholic liver disease: mechanisms of injury and targeted treatment. Nature Reviews. Gastroenterology & Hepatology, 12(4), 231–242.
Funding
This work is financially supported by the National Natural Science Foundation of China (31700015), Fundamental Research Funds for the Central Universities (JZ2018HGTB0244), and Anhui Natural Science Foundation (1808085QC66).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statement
The article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 290 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Zong, S. & Li, J. Attenuation Effects of Bulk and Nanosized ZnO on Glucose, Lipid Level, and Inflammation Profile in Obese Mice. Appl Biochem Biotechnol 190, 475–486 (2020). https://doi.org/10.1007/s12010-019-03115-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03115-w