Abstract
The aim of this study was to perform the adaptation of Lactobacillus paracasei NRRL B-4564 to substrate through adaptive evolution in order to ensure intensive substrate utilization and enhanced L (+)-lactic acid (LA) production on molasses-enriched potato stillage. To evaluate the strain response to environmental conditions exposed during the adaptation process and to select the best adapted cells, the antioxidant activity and LA-producing capability were assessed in batch fermentation. The most promising adapted strain was further used in a pulsed fed-batch mode. Among three selected adapted strains, L. paracasei A-22 showed considerably improved antioxidant capacity, demonstrating more than onefold higher 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging rates compared to parent strain. This strain also exhibited superior LA production in batch fermentation and reached 89.4 g L−1 of LA, with a yield of 0.89 g g−1, a productivity of 1.49 g L−1 h−1, and an optical purity greater than 99%. Furthermore, in fed-batch mode L. paracasei A-22 resulted in 59% higher LA concentration (169.9 g L−1) compared to parent strain (107.1 g L−1). The strain adaptation to molasses environment, performed in this study, is a rather simple and promising method for enhancement of LA production on the complex agro-industrial substrate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lactic acid bacteria (LAB) are a group of industrially important microorganisms that are extensively used in various applications, ranging from the fermentation of food and beverages to production of valuable products such as bacteriocins, exopolysaccharides, and lactic acid (LA). Over the past years, fermentative production of LA has attracted a considerable attention, mostly due to increasing demand for LA as a starting material for polylactic acid (PLA), a biodegradable and biocompatible polymer suitable for food packaging, drug delivery systems, children toys production, etc. [1].
In industrial bioprocesses, LAB are challenged by variety of adverse and fluctuating conditions, such as acid, temperature, oxidative, osmotic and nutrient stresses, etc. [2]. To ensure their efficient industrial applications, a resistance of microbial strains to stress conditions often needs to be improved [3]. Among different physiological features, improving the antioxidant properties was found to be an effective approach for increasing general robustness of LAB against multiple stresses [4]. Some LAB have evolved protection systems, including both non-enzymatic components and antioxidant enzymes in order to overcome the damaging effects of reactive oxygen species (ROS) generated during stressful conditions [2, 4]. Low-intensity oxidative stress can trigger adaptive response and may be beneficial due to strengthening of antioxidant protective potential of the microorganism [5, 6], suggesting that gradually increasing stress conditions could improve the strain robustness. Ability of microorganisms to rapidly adapt to different environmental conditions has been successfully applied in order to evolve microbial strains with desired characteristics for different biotechnological purposes [7]. Thus, the adaptation of producing microorganisms to high concentrations of specific sugars or fermentation inhibitors has been suggested to enhance bioethanol and xylitol production [8,9,10]. However, data on development of adapted LAB used for LA production are insufficiently presented in literature, although essential for their efficient application in large-scale fermentation, especially on cheap and renewable substrates with a complex composition.
Sugar beet and cane molasses are relatively inexpensive industrial by-products which have been used for production of ornithine, succinic acid [11], lysine [12], polysaccharides [13, 14], and LA [15]. In these fermentations, molasses was subjected to different pretreatments (cation exchange resin, sulfuric acid, tricalcium phosphate, potassium ferrocyanide, and EDTA treatments) in order to remove metal ions, dark-colored substances, and other compounds which may inhibit the growth of microorganisms and enzymes associated with product biosynthesis [11,12,13,14,15]. The adaptation of microorganisms used for fermentation to molasses could be a simpler and economically more favorable approach for improvement of fermentation efficiency. In order to avoid or reduce substrate-associated osmotic stress and achieve high productivities, fed-batch fermentation has been widely studied and applied in different fermentation processes [16]. It was proved to be more efficient method for LA production on synthetic substrates compared to batch process [17, 18]. However, the studies of fed-batch fermentation on waste substrates are still limited, mainly because of high dry matter content and complex structure of waste substrates, as well as difficulties to establish an adequate feeding strategy [19].
The main objective of this study was to develop the process for effective adaptation of L. paracasei NRRL B-4564 to sugar beet molasses in order to ensure intensive substrate utilization and enhanced LA production on molasses-enriched distillery stillage. To evaluate the strain response to environmental conditions exposed during the adaptation process, an antioxidant capacity of L. paracasei was assessed. Further, the adapted L. paracasei strains were challenged and compared in batch fermentation mode. Finally, the best adapted strain was selected and used in pulsed fed-batch fermentation in order to improve efficiency of the fermentation.
Materials and Methods
Preparation of Fermentation and Adaption Media
Distillery stillage remained after bioethanol production on wasted potato was obtained from Reahem ethanol plant (Reahem d.o.o., Srbobran, Serbia). Sugar beet molasses remained after the final crystallization stage and separation of raw sugar was provided from Alpis-SLC ethanol plant (Swan lake d.o.o., Belgrade, Serbia). Potato stillage had the following composition: total sugars, 1.57% (w/w); total nitrogen, 0.18% (w/w); alpha amino nitrogen, 190.46 mg L−1; lipids, 0.31% (w/w); ash, 0.89% (w/w). Sugar beet molasses consisted of total sugars, 53.16% (w/w); total nitrogen, 1.31% (w/w); alpha amino nitrogen, 800.77 mg L−1; lipids, 0.28% (w/w); and ash, 11.97% (w/w) [20].
In order to obtain initial sugar concentration of around 100 g L−1, 200 mL of the stillage and 32 g of molasses were mixed. This optimal initial sugar concentration was determined in our previous study [20]. After adjustment of pH in the substrate to 6.5 with 30% NaOH (Sigma-Aldrich, USA), the fermentation media was sterilized at 121 °C for 15 min and used for batch and fed-batch fermentation.
Modified Man Rogosa Sharpe (MRS) broth containing increasing concentration of sugar beet molasses (5–25% w/v) was used as an adaptation media. Modified MRS broth with molasses had the following composition: peptone, 10 g L−1; meat extract, 10 g L−1; yeast extract, 5 g L−1; K2HPO4, 2 g L−1; C2H9NaO5, 5 g L−1; C6H17N3O7, 2 g L−1; total sugars from molasses, approximately 25–125 g L−1; distilled water, 1 L. After adjustment of pH of the substrate to 6.5 with 30% NaOH solution, the medium was sterilized at 121 °C for 15 min and used as adaptation media.
Microorganism
L. paracasei NRRL B-4564, a homofermentative L (+)-lactic acid producing strain, used in these experiments was purchased from Northern Regional Research Laboratory (NRRL, Peoria, USA). This strain was selected as the most promising for LA production on a combined distillery stillage and sugar beet molasses substrate in our previous study [20]. The parent culture was propagated under microaerophilic static conditions using Anaerocult® C bags (Merck KGaA, Darmstadt, Germany) at 37 °C for 18 h in MRS broth before inoculation to fermentation medium.
Strain Adaptation
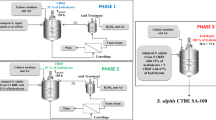
After preliminary studies, the following adaptation protocol was established and used for L. paracasei NRRL B-4564 strain in order to select random mutants for enhanced LA production. A detailed adaptation process performed in this study is shown in Fig. 1.
The first phase of the adaptation experiment was conducted in batch mode and consisted of subsequent transfer of L. paracasei NRRL B-4564 strain to adaptation media containing increasing concentration of sugar beet molasses (5%, 10%, 15%, 20%, and 25% w/v). The culture was propagated with shaking (100 rpm, KS 4000i control, IKA®, Staufen, Germany) at 41 °C in 50 mL of adaptation media with inoculum concentration of 5% (v/v) and under microaerophilic conditions. After reaching the late exponential growth phase (20–24 h), the culture was transferred to a fresh adaptation media with higher molasses concentration for consecutive cultivation. The culture was grown three times at the same sugar beet molasses concentration before exposure to the higher one. The first adaptation phase was carried out for 15 days. After this process, the culture was streaked onto modified MRS agar supplemented with 10% (w/v) of sugar beet molasses and incubated at 37 °C for 48 h under anaerobic conditions. After incubation, the biggest colony designated as A-15 was selected and used for further adaptation procedure.
The second adaptation phase was conducted in fed-batch mode. Isolated colony from previous adaptation stage was inoculated in modified MRS media containing 25% (w/v) of molasses. After depletion of sugar below 70 g L−1, a fresh modified MRS media was fed in order to maintain the total sugar concentration at around 125 g L−1. During this adaptation phase, the samples were aseptically withdrawn every 24 h, and sugar consumption and a number of living cells were further analyzed. The pH value of the media was readjusted to 6.5 by the addition of sterile 30% NaOH solution at 24 h intervals. Adaptation was stopped after significant decrease in viable cell number (bellow 105 CFU mL−1), and slower sugar consumption and lowering of pH were observed. The culture was streaked onto modified MRS agar containing 10% (w/v) of sugar beet molasses after 7 and 14 days of second adaptation phase and the biggest colonies designated as A-22 and A-29 were selected, respectively.
The three selected strains (A-15, A-22, and A-29) were further characterized regarding their antioxidant activity and LA-producing performance.
Determination of Antioxidant Activity of Parent and Adapted Strains
Preparation of Intact Cells and Intracellular Cell-Free Extracts
For evaluation of antioxidant activity parent, L. paracasei NRRL B-4564 strain was grown in MRS broth and modified MRS broth supplemented with 5% (w/v) of sugar beet molasses, while resulting three adapted strains were grown only in modified MRS broth supplemented with 5% (w/v) of molasses. After 18 h of incubation at 37 °C under microaerophilic static conditions, the bacterial cells were harvested by centrifugation (6000×g, 10 min, centrifuge: Sigma® model 2–16, Shropshire, UK), washed twice in deionized water, and resuspended in deionized water to make bacterial cell count to 109 CFU mL−1. The intracellular cell-free extracts were prepared by the method of Lin and Yen [21]. Cell disruption was performed by ultrasonic homogenizer (Sonopuls HD 2200, BANDELIN electronic, Berlin, Germany) for five 1-min intervals (1 min on/1 min off, 35% amplitude) ensuring a constant cooling. Cell debris was removed by centrifugation (8000×g, 10 min, 4 °C) and the resulting supernatant was used for antioxidant activity assay.
DPPH Free Radical-Scavenging Activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging ability of parent and adapted strains was determined according to the method described by Li et al. [22] with some modifications. Briefly, 1 mL of sample (intact cells or intracellular cell-free extracts) was added to 1 mL freshly prepared DPPH solution (0.15 mM in methanol). The mixture was mixed vigorously and incubated at room temperature in the dark for 30 min. The blanks contained the sample and methanol, while the controls included methanol and DPPH solution. After centrifugation (8000×g, 10 min, 4 °C), the scavenged DPPH free radical was analyzed by measuring the decrease in absorbance at 517 nm. The scavenging ability was defined as follows:
Batch and Fed-Batch Fermentation of Molasses-Enriched Potato Stillage
Lactic acid production by parent and adapted L. paracasei (A-15, A-22, and A-29) strains was firstly evaluated and compared in batch fermentation mode. Batch fermentations were performed with shaking (100 rpm, KS 4000i control, IKA®, Staufen, Germany) in 500 mL flasks with 200 mL of the fermentation media. Parent strain was propagated under microaerophilic static conditions at 37 °C for 18 h in MRS broth before inoculation to fermentation media, while adapted strains (A-15, A-22, and A-29) were propagated at the same conditions in modified MRS broth supplemented with 5% (w/v) of molasses. The fermentations were initiated by addition of 10% (v/v) of the inoculum and conducted at temperature of 41 °C, under microaerophilic conditions using gas pack system Anaerocult® C bags (Merck KGaA, Darmstadt, Germany).
The most promising strain in batch mode, L. paracasei A-22, was further used in pulsed fed-batch fermentation and compared with its parent strain. Fed-batch fermentations were performed with shaking (100 rpm, KS 4000i control, IKA®, Staufen, Germany) under microaerophilic conditions. The fermentations were initiated by addition of 10% (v/v) of inoculum and performed in flasks with starting working volume of 200 mL of fermentation media at temperature of 41 °C. A feeding substrate composed of combined potato stillage and sugar beet molasses media with a total sugar concentration of 200 g L−1 was supplied after 12, 24, 36, and 48 h of fermentation, in order to maintain the total sugar concentration at around 100 g L−1.
During the batch and fed-batch fermentations, the pH was adjusted to 6.5 by the addition of sterile 30% NaOH solution at 4-h intervals. The samples were aseptically withdrawn and the substrate consumption, LA concentration, and a number of living cells were further determined.
Methods of Analysis
Total sugar concentration was estimated using 3,5-dinitrosalicylic acid method [23], after sample hydrolysis with HCl in order to break down sucrose into glucose and fructose. The sample solution was hydrolyzed in 10 M HCl at 100 °C for 10 min and then neutralized with NaOH solution. A calibration curve was obtained at 505 nm using standard sucrose solutions. The concentration and optical purity of LA was determined by enzymatic method (L-/D-Lactic acid assay, Megazyme®, Wicklow, Ireland). LA yield determined in the study represents mass (g) of LA produced per mass (g) of initial sugar present in the media. LA yield coefficient was expressed as mass (g) of LA produced per mass (g) of sugar consumed. LA productivity represents mass (g) of produced LA per volume (L) of fermentation media in fermentation time (h). A number of viable cells was estimated using pour plate technique on MRS agar after incubation for 48 h at 37 °C. Vanillin and 5-hydroxymethylfurfural (HMF) in sugar beet molasses were determined by Dionex Ultimate 3000 Thermo Scientific (Waltham, USA) HPLC system. A reverse phase column (Hypersil GOLD C18, 150 mm × 4.6 mm, 5 μm) at 25 °C was employed. The HPLC analysis was performed according to the method of Barroso et al. [24]. Detection was achieved by UV detector at 280 nm. All chemicals used in experiments were analytical grade.
Statistical Analysis
The experiments were done in triplicates. All values are expressed as means ± standard deviation. Mean values of treatments were compared by the analysis of variance. One-way ANOVA followed by Tukey’s test was applied to evaluate the effect of investigated parameters. Differences were considered significant at p < 0.05.
Results and Discussion
The Effect of Adaptive Evolution on Antioxidant Activity of L. paracasei
To ensure an efficient LA production on a complex substrate, the robust microbial strain is highly required, regarding both a broad range of substrate utilization capability and resistance to different adverse compounds or stressful conditions. In order to improve LA production on agro-industrial waste substrate, L. paracasei NRRL B-4564 was exposed to gradually increasing concentration of sugar beet molasses. To illustrate the cellular response to environmental conditions, antioxidant activity of parent and adapted strains selected during the adaptation was further evaluated. The antioxidant activities of parent strain upon exposure to different environment (MRS broth and modified MRS broth supplemented with molasses) and adapted strains (A-15, A-22, A-29) are presented in Fig. 1.
Scavenging of DPPH free radical is attributed to the hydrogen-donating abilities of antioxidants and is the most frequently used method for assessment of antioxidant capacity [25]. The results of present study have shown that the intact cells as well as intracellular cell-free extracts of all tested strains had considerable hydrogen-donating ability and behaved as antioxidants. In general, the strain exposure to higher molasses concentration elicited higher antioxidant activity in L. paracasei. As shown in Fig. 2, parent strain propagated in the presence of molasses demonstrated DPPH scavenging rates of 57.1% (intact cells) and 41.8% (intracellular cell-free extract), which were significantly higher than those of parent strain propagated in a suitable growth media such as MRS broth (Fig. 2). The increase of antioxidant capacity of tested strains subjected to sugar beet molasses indicates that the strain exposure to molasses induced synthesis of antioxidant metabolites and enzymes involved in DPPH free radical scavenging. It has been shown that heavy metals, furan aldehydes, and different phenolic compounds induced the accumulation of ROS (hydrogen peroxide, superoxide anion, and hydroxyl radical) in many bacteria and yeast [26,27,28]. It could be assumed that the improvement of antioxidant defense capacity in L. paracasei observed in this study was the response to the variety of pro-oxidative compounds present in molasses such as furan aldehydes (furfural, HMF), phenolic compounds (vanillin, vanillic acid), volatile compounds (hexanol, heptanol), metal ions, etc. [29, 30].
Although molasses has numerous compounds favorable for microbial growth, it also contains compounds that cannot be used or converted by microorganisms and some of the unfavorable components may inhibit the growth and metabolism of microorganisms. Inhibitory effect of furfural, HMF, acetic acid, syringaldehyde, and vanillin on ethanol [26, 31] or LA [32, 33] producing strains has already been studied. Among reported inhibitory compounds, HMF and vanillin were detected in sugar beet molasses in significant quantities. Most of the inhibitory compounds in molasses are formed during sugar refining process, and their concentration is highly dependent on the production process and can vary greatly among sugar refineries. The amounts of HMF (38.70 mg kg−1) and vanillin (25.49 mg kg−1) detected in molasses were slightly higher than previously reported values for sugar beet molasses (20 mg kg−1 and 17.41 mg kg−1, respectively) [34, 35].
Adapted L. paracasei strains (A-15, A-22, and A-29) demonstrated even higher DPPH radical-scavenging ability, which was in the range of 67.1–85.9% for intact cells and 48.5–61.7% for intracellular cell-free extracts (Fig. 2). Among three adapted strains, L. paracasei A-22 exhibited the highest capability to remove DPPH free radical which was more than onefold higher compared to parent L. paracasei propagated in MRS broth. As can be seen in Fig. 2, A-29 strain selected at the end of adaptation experiment showed loss of scavenging ability compared to A-22 strain (p = 0.00196 ˂ 0.05 for intracellular cell-free extracts and p = 0.05251 > 0.05 for intact cells), suggesting that the exposure to high molasses concentration over longer period resulted in a decrease in antioxidant defense capability of L. paracasei. Here, observed pattern of the stress-induced response demonstrated by L. paracasei is following a kinetics of intensity-based classification of oxidative stress reported by Lushchak [5]. Namely, the activity of antioxidant and associated enzymes in living organisms is dependent on the intensity of oxidative stress. At low-intensity oxidative stress, the activity of antioxidant or associated enzymes could reach a certain maximum, and further with increase of ROS concentration return to the initial level [5].
In order to investigate the potential of adapted strains to produce LA on molasses-enriched potato stillage, three adapted strains (A-15, A-22, and A-29) were further evaluated in batch fermentation and compared with parent L. paracasei.
Batch Fermentation by Parent and Adapted Strains
Adapted and parent strains were investigated for LA production in batch fermentations on molasses-enriched potato stillage and the results are presented in Fig. 3. The main parameters of LA fermentation obtained by adapted and parent strains are shown in Table 1.
The results show that the LA-producing ability was related to their antioxidant capacity. The strain A-22 showed significantly superior LA-producing ability and completed the fermentation much faster than the parent strain (Fig. 3a). During the first 48 h, this strain utilized about 80% of total sugars present in the media, while parent L. paracasei utilized only 56% of total sugars. Final volumetric LA productivity achieved at 60 h of batch fermentation with adapted A-22 strain (1.49 g L−1 h−1) was 31% higher than that obtained with parent L. paracasei (1.14 g L−1 h−1).
Similar trends of sugar consumption and LA production by adapted A-15 and A-29 strains were observed and there was no statistically significant difference between these two adapted strains (p = 0.98781 > 0.05). The LA-producing performance of examined strains showed good agreement with their antioxidant capability, suggesting that improved LA production could be a result of better protection against the harmful compounds present in the media and the effective strain acclimatization to waste substrate. Figure 3b illustrates the growth rate of parent and adapted L. paracasei during batch fermentation. Similarly to production of LA, the growth of adapted cells was also better compared to that of parent cells. Among three selected strains, the strain A-22 showed significantly improved growth performance due to previous effective cell adjustment to the growth environment. Yang et al. [36] studied LA production on mixed bakery waste by engineered and subsequently adapted Thermoanaerobacterium sp. The strain adaptation to high sugar medium in that study led to the improvement in substrate uptake rate and finally resulted in LA concentration of 77.7 g L−1 and productivity of 1.30 g L−1 h−1 [36]. Similarly, the adaptation of Rhizopus oryzae has been demonstrated on corncob hydrolysate rich in xylose in order to improve the efficiency of substrate utilization and enhance LA production [37].
Based on the findings obtained in this study, adapted A-22 strain was chosen for assessment of fed-batch fermentation in order to further improve efficiency of the fermentation.
Fed-Batch Fermentation by Parent and Adapted A-22 Strain
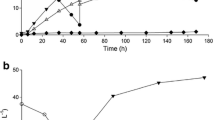
In these experiments, pulsed fed-batch fermentation by parent and adapted A-22 strain was studied and compared. The kinetics of LA fermentation in fed-batch mode on sugar beet molasses-enriched potato stillage is presented in Fig. 4, while the main parameters of fed-batch fermentations are presented in Fig. 5.
During the first 60 h of fed-batch fermentation by adapted strain, a maximal productivity of more than 2 g L−1 h−1 and LA concentration of 125.3 g L−1 were achieved. Also, a high number of adapted L. paracasei cells of more than 1010 CFU mL−1 was obtained during the first 60 h, and after that time, a slight decrease in viable cell number was noticed (Fig. 4b). Since LA production is dependent on cell growth, lower rates of sugar consumption and LA production were observed from 60 to 120 h, during that time 44.6 g L−1 of LA was produced. On the other hand, during the first 60 h of fed-batch fermentation by parent strain, LA concentration of 88.5 g L−1 was obtained and a slight increase of only 18.5 g L−1 of LA towards the end of fermentation was achieved. In addition to lower LA-producing ability, the growth kinetics of parent L. paracasei was also inferior compared to adapted strain, showing a considerable decrease in viable cell number after the third cycle of feeding (Fig. 4a).
The adapted strain showed significantly enhanced LA and biomass producing performance which enabled performing one more feeding cycle compared to its parent strain. This indicates an improved tolerance of adapted L. paracasei to high level of inhibitory compounds accumulated in fermentation media during substrate feeding. Final LA concentration obtained in fed-batch mode by adapted strain (169.9 g L−1) was 59% higher than in fed-batch fermentation by parent strain (107.1 g L−1). The high LA concentration attained in fed-batch fermentation is significant for a subsequent step of LA extraction from the complex fermentation media, since higher concentration of LA in the media enables more efficient recovery of the produced LA [38]. Also, an overall LA yield of 0.79 g g−1 achieved in the fermentation by adapted L. paracasei was significantly higher (p = 0.04967 ˂ 0.05) compared to that obtained by its parent strain (0.67 g g−1). Rather high residual sugar concentration of 20.74 g L−1 observed at the end of fermentation by adapted L. paracasei (Fig. 4b) was a consequence of presence of unfermentable sugars in the molasses, as well as of the inhibition by the product (LA). However, this residual sugar concentration was significantly lower than that obtained at the end of fermentation by parent L. paracasei (41.16 g L−1).
Overall analysis of the results achieved in batch and fed-batch fermentation modes indicates that the prolonged time of the fed-batch process differently affects fermentation parameters. The maximal productivities and biomass production are obtained within the first 60 h, after that time, these parameters are starting to decrease while LA concentration and LA yield coefficients are still increasing towards the end of fermentation. Although the superiority of the fed-batch process to batch process is obvious, a precise determination of the fed-batch fermentation time should be the result of an economic analysis which should take into consideration all relevant factors such as production costs, substrate, and product costs as well as the LA separation costs.
Adaptive evolution of L. paracasei led to significant improvement of fermentation and growth rates. However, it should be pointed out that the mechanism of improved tolerance and adaptation of L. paracasei to molasses environment remained unknown and the genetic basis of the improved phenotype remains yet to be determined. As reported earlier, the adaptation of microorganisms to different harmful compounds could be attributed to the synthesis of enzymes or cofactors which participate in reduction of inhibitory components and allow more effective substrate utilization [7]. In addition to these changes, the improvement of LA-producing performance of adapted L. paracasei could be the result of adaptive changes of the oxidative stress response, as demonstrated in the present study. The effects of furan aldehydes on microbial cells are mainly related to microbial growth inhibition by damaging DNA and/or inhibiting glycolysis pathway and hexsokinases responsible for phosphorylation of six-carbon sugars, changes in the metabolic pathways, re-direction of energy to fix the damage, etc. [32]. The process by which furans can be metabolized by the cells has already been studied for several yeast and LAB strains [31,32,33]. L. plantarum was able to convert furans, including HMF, to less inhibitory components, and at the same time, to produce LA in fermentation of hemicellulosic hydrolysate [32]. On the other hand, phenolic compounds, including vanillin, strongly depressed expression of the genes encoding LA dehydrogenase from Pediococcus acidilactici and thus inhibited LA production even at pretty low concentrations (0.3 g L−1) [33].
Table 2 illustrates the results of the present paper and literature data reported on fed-batch fermentations of different agro-industrial waste substrates.
The LA concentration of 169.9 g L−1 obtained on the molasses-enriched stillage using adapted L. paracasei was superior to the results presented on various waste substrates. Also, in many studies of fed-batch fermentation on different synthetic and rather expensive substrates [17, 18, 47], significantly lower LA concentrations and productivities were reported than in our study. Besides the effective adaptation performed, this could also be attributed to a suitable chemical composition of the waste substrate based on potato stillage and sugar beet molasses, which is a great source of sugars (beet molasses), nitrogen [20], and essential minerals such as Mg and Mn (potato stillage) required for growth of fastidious LAB and efficient LA production [48].
Significant problems in using molasses as a substrate in fed-batch fermentations are related to a high content of potentially adverse compounds. In order to avoid a negative effect of these compounds, a co-feeding of glucose and cane molasses was proposed as an effective strategy for LA production by Bacillus coagulans H-1 [46]. Here, presented adaptation of LAB to waste substrate based on sugar beet molasses-enriched potato stillage resulted in improved antioxidant capacity of producing strain, significantly enhanced substrate utilization, and consequently increased LA and biomass production. Thus, it could be suggested as a simple method for improvement of strain robustness and enhancement of utilization of waste substrates for production of high-value bio-based products such as LA and LAB biomass.
Conclusions
Among three adapted strains evolved by a simple adaptation procedure using sugar beet molasses as a challenging agent, L. paracasei A-22 was the most promising regarding both antioxidant capacity and LA-producing ability. The adapted strain showed significantly enhanced cellular growth and fermentation performance, resulting in 31% higher LA productivity in batch mode compared to parent strain. In addition, increase in effectiveness of LA production was achieved in pulsed fed-batch fermentation with final LA concentration of 169.9 g L−1 obtained after four successive cycles of feeding. The strain adaptation to molasses environment could be applied as a feasible strategy for efficient and sustainable utilization of a complex agro-industrial waste substrate for the production of high-value products such as LA and LAB biomass.
References
Madhavan Nampoothiri, K., Nair, N. R., & John, R. P. (2010). An overview of the recent developments in polylactide (PLA) research. Bioresource Technology, 101(22), 8493–8501.
Van De Guchte, M., Serror, P., Chervaux, C., Smokvina, T., Ehrlich, S. D., & Maguin, E. (2002). Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek, 82(1/4), 187–216.
Zhang, Y., Zhu, Y., Zhu, Y., & Li, Y. (2009). The importance of engineering physiological functionality into microbes. Trends in Biotechnology, 27(12), 664–672.
Zhang, Y., & Li, Y. (2013). Engineering the antioxidative properties of lactic acid bacteria for improving its robustness. Current Opinion in Biotechnology, 24(2), 142–147.
Lushchak, V. I. (2014). Free radicals, reactive oxygen species, oxidative stress and its classification. Chemico-Biological Interactions, 224, 164–175.
Lushchak, V. I. (2011). Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 153(2), 175–190.
Dragosits, M., & Mattanovich, D. (2013). Adaptive laboratory evolution – principles and applications for biotechnology. Microbial Cell Factories, 12(1), 64.
Sunwoo, I. Y., Kwon, J. E., Nguyen, T. H., Ra, C. H., Jeong, G. T., & Kim, S. K. (2017). Bioethanol production using waste seaweed obtained from Gwangalli beach, Busan, Korea by co-culture of yeasts with adaptive evolution. Applied Biochemistry and Biotechnology, 183(3), 966–979.
Misra, S., Raghuwanshi, S., & Saxena, R. K. (2013). Evaluation of corncob hemicellulosic hydrolysate for xylitol production by adapted strain of Candida tropicalis. Carbohydrate Polymers, 92(2), 1596–1601.
Tomás-Pejó, E., Ballesteros, M., Oliva, J. M., & Olsson, L. (2010). Adaptation of the xylose fermenting yeast Saccharomyces cerevisiae F12 for improving ethanol production in different fed-batch SSF processes. Journal of Industrial Microbiology and Biotechnology, 37(11), 1211–1220.
Xu, S., Hao, N., Xu, L., Liu, Z., Yan, M., Li, Y., & Ouyang, P. (2015). Series fermentation production of ornithine and succinic acid from cane molasses by Corynebacterium glutamicum. Biochemical Engineering Journal, 99, 177–182.
He, X., Chen, K., Li, Y., Wang, Z., Zhang, H., Qian, J., & Ouyang, P. (2015). Enhanced l-lysine production from pretreated beet molasses by engineered Escherichia coli in fed-batch fermentation. Bioprocess and Biosystems Engineering, 38(8), 1615–1622.
Küçükaşik, F., Kazak, H., Güney, D., Finore, I., Poli, A., Yenigün, O., Nicolaus, B., & Öner, E. T. (2011). Molasses as fermentation substrate for levan production by Halomonas sp. Applied Microbiology and Biotechnology, 89(6), 1729–1740.
Roukas, T. (1998). Pretreatment of beet molasses to increase pullulan production. Process Biochemistry, 33(8), 805–810.
Kotzamanidis, C., Roukas, T., & Skaracis, G. (2002). Optimization of lactic acid production from beet molasses by Lactobacillus delbrueckii NCIMB 8130. World Journal of Microbiology and Biotechnology, 18(5), 441–448.
Lee, J., Lee, S. Y., Park, S., & Middelberg, A. P. (1999). Control of fed-batch fermentations. Biotechnology Advances, 17(1), 29–48.
Abdel-Rahman, M. A., Tashiro, Y., & Sonomoto, K. (2013). Recent advances in lactic acid production by microbial fermentation processes. Biotechnology Advances, 31(6), 877–902.
Hofvendahl, K., & Hahn-Hägerdal, B. (2000). Factors affecting the fermentative lactic acid production from renewable resources1. Enzyme and Microbial Technology, 26(2-4), 87–107.
Li, Z., Lu, J., Zhao, L., Xiao, K., & Tan, T. (2010). Improvement of L-lactic acid production under glucose feedback controlled culture by Lactobacillus rhamnosus. Applied Biochemistry and Biotechnology, 162(6), 1762–1767.
Mladenović, D., Djukić-Vuković, A., Kocić-Tanackov, S., Pejin, J., & Mojović, L. (2016). Lactic acid production on a combined distillery stillage and sugar beet molasses substrate. Journal of Chemical Technology and Biotechnology, 91(9), 2474–2479.
Lin, M. Y., & Yen, C. L. (1999). Antioxidative ability of lactic acid bacteria. Journal of Agricultural and Food Chemistry, 47(4), 1460–1466.
Li, S., Zhao, Y., Zhang, L., Zhang, X., Huang, L., Li, D., Niu, C., Yang, Z., & Wang, Q. (2012). Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chemistry, 135(3), 1914–1919.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428.
Barroso, C. G., Rodriguez, M. C., Guillen, D. A., & Perez-Bustamante, J. A. (1996). Analysis of low molecular mass phenolic compounds, furfural and 5-hydroxymethylfurfural in Brandy de Jerez by high-performance liquid chromatography-diode array detection with direct injection. Journal of Chromatography A, 724(1-2), 125–129.
Mishra, V., Shah, C., Mokashe, N., Chavan, R., Yadav, H., & Prajapati, J. (2015). Probiotics as potential antioxidants: a systematic review. Journal of Agricultural and Food Chemistry, 63(14), 3615–3626.
Allen, S. A., Clark, W., McCaffery, J. M., Cai, Z., Lanctot, A., Slininger, P. J., Liu, Z. L., & Gorsich, S. W. (2010). Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnology for Biofuels, 3(1), 2.
Solioz, M., Mermod, M., Abicht, H. K., & Mancini, S. (2011). Responses of lactic acid bacteria to heavy metal stress. In E. Tsakalidou & K. Papadimitriou (Eds.), Stress responses of lactic acid bacteria (pp. 163–195). Boston, MA: Springer.
Park, H. S., Um, Y., Sim, S. J., Lee, S. Y., & Woo, H. M. (2015). Transcriptomic analysis of Corynebacterium glutamicum in the response to the toxicity of furfural present in lignocellulosic hydrolysates. Process Biochemistry, 50(3), 347–356.
Taherzadeh, M. J., & Karimi, K. (2011). Fermentation inhibitors in ethanol processes and different strategies to reduce their effects. In: Biofuels: alternative feedstocks and conversion processes (Pandey, A., Larroche, Ch., Ricke, S.C., Dussap, C-G. Gnansounou, E., eds.), Academic Press, pp. 287–311.
Fattohi, N. (1990). Investigation into the presence of volatile secondary constituents of beet molasses with inhibitory effect on yeast fermentation and baker’s yeast quality. Zuckerindustrie, 115(5).
Almeida, J. R., Modig, T., Petersson, A., Hähn-Hägerdal, B., Lidén, G., & Gorwa-Grauslund, M. F. (2007). Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. Journal of Chemical Technology and Biotechnology, 82(4), 340–349.
de Oliveira, R. A., Rossell, C. E. V., Venus, J., Rabelo, S. C., & Maciel Filho, R. (2018). Detoxification of sugarcane-derived hemicellulosic hydrolysate using a lactic acid producing strain. Journal of Biotechnology, 278, 56–63.
Yi, X., Zhang, P., Sun, J., Tu, Y., Gao, Q., Zhang, J., & Bao, J. (2016). Engineering wild-type robust Pediococcus acidilactici strain for high titer L-and D-lactic acid production from corn stover feedstock. Journal of Biotechnology, 217, 112–121.
Filipčev, B., Mišan, A., Šarić, B., & Šimurina, O. (2016). Sugar beet molasses as an ingredient to enhance the nutritional and functional properties of gluten-free cookies. International Journal of Food Sciences and Nutrition, 67(3), 249–256.
Valli, V., Gómez-Caravaca, A. M., Di Nunzio, M., Danesi, F., Caboni, M. F., & Bordoni, A. (2012). Sugar cane and sugar beet molasses, antioxidant-rich alternatives to refined sugar. Journal of Agricultural and Food Chemistry, 60(51), 12508–12515.
Yang, X., Zhu, M., Huang, X., Lin, C. S. K., Wang, J., & Li, S. (2015). Valorisation of mixed bakery waste in non-sterilized fermentation for L-lactic acid production by an evolved Thermoanaerobacterium sp. strain. Bioresource Technology, 198, 47–54.
Bai, D. M., Li, S. Z., Liu, Z. L., & Cui, Z. F. (2008). Enhanced l-(+)-lactic acid production by an adapted strain of Rhizopus oryzae using corncob hydrolysate. Applied Biochemistry and Biotechnology, 144(1), 79–85.
Peinemann, J. C., & Pleissner, D. (2018). Material utilization of organic residues. Applied Biochemistry and Biotechnology, 184(2), 733–745.
Djukić-Vuković, A., Mojović, L., Vukašinović-Sekulić, M., Nikolić, S., & Pejin, J. (2013). Integrated production of lactic acid and biomass on distillery stillage. Bioprocess and Biosystems Engineering, 36(9), 1157–1164.
Marques, S., Gírio, F. M., Santos, J. A. L., & Roseiro, J. C. (2017). Pulsed fed-batch strategy towards intensified process for lactic acid production using recycled paper sludge. Biomass Conversion and Biorefinery, 7(2), 127–137.
Ouyang, J., Ma, R., Zheng, Z., Cai, C., Zhang, M., & Jiang, T. (2013). Open fermentative production of L-lactic acid by Bacillus sp. strain NL01 using lignocellulosic hydrolyzates as low-cost raw material. Bioresource Technology, 135, 475–480.
Wang, L., Zhao, B., Liu, B., Yu, B., Ma, C., Su, F., Hua, D., Li, Q., Ma, Y., & Xu, P. (2010). Efficient production of L-lactic acid from corncob molasses, a waste by-product in xylitol production, by a newly isolated xylose utilizing Bacillus sp. strain. Bioresource Technology, 101(20), 7908–7915.
Hu, J., Zhang, Z., Lin, Y., Zhao, S., Mei, Y., Liang, Y., & Peng, N. (2015). High-titer lactic acid production from NaOH-pretreated corn stover by Bacillus coagulans LA204 using fed-batch simultaneous saccharification and fermentation under non-sterile condition. Bioresource Technology, 182, 251–257.
Ge, X. Y., Qian, H., & Zhang, W. G. (2009). Improvement of l-lactic acid production from Jerusalem artichoke tubers by mixed culture of Aspergillus niger and Lactobacillus sp. Bioresource Technology, 100(5), 1872–1874.
Wang, L., Zhao, B., Li, F., Xu, K., Ma, C., Tao, F., Li, Q., & Xu, P. (2011). Highly efficient production of d-lactate by Sporolactobacillus sp. CASD with simultaneous enzymatic hydrolysis of peanut meal. Applied Microbiology and Biotechnology, 89(4), 1009–1017.
Xu, K., & Xu, P. (2014). Efficient production of L-lactic acid using co-feeding strategy based on cane molasses/glucose carbon sources. Bioresource Technology, 153, 23–29.
Zhang, Y., & Vadlani, P. V. (2013). D-lactic acid biosynthesis from biomass-derived sugars via Lactobacillus delbrueckii fermentation. Bioprocess and Biosystems Engineering, 36(12), 1897–1904.
Cheng, X., Dong, Y., Su, P., & Xiao, X. (2014). Improvement of the fermentative activity of lactic acid bacteria starter culture by the addition of Mn2+. Applied Biochemistry and Biotechnology, 174(5), 1752–1760.
Acknowledgements
Authors acknowledge Milica Carević, PhD, for help in HPLC analysis.
Funding
Research presented in this paper was funded by the Ministry of Education, Science and Technological Development, Republic of Serbia, project number TR 31017 and Scientific Project#1 between People’s Republic of China and the Republic of Serbia 2017–2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Mladenović, D., Pejin, J., Kocić-Tanackov, S. et al. Enhanced Lactic Acid Production by Adaptive Evolution of Lactobacillus paracasei on Agro-industrial Substrate. Appl Biochem Biotechnol 187, 753–769 (2019). https://doi.org/10.1007/s12010-018-2852-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2852-x