Abstract
Levan is a homopolymer of fructose with many outstanding properties like high solubility in oil and water, strong adhesiveness, good biocompatibility, and film-forming ability. However, its industrial use has long been hampered by costly production processes which rely on mesophilic bacteria and plants. Recently, Halomonas sp. AAD6 halophilic bacteria were found to be the only extremophilic species producing levan at high titers in semi-chemical medium containing sucrose, and in this study, pretreated sugar beet molasses and starch molasses were both found to be feasible substitutes for sucrose. Five different pretreatment methods and their combinations were applied to both molasses types. Biomass and levan concentrations reached by the Halomonas sp. AAD6 cells cultivated on 30 g/L of pretreated beet molasses were 6.09 g dry cells/L and 12.4 g/L, respectively. When compared with literature, Halomonas sp. was found to stand out with its exceptionally high levan production yields on available fructose. Molecular characterization and monosaccharide composition studies confirmed levan-type fructan structure of the biopolymers. Rheological properties under different conditions pointed to the typical characteristics of low viscosity and pseudoplastic behaviors of the levan polymers. Moreover, levan polymer produced from molasses showed high biocompatibility and affinity with both cancerous and non-cancerous cell lines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The interest in exopolysaccharides (EPSs) has increased considerably in recent years, as they are candidates for many commercial applications in different industrial sectors like food, petroleum, and pharmaceuticals. EPSs have several advantages over chemical equivalents including biocompatibility and biodegradability. In spite of the advantages, fermentation must be cost competitive with chemical synthesis, and many of the potential applications that have been considered for EPSs depend on whether they can be produced economically. Fermentation medium can represent almost 50% of the cost for a microbial fermentation (Van Hoek et al. 2003). Employing complex media for growth is not economically attractive because of the high amount of expensive nutrients such as yeast extract, peptone, and salts. Hence to achieve high production yields as well as to compete with synthetic petrochemical products in performance and cost, it is a prerequisite to design an optimal cost-effective production medium. Much effort in fermentation process optimization has been made to produce the biopolymers economically from several inexpensive waste substrates, thereby decreasing their production costs (Nicolaus et al. 2010).
Like starch, fructans are another form of reserve carbohydrates in plants. Whereas levan-type fructans are long linear homopolymers of β(2-6)-linked fructose residues, inulin is a shorter β(1-2)-linked fructose polymer. Interacting with phospholipids and hence protecting lipids from undergoing phase transition, fructans are reported to maintain the integrity of membranes during temperature changes, drought, and salt stress (Livingston et al. 2009). As a water-soluble, strongly adhesive, and film-forming biopolymer, levan has many valuable properties like low viscosity, high solubility in oil, compatibility with salts and surfactants, stability to heat, acid and alkali, high holding capacity for water and chemicals, and good biocompatibility. Hence, levan has many potential uses as emulsifier, stabilizer and thickener, encapsulating agent, osmoregulator, and cryoprotector in a number of industries such as food, cosmetics, pharmaceutical, and chemical. Moreover, in medicine, levan is used as plasma substitute, prolongator of drug activity, radio protector, antitumor, and antihyperlipidemic agent (Kang et al. 2009).
These properties distinguishing levan from other polysaccharides have long been of scientific interest. However, due to its high cost, levan has only been produced and utilized in small quantities. Therefore, high-level levan-producing microbial systems may have great industrial importance. Recently, Halomonas sp. has been reported as a high-level levan producer microorganism for the first time by our research group (Poli et al. 2009). Since Halomonas sp. is the only extremophile producing levan at high titers, considerable research effort has been directed to combine the advantages of osmoadaptation and halophilicity to favor a cost-effective and environmentally friendly levan production process.
Due to the fact that sucrose has been shown to promote levan synthesis by Halomonas sp. (Poli et al. 2009), sugar beet molasses was chosen to be used as an inexpensive and renewable substitute for sucrose within the scope of this study. Starch molasses was also used to ascertain the importance of molasses type. Different pretreatment methods and fermentation conditions were studied to optimize microbial growth and biopolymer production. Growth and levan production profiles of the Halomonas sp. cultures revealed growth-associated production at levels considerably higher than other levan producer microbial systems utilizing low-cost fermentation substrates. Levan-type fructan structure of the recovered biopolymers was demonstrated by monosaccharide composition analysis and molecular characterization studies. Rheological properties of the polymers were investigated under different conditions, and results revealed that these levan polymers have the characteristic low viscosity and appear to exhibit pseudoplastic behaviors. Biological activity studies revealed that levan polymer produced from molasses possesses high biocompatibility and affinity with HeLa human epithelial cervical cancer and L929 mouse fibroblast cell lines.
Materials and methods
Microorganism
Halophilic bacterial strain Halomonas sp. AAD6 (JCM15723, DSM 21644, GenBank accession number DQ131909) used in this study was isolated from Çamaltı Saltern Area in Turkey (latitude, 38°25′N; longitude, 27°08′E).
Chemicals
All chemicals and solutions used in this study were supplied by Merck (Germany), Sigma (USA), Difco (USA), and Fluka (Switzerland). Activated carbon (Norit SA4 PAC) was supplied by Norit Inc. (USA).
Molasses
Within the scope of this study, two different types of molasses were used for biopolymer production—sugar beet molasses (BM), which is a byproduct of the manufacture of sucrose from sugar beet, and starch molasses (SM), which is a byproduct of dextrose manufacture from starchy substrates like corn. BM was supplied by Kütahya Sugar Factory (Kütahya, Turkey), and SM containing 50.6% total sugars and 20% glucose was provided by Akmaya Yeast Factory (Avcilar, Turkey). Average composition of sugar beet molasses used in this study was reported to contain 48–51% sucrose, 15–18% moisture, 11–13% ash, 8% betaine, 3.61% potassium, 0.9% chloride, 0.53% calcium, 0.45% sodium, 0.27% sulfate, 0.09% nitrate, 3% carbohydrate, and 7.5% other organic compounds (Kütahya Sugar Factory, Turkey).
Pretreatments of molasses
Starch and sugar beet molasses were subjected to different pretreatment methods like clarification, pH adjustment, sulfuric acid, tricalcium phosphate, and activated carbon treatments as well as their different combinations. Before and after the pretreatments, both sugar beet and starch molasses were analyzed for their total carbohydrate concentration by the phenol/sulfuric acid method (Dubois et al. 1956) and then diluted with distilled water to an appropriate final carbohydrate concentration.
Clarification (CL)
Both molasses were centrifuged at 5,000×g for 15 min, and the clear supernatants were directly used in the experiments without any pH adjustment.
Clarification and pH pretreatment (CpH)
Both molasses were centrifuged at 5,000×g for 15 min, and the pH of the clear supernatants were adjusted to 7.0 and then used for fermentation.
Sulfuric acid pretreatment (H)
First, pH of both molasses was adjusted to 3.0 with concentrated H2SO4 and allowed to mix for 24 h. After centrifugation at 5,000×g for 15 min, the pH of the clear, acid-treated supernatants was adjusted to 7.0 and then used as the carbon source for the experiments.
Activated carbon pretreatment (AC)
Three percent (w/v) activated carbon was added to the molasses, and after 1 h of mixing, insoluble parts were removed by centrifugation at 5,000×g for 15 min. The supernatants were subjected to the same procedure for the second time and then their pH values were adjusted to 7.0 for use in fermentation.
Tricalcium phosphate pretreatment (TCP)
Both molasses were treated with 2% (w/v) tricalcium phosphate (TCP) followed by autoclaving at 105 °C for 5 min, cooling down to room temperature, and then clarification by centrifugation at 5,000×g for 15 min. The supernatants were used in the experiments after their pH was adjusted to 7.0.
Tricalcium phosphate and sulfuric acid pretreatment (TCPH)
TCP pretreated supernatants were acidified with concentrated H2SO4 by mixing for 24 h at pH 3.0. Afterward, they were centrifuged at 5,000×g for 15 min, their pH were adjusted to 7.0, and then used as carbon source for fermentation.
Sulfuric acid and activated carbon pretreatment (HAC)
Acid-treated liquors obtained as explained above were subjected to 3% (w/v) activated carbon pretreatment (AC).
Tricalcium phosphate, sulfuric acid, and activated carbon pretreatment (TCPHAC)
Molasses solutions pretreated with tricalcium phosphate followed by acidification with sulfuric acid (TCPH) were subjected to 3% (w/v) activated carbon pretreatment as explained before.
Media and cultivation conditions
Pretreated molasses were sterilized at 121 °C for 3 min and then added to sterile optimum semi-chemical medium (7 g/L K2HPO4, 2 g/L KH2PO4, 0.1 g/L MgSO4·7H2O, 1 g/L (NH4)2SO4, 0.5 g/L peptone, 137.2 g/L NaCl) (Poli et al. 2009) at a certain final carbohydrate concentration. To prepare preculture, sterile liquid medium was inoculated with 1% (v/v) frozen glycerol culture and incubated at 37 °C at 180 rpm for 24 h in an orbital shaker. One per cent (v/v) of this preculture was used as an inoculum.
Batch shake flask experiments were carried out in 250-mL Erlenmeyer flasks with medium volumes up to one third of the flask volume. To investigate the levan production profiles, 1-L Erlenmeyer flasks containing 300 ml culture media were used. Certomat BS1 (80 × 42 cm tray) and New Brunswick Scientific Excella E 24 (46 × 46 cm tray) orbital shaker incubators were used with the set agitation rate of 180 rpm and the set temperature of 37 °C.
Analytical methods
Cell growth was monitored by measuring offline the optical densities at 660 nm using Lambda35 UV/Vis spectrophotometer. Biomass concentration in terms of dry cell weight (DCW) per liter was determined gravimetrically using harvested cells which were washed with distilled water and then dried at 100 °C until constant cell dry weight was achieved. To determine the total carbohydrate concentration, EPS samples were dissolved in ultra pure distilled water (1% w/v). Carbohydrate content was determined using phenol/sulfuric acid method using glucose as standard (Dubois et al. 1956). Protein concentration was determined by the Bradford test using bovine serum albumin as standard (Bradford 1976). Spectrophotometric measurement at 260 nm was used to determine the quantity of nucleic acids in the EPS samples. Heavy metals were analyzed by using PerkinElmer AAnalyst 300 atomic absorption spectrophotometer.
Isolation and purification of EPS
For the purification of EPS, cells at their early stationary phase of growth were harvested by centrifugation at 13,000×g for 20 min, and the supernatant phases were treated with two volumes of ethanol, held at −18 °C overnight, and then centrifuged at 15,000×g and 4 °C for 30 min using a refrigerated centrifuge. The pellets were dissolved in hot distilled water, dialyzed against several runs of distilled water for 3 days, lyophilized, and then weighed. The EPS samples were analyzed for total carbohydrate, protein, and nucleic acid contents.
Chemical characterization
For the sugar analysis, lyophilized samples (3–4 mg) were hydrolyzed with 0.5 M trifluoroacetic acid (TFA) at 120 °C for 1.5 h. The sugar composition of the EPS was analyzed by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE-PAD) using standards for identification and calibration curves (Manca et al. 1996). Fourier transform-infrared (FT-IR) spectroscopy spectra of EPS were obtained with Nicolet 6700 FT-IR spectrometer between 400 and 4,000 wave numbers (per centimeter).
Rheological studies
Lyophilized EPS samples and standard polymers including xanthan (Sigma no. G1253), sodium alginate (Sigma no. A2033), and pullulan (Sigma no. P4516) were dissolved in either distilled water or 10% NaCl solution at varying concentrations, and the shear viscosity measurements of the aqueous polymer solutions were recorded using AR 1500ex rheometer (TA Instruments, USA) at 20 and 37 °C. The shear stress used was in the range of 0.1–1.0 Pa for low, 1.0–10.0 Pa for medium, and 5.0–15.0 Pa for high stress conditions, respectively.
Biological activity studies
A cancerous cell line HeLa (human cervical cancer cells) and a non-cancerous cell line L929 (mouse fibroblast cells) were used in this study, and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2 H tetrazolium bromide (MTT) cell proliferation assay was employed to assess the cell viability. L929 and HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum and 100 U/mL penicillin/streptomycin. Both cells were seeded in a 96-well plate at the concentration of 4 × 103 cell/well with triplicate and incubated overnight under 5% CO2 in a humidified atmosphere at 37 °C. Cell culture media contained 1, 5, and 50 μg of pure levan, and cultures without levan were used as control. Cell viability was tested by MTT assay after 24, 48, and 72 h of incubation. The MTT assay was carried out using a modification of the method of Mossman (1983). Briefly, 20 μl of MTT (5 mg/mL) was added into each well followed by 2-h incubation at 37 °C in 5% CO2 incubator. Subsequently, optical density, which is directly correlated with cell quantity, was read at 490 nm using EL×800 universal microplate reader (Bio-TEK instruments, Inc., USA). Cells cultured with medium alone were used as control, and the cell viability was considered as 100%. The absorbance of the DMSO blank was subtracted from all values. Absorbance measured in the MTT assay was converted to percent cell viability.
Results
Pretreatment of molasses
Sugar beet molasses and starch molasses were subjected to five different physical and chemical pretreatment methods, i.e., clarification, pH adjustment, sulfuric acid, tricalcium phosphate, and activated carbon treatment. Together with combinations of these methods, eight different pretreatment procedures were applied to both sugar beet and starch molasses making a total of 16 different pretreated molasses samples. All pretreatments involved a final clarification step before their use as carbon source in the experiments. With the exception of CL, the final pH values of all the pretreated molasses samples were adjusted to 7.0 and then used for microbial fermentation. The initial pH values of the untreated molasses samples were found to vary within the range of 7.35– 7.65. Total carbohydrate content of untreated sugar beet and starch molasses were found to be around 550 and 500 g/L, respectively. Moreover, negligible differences in carbohydrate contents of the samples before and after the pretreatments suggested the fact that pretreatments have not affected the sugar contents of the molasses as also reported by Kundu et al. (1984). To investigate the effect of pretreatments on the removal of heavy metal from the molasses, the pretreated samples were analyzed for their iron, zinc, and nickel content and percent changes in each pretreatment were calculated. The pretreatments applied in this study and changes in heavy metal contents are summarized in Fig. 1.
Generally, iron, zinc, and nickel concentrations were within 0.5–13, 0.3–2.3, and 0.4–0.6 mg/L ranges, respectively. The clarified and pH-adjusted molasses samples indicated that whereas starch molasses was richer in its iron content, sugar beet molasses was richer in its zinc content. No significant differences between the two types of molasses could be detected about their nickel content (results not shown).
As shown in Fig. 1, when CL and CpH treatments were compared, pH adjustment was found not to change the heavy metal concentration in both molasses types as expected. In terms of iron, zinc, and nickel removal, effect of the strong acid pretreatment (H) on both molasses was also low. About 10% decrease in iron content was observed whether the acid treatment was applied to clarified or TCP-treated starch molasses samples. Iron in the clarified beet molasses was 21% reduced by acid treatment; however, a slight increase (8%) in iron content was observed in the TCP-treated beet molasses. This could be most probably because of the fact that TCP treatment already removed more than 70–80% of the iron content of both types of molasses. Not only iron, but also zinc was removed up to 70% from both molasses by the TCP treatment.
Effect of pretreatments on microbial levan production
To evaluate the effect of pretreatments on the growth and levan production, fermentation experiments were conducted with shake flask cultures where pretreated molasses samples were added aseptically to the 75 ml optimum semi-chemical medium at 10 and 30 g/L of final carbohydrate concentrations. Microbial production was terminated 5–10 h after the cultures entered their stationary phase of growth with negligible changes in the turbidity (OD660nm) of the cultures. Dry cell mass and levan yields of the cultures were summarized in Table 1.
Biomass and levan yields of clarified (CL), acid-treated (H), and pH-adjusted (CpH) starch molasses cultures were clearly distinguishable from the other ones. As also mentioned before, CL, CpH, and H treatments were found not to change the heavy metal concentration in both molasses types. Hence, the obvious improvement in growth may result from TCP or AC treatments that changed the starch molasses composition in favor of the metabolic needs of Halomonas sp. AAD6 cells. In sugar beet molasses, TCPH and TCPHAC cultures reached higher biomass concentrations than the others suggesting the importance of additional TCP treatment over H or HAC for beet molasses. TCPHAC pretreatment for beet molasses gave the highest amount of cellular mass whereas activated carbon (AC) pretreatment was the best pretreatment for bacterial growth in starch molasses.
At 10 g/L, highest levan concentrations were reached by cultures grown in TCPHAC-pretreated sugar beet and starch molasses with yields of 4.19 and 3.68 g/L, respectively. Since the growth and levan yields of both types of molasses were close, they were also compared at a higher initial concentration (30 g/L). At 30 g/L HAC and TCPHAC, about 70% more levan concentrations could be reached with the beet molasses when compared with starch molasses. The levan yields on biomass ranged between 0.50 to 1.31 g of levan per gram of DCW for beet molasses with the highest yields obtained from the TCPH and TCPHAC cultures. For the starch molasses though, besides CL, CpH, and H, the yields were found to vary within a narrow range of 0.61–0.81 g of levan per gram of DCW (Table 1).
All levan samples, both from beet and starch molasses, were composed 90–100% of carbohydrate molecules excluding of acid pretreated (H) molasses of both kinds where the carbohydrate content dropped to 80% (results not shown). This drop was most probably because of cell lysis, as also verified by the high nucleic acid and protein contents of these samples. Similar observation could also be made for the CpH-treated starch molasses with 90% carbohydrate content (results not shown).
Effect of color removal by repeated activated carbon treatment
In order to investigate the effect of color removal and some trace metals (Fe, Ni, Zn) in beet molasses on biomass growth and levan production, clarified sugar beet molasses (BM CpH) was subjected to multiple runs of 2% (w/v) AC pretreatment. Color removal was followed by measuring the optical density of the pretreated molasses samples at 395 nm (Ahmadi et al. 2006). Samples were added to chemical media at 10 g/L final carbohydrate concentration, and stationary phase Halomonas sp. AAD6 shake flask cultures were analyzed for biomass and levan production. Results are tabulated in Table 2 together with the heavy metal composition of AC-treated sugar beet molasses samples.
As expected with repeated cycles of AC treatment, coloring substances were effectively adsorbed on AC resulting in up to 90% color removal. In accordance with earlier results (Fig. 1), dissolved iron (Fe+2) concentration increased with number of cycles, however, with decreasing efficiencies so that three and four cycles of pretreatment only resulted in 38% and 10% increases in iron concentration, respectively. Nickel concentration was found to change ±5% resulting in only minor changes, similar to previous observations. Zinc was effectively removed in three cycles of pretreatment, and an additional fourth cycle did not result in a detectable change in zinc content.
Cultures grown on two times treated BM reached stationary phase after 75 h, and additional 40 h of fermentation did not result in any change in culture turbidity. On the other hand, although cultures grown in three and four cycles of AC-treated molasses were found to reach higher optical densities after 120 h of fermentation, culture turbidity was found to decline rapidly within the following 30 h. Hence, prolonged incubation had a negative effect on the maintenance of cellular integrity resulting in cell lysis and associated lower absorbency values. This hypothesis was strengthened by the high nucleic acid and protein and low carbohydrate contents of the samples indicating that cellular proteins and DNAs were released to the broth because of lysis and co-precipitated with EPSs (results not shown).
Monosaccharide analysis
Monosaccharide composition of the EPSs was analyzed by HPAE-PAD, and as shown in Table 3, all the EPSs were mainly composed of fructose units with trace amounts of glucose. Hence, all samples were actually fructans, and presence of glucose could be attributed to the bacterial fructan biosynthesis where sucrose acts as acceptor for the initial polymerization by the levansucrase enzyme resulting in a glucosyl residue at the end of each chain (Ozimek et al. 2006).
Structural characterization of EPSs
FT-IR spectra of the levan produced by cultures growing on TCPHAC-pretreated sugar beet molasses were measured to determine their structural differences from levan produced by Halomonas sp. AAD6 on pure sucrose as well as from commercial levan produced by Zymomonas mobilis (Fig. 2). In these spectra, the strong bands around 3,300 cm−1 were assigned to the hydroxyl (OH) stretching vibration of the polysaccharides, and the two bands existing around 2,900 and 2,950 cm−1 were because of carbon–hydrogen (C–H) stretching vibration which indicate the existence of fructose residue (Liu et al. 2010). The bands in the region of 1,430 and 1,200 cm−1 were assigned to C–H plane deformation vibration combined with aromatic skeletal vibrations (Schwanninger et al. 2004; Liu et al. 2010). The bands between 1,120 cm and 1,020 cm−1 were dominated by the stretching vibrations of the glycosidic linkage contributions of C–O–C and C–O–H (Wu et al. 2009). A characteristic absorption around 930 cm−1 resulted from the stretching vibration of pyran ring (Schwanninger et al. 2004). The high level of resemblance observed in the spectra of the samples suggested the fact that these BM TCPHAC polymers were in fact levan-type polysaccharides.
FT-IR spectra of commercial levan and levan produced by Halomonas sp. on pure sucrose (Poli et al. 2009) and on pretreated molasses
Effect of initial sugar concentration
In order to determine the optimum initial substrate concentration, preliminary shake flask experiments were conducted in optimum medium containing TCPHAC-treated sugar beet molasses at 50, 100, 150, and 200 g/L of concentrations. The growth of the cultures were followed, and the turbidity of the stationary phase cultures measured as OD660nm was found to have decreased from 10.8 (for 30 g/L) down to 2.77, 0.136, 0.144, and 0.110 for 50, 100, 150 and 200 g/L of initial sugar concentrations, respectively. This could be because of the inhibitory osmotic stress that the cells were exposed to high salinity and sugar concentrations, as suggested by Liu et al. (2008). Same experiments were performed with 45 g/L of initial sugar concentration, and the biomass concentration (4.68 g DCW/L) and levan yields (6.86 g/L) were both lower than 5.75 g DCW/L of biomass and 7.56 g/L of levan yields obtained with 30 g/L of initial concentration of TCPHAC-pretreated beet molasses (Table 1).
Levan production profiles
Time course of the growth and levan production of Halomonas sp. AAD6 cells cultivated on 30 g/L TCPHAC-pretreated beet molasses is shown in Fig. 3. During microbial production, samples were taken and polymers were purified from the culture supernatants by alcohol precipitation and dialysis. Levan production was found to be growth-associated as also reported earlier for production from sucrose containing semi-chemical medium (Poli et al. 2009). Biomass and levan concentrations reached by the cultures after 210 h of fermentation period were 6.09 g DCW/L and 12.4 g/L, respectively. All the polymer samples were found to contain mainly carbohydrate (95–99%) with less than 0.5% protein and 1% nucleic acid.
Rheological analysis of levan produced by Halomonas sp.
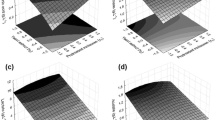
In order to understand rheological characteristics of levan produced from pretreated sugar beet molasses, flow characteristics of 1% BM TCPHAC aqueous solutions prepared with either distilled water or 10% NaCl were measured at two different temperatures (20 and 37 °C). To understand the thixotropic and shear thinning properties of the samples, steady shear stress was measured over a range of shear rates of 10–200 s−1 at the aforementioned temperatures and salt concentration (Fig. 4). BM TCPHAC aqueous solutions have shown the characteristic of low viscosity which in turn is in good agreement with earlier reported 0.13–0.38 dL/g range for the intrinsic viscosity of levan from Microbacterium laevaniformans (Bae et al. 2008). These intrinsic viscosity values were quite low compared with typical intrinsic viscosities ranging from 1 dL/g for compact coil or flexible chains (dextran) to 20 dL/g for extended chains (alginate) and up to 50 dL/g (xanthan).
The shear stress measurements for the biopolymer solutions indicated that the shear stress versus shear rate curves were characterized by increased shear stress with increasing shear rate (Fig. 5). As a consequence, all biopolymer samples appeared to exhibit pseudoplastic behaviors. Furthermore, the shear stress versus shear rate curves were fitted into the Oswald–de Waele model (σ = K·γ n), where σ is the shear stress (Pa), γ is the shear rate (1/s), K is the consistency coefficient (Pa sn), and n is the flow behavior index. Oswald–de Waele model parameters for BM TCPHAC were given in Table 4 together with those of xanthan, pullulan, and alginic acid. The flow behavior indices, n, which provide the degree of pseudoplasticity were determined to be less than 1, which verified the pseudoplastic behaviors of the polymer samples. Moreover, the consistency coefficient (K), which is a measure of viscosity, was found to vary considerably with the type of sample. As expected, its value for xanthan was highest within the range 2.7–5.3 Pa sn. The corresponding values for the levan samples were closer to alginic acid.
Biological activity
Levan produced by Halomonas sp. AAD6 grown in semi-chemical medium was subjected to several biocompatibility tests with osteoblast cells isolated from the calvaria of Wistar rats and mouse monocyte/macrophage cell line J774. Results showed that this EPS did not affect cellular viability and proliferation of osteoblasts and murine macrophages. Moreover, the protective effect of the polymer against the toxic activity of avarol implied its additional use as an anti-cytotoxic agent (Poli et al. 2009).
To assess the biocompatibility of levan from Halomonas sp. produced from beet molasses, basic in vitro tests were performed with HeLa and L929 cell lines. Cell cultures were stimulated with BM TCPHAC samples, and after 24, 48, and 72 h, MTT cell proliferation assay was employed to assess the cell viability. No significant difference in viability for 24 and 48 h of incubation for both cell lines was detected when they were incubated in the presence of 1, 5, and 50 μg of levan (Fig. 6). However, after 72 h, a significant cell proliferation was detected for HeLa cells at low polymer concentrations. Although in vitro antitumor activity of levan produced by Gluconoacetobacter xylinus, M. laevaniformans, Rahnella aquatilis, and Z. mobilis has been shown against SNU-1 (human stomach carcinoma cell) and Hep G2 (hepatocellular carcinoma cell) tumorogenic cell lines (Yoo et al. 2004), no such antitumor effect could be detected for levan produced in this study. On the contrary, levan polymer showed high biocompatibility and affinity with both cell lines.
Discussion
Due to its many advantages like high sucrose and other nutrient contents, low cost and ready availability, and ease of storage, molasses has long been used as a substrate for fermentative production of commercial polysaccharides such as curdlan (Lee et al. 2003), xanthan (Kalogiannis et al. 2003), dextran (Vedyashkina et al. 2005), scleroglucan (Survase et al. 2007), and gellan (Banik et al. 2007). In this study, molasses has been shown to be an economical and renewable alternative to sucrose for the microbial levan production by Halomonas sp.
It is known that trace elements like Fe, Ni, and Zn are essential for microbial growth (Zhang et al. 2003; Patidar and Tare 2006; Müller 2009). Since levan production process in the Halomonas system is growth-associated (Poli et al. 2009), it is highly linked to the growth conditions of the cultures. Therefore, heavy metal composition of the pretreated molasses samples was analyzed in this study, and TCP was found to be an effective compound for selective removal of iron and zinc from molasses or other mixtures of comparable composition. Similar results were also reported in the literature. For citric acid production using Aspergillus niger T55, 81% of iron in the cane molasses was removed by both TCP and TCPH methods and zinc removal was 84% by TCP and 96% by TCPH pretreatments (Kundu et al. 1984). Also, for the citric acid production from a novel A. niger strain, 9.5% iron removal and 56% zinc removal by TCP method were reported (Lotfy et al. 2007). Heavy metals like iron, zinc, and nickel enter the apatite crystal structure of TCP (Ca3(PO4)2) by replacing the Ca atom and causing some distortion in the crystal structure (Yin et al. 2002).
Whereas TCP treatment was not effective for nickel removal, AC adsorbed 20–40% of the nickel from the treated samples. Removal of Zn, on the other hand, remained within 4–14% range. As such, in the TCPHAC-treated samples, from the three consecutive steps, TCP step removed Zn and AC adsorbed Ni from the clarified molasses. After the AC treatment, a drastic increase in the dissolved iron (Fe+2) content was observed (Fig. 1). This could be because of the reduction of iron from its impregnated Fe+3 form to its soluble form, since this increase in soluble iron was more profound when acid-treated samples were subjected to AC treatment to yield HAC and TCPHAC.
In both molasses types, pretreatments like clarification and pH adjustment were not adequate as also revealed by the low levan production yields. This is an expected situation because of the retained undesirable constituents (e.g., heavy metals, impurities) which influence the growth of microorganism and associated polysaccharide production (Kundu et al. 1984; Roukas 1998; Jiang et al. 2009). The growth promoting effects of the AC and TCP pretreatments of starch molasses were also reflected in the levan production yields which were comparable to sugar beet molasses, making starch molasses also a potential fermentation substrate besides sugar beet.
Generally, biopolymer amounts that were produced from pretreated sugar beet molasses were higher than those produced from starch molasses, most probably because of their predominant monosaccharides which are sucrose and glucose for sugar beet and starch molasses, respectively. This result is also in accordance with the reported fact that sucrose was the best carbon source for both biomass and EPS production (Poli et al. 2009).
In this study, removal of heavy metals and increases in iron concentration most probably affected the cellular metabolism that did not result in any growth impairment but rather resulted in diminished maintenance of the cellular integrity and lower EPS yields. These observations pointed to the important fact that besides microbial growth, EPS production profiles should also be considered and fermentation time should be decided accordingly.
The diauxic growth and associated polymer production profiles observed in Fig. 3 suggested the preferential utilization of various carbon sources present in molasses which are predominantly sucrose, but there are also significant amounts of glucose and fructose and lower amounts of raffinose as well. Whereas biomass formation was reported in the presence of all these four sugars, only Halomonas sp. AAD6 cells grown in the presence of sucrose and raffinose were found to produce high and low amounts of levan, respectively (Poli et al. 2009). In the first phase of the diauxic profile with 0.0868 ± 0.04 g of levan per liter per hour of levan productivity, the microbial system was found to produce 8.9 g/L of levan within 100 h of fermentation period. After a lag period where metabolic pathways were probably induced for the utilization of a less favored carbon source like raffinose, cultures entered the second phase of production where productivity decreased to 0.0299 ± 0.05 g of levan per liter per hour. Further studies on levan production process should be performed with bioreactor cultures where many important fermentation parameters such as biomass, levan production, dissolved oxygen, and sugar profiles could be followed. Such data would provide more in-depth understanding of the re-organization of metabolism of the Halomonas sp. AAD6 cultures in the presence of different sugars.
Based on the theoretical yield on available fructose (0.526 g of levan per gram of sucrose), the levan yield of this study is 79% of the theoretical yield assuming that all the 30 g/L of initial carbohydrate is sucrose. As shown in Table 5, besides Halomonas sp., optimization of levan production by use of low-cost fermentation substrates is reported for only two microbial systems, namely Paenibacillus polymyxa (NRRL-18475) (Han and Watson 1992) and Z. mobilis (Oliveira et al. 2007). For the microbial production with P. polymyxa NRRL B-18475, sugar cane syrup and sugar beet molasses resulted in very low levan yields. Therefore, peptone was added to the cane syrup, and beet molasses was subjected to various expensive pretreatments like passing it through gel filtration and anion-exchange columns in order to increase the levan yields to levels comparable with sucrose (Han and Watson 1992). Recently, a statistical optimization study on levan production by a very similar strain, P. polymyxa NRRL B-18475, was reported to yield 35.3 g/L of levan in sucrose containing complex medium (Liu et al. 2010). Average levan productivity of Halomonas sp. AAD6 cultures (1.42 g/L/day) was found to be comparable to that of P. polymyxa NRRL B-18475 cultures grown on sugar cane syrup (Table 5). To produce levan from Z. mobilis, both sugar cane molasses and sugar cane syrup were clarified by centrifugation followed by filtration and then used at 250 g/L of carbohydrate concentration; however, theoretical yields are lower than the other two microbial systems. When the levan yields in Table 5 are compared, Halomonas sp. stands out with its exceptionally high levan production yields on available fructose.
Microbial system used and culture conditions are the two main established factors for the chemical structure and size of levan which in turn are closely related to the biological activity. Yoon et al. (2004) investigated the effect of branching degree on the antitumor activity of levan from M. laevaniformans by stepwise enzymatic debranching and reported a decreasing activity with decreasing degree of branching. Considering the crucial role of the branch structure in levan’s antitumor activity, absence of antitumor activity of levan from Halomonas sp. could be attributed to the lack of branching in its chemical structure.
Like every emerging technology, difficult engineering and economic hurdles stand in the way of biopolymer commercialization efforts. As such, levan is a very expensive polymer and therefore any reduction in its production cost has high industrial importance. Halomonas sp. AAD6 was found to be a promising microorganism for levan production, and with this study, about ten-fold increase in theoretical levan yields was achieved by use of low-cost substrates like sugar beet molasses and starch molasses as substitutes for sucrose. Current studies are focused on the optimization of fermentation conditions for reproducible and large-scale levan production by Halomonas sp. bioreactor cultures.
References
Ahmadi M, Vahabzadeh F, Bonakdarpour B, Mehranian M (2006) Empirical modeling of olive oil mill wastewater treatment using loofa-immobilized Phanerochaete chrysosporium. Process Biochem 41:1148–1154. doi:10.1016/j.procbio.2005.12.012
Bae IY, Oh IK, Lee S, Yoo SH, Lee HG (2008) Rheological characterization of levan polysaccharides from Microbacterium laevaniformans. Int J Biol Macromol 42:10–13. doi:10.1016/j.ijbiomac.2007.08.006
Banik RM, Santhiagu A, Upadhyay SN (2007) Optimization of nutrients for gellan gum production by Sphingomonas paucimobilis ATCC-31461 in molasses based medium using response surface methodology. Bioresour Technol 98(4):792–797. doi:10.1016/j.biortech.2006.03.012
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Dubois MA, Gilles KA, Hamilton JK, Robers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. doi:10.1021/ac60111a017
Han YW, Watson MA (1992) Production of microbial levan from sucrose, sugarcane juice and beet molasses. J Ind Microbiol 9:257–260. doi:10.1007/BF01569633
Jiang L, Wang J, Liang S, Wang X, Cen P, Xu Z (2009) Butyric acid fermentation in a fibrous bed bioreactor with immobilized Clostridium tyrobutyricum from cane molasses. Bioresour Technol 100:3403–3409. doi:10.1016/j.biortech.2009.02.032
Kalogiannis SG, Iakovidou M, Liakopoulou-Kyriakides DA, Kyriakidis, Skaracis GN (2003) Optimization of xanthan gum production by Xantomonas campestris grown in molasses. Process Biochem 39:249–256. doi:10.1016/S0032-9592(03)00067-0
Kang SA, Jang KH, Seo JW et al (2009) Levan: applications and perspectives. In: Rehm BHA (ed) Microbial production of biopolymers and polymer precursors. Caister Academic Press, Norwich, pp 145–161
Kundu S, Panda T, Majumdar SK, Guha B, Bandyopadhyay KK (1984) Pretreatment of Indian cane molasses for increased production of citric acid. Biotechnol Bioeng 26:1114–1121. doi:10.1002/bit.260260915
Lee SY, Park SJ, Lee Y, Lee SH (2003) Economic aspects of biopolymer production. In: Steinbuchel A (ed) Biopolymers. VCH-Wiley, Weinheim, pp 307–337
Liu YP, Zheng P, Sun ZH, Ni Y, Dong JJ, Zhu LL (2008) Economical succinic acid production from cane molasses by Actinobacillus succinogenes. Bioresour Technol 99:1736–1742. doi:10.1016/j.biortech.2007.03.044
Liu J, Luo H, Ye Y, Sun Z, Zeng LuX (2010) Medium optimization and structural characterization of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr Polym 79:206–213. doi:10.1016/j.carbpol.2009.07.055
Livingston DP, Hincha DK, Heyer AG (2009) Fructan and its relationship to abiotic stress tolerance in plants. Cell Mol Life Sci 66:2007–2023. doi:10.1007/s00018-009-0002-x
Lotfy WA, Ghanem KM, El-Helow ER (2007) Citric acid production by a novel Aspergillus niger isolate: I. Mutagenesis and cost reduction studies. Bioresour Technol 98:3464–3469. doi:10.1016/j.biortech.2006.11.007
Manca MC, Lama L, Improta R, Esposito E, Gambacorta A, Nicolaus B (1996) Chemical composition of two exopolysaccharides from Bacillus thermoantarcticus. Appl Environ Microbiol 62:3265–3269
Mossman T (1983) Rapid colorimetric assay for cellular growth and survivals: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. doi:10.1016/0022-1759(83)90303-4
Müller B (2009) Impact of the bacterium Pseudomonas fluorescens and its genetic derivatives on vermiculite: effects on trace metals contents and clay mineralogical properties. Geoderma 153:94–103. doi:10.1016/j.geoderma.2009.07.025
Nicolaus B, Kambourova M, Toksoy Öner E (2010) Exopolysaccharides from extremophiles: from fundamentals to biotechnology. Environ Technol 31(10):1145–1158. doi:10.1080/09593330903552094
Oliveira MR, Silva RSSF, Buzato JB, Celligoi MAPC (2007) Study of levan production by Zymomonas mobilis using regional low-cost carbohydrate sources. Biochem Eng J 37:177–183. doi:10.1016/j.bej.2007.04.009
Ozimek LK, Kralj S, van der Maarel MJEC, Dijkhuizen L (2006) The levansucrase and inulosucrase enzymes of Lactobacillus reuteri 121 catalyse processive and non-processive transglycosylation reactions. Microbiology 152:1187–1196. doi:10.1099/mic.0.28484-0
Patidar SK, Tare V (2006) Effect of nutrients on biomass activity in degradation of sulfate laden organics. Process Biochem 41:489–495. doi:10.1016/j.procbio.2005.07.001
Poli A, Kazak H, Gürleyendağ B, Tommonaro G, Pieretti G, Toksoy Öner E, Nicolaus B (2009) High level synthesis of levan by a novel Halomonas species growing on defined media. Carbohydr Polym 78:651–657. doi:10.1016/j.carbpol.2009.05.031
Roukas T (1998) Pretreatment of beet molasses to increase pullulan production. Process Biochem 33:805–810. doi:10.1016/S0032-9592(98)00048-X
Schwanninger M, Rodrigues JC, Pereira H, Hinterstoisser B (2004) Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib Spectrosc 36:23–40. doi:10.1016/j.vibspec.2004.02.003
Survase SA, Saudagar PS, Singhal RS (2007) Use of complex media for the production of scleroglucan by Sclerotium rolfsii MTCC 2156. Bioresour Technol 98(7):1509–1512. doi:10.1016/j.biortech.2006.05.022
van Hoek P, Aristidou A, Hahn JJ, Patist A (2003) Fermentation goes large scale. Chem Eng Prog 99:37–42
Vedyashkina TA, Revin VV, Gogotov IN (2005) Optimizing the conditions of dextran synthesis by the bacterium Leuconostoc mesenteroides grown in a molasses-containing medium. Appl Biochem Microbiol 41(4):361–364. doi:10.1007/s10438-005-0061-1
Wu M, Wu Y, Zhou J, Pan Y (2009) Structural characterization of a water-soluble polysaccharide with high branches from the leaves of Taxus chinensis var. mairei. Food Chem 113:1020–1024. doi:10.1016/j.foodchem.2008.08.055
Yin X, Calderin L, Stott MJ, Sayer M (2002) Density functional study of structural, electronic and vibrational properties of Mg- and Zn-doped tricalcium phosphate biomaterials. Biomaterials 23:4155–4163. doi:10.1016/S0142-9612(02)00199-0
Yoo SH, Yoon EJ, Cha J, Lee HG (2004) Antitumor activity of levan polysaccharides from selected microorganisms. Int J Biol Macromol 34:37–41. doi:10.1016/j.ijbiomac.2004.01.002
Yoon EJ, Yoo SH, Cha J, Lee HG (2004) Effect of levan’s branching structure on antitumor activity. Int J Biol Macromol 34:191–194. doi:10.1016/j.ijbiomac.2004.04.001
Zhang Y, Zhang Z, Suzuki K, Maekawa T (2003) Uptake and mass balance of trace metals for methane producing bacteria. Biomass Bioenerg 25:427–433. doi:10.1016/S0961-9534(03)00012-6
Acknowledgements
This research has been financially supported by TUBITAK through project no. MAG-108 M193 and by the Ministry of Foreign Affair (MAE, Italy). Fellowship of Faruk Küçükaşık (project no. MAG-108M193) provided by TUBITAK is gratefully acknowledged. Authors thank Bahar Gurleyendag and Dr. Seval Genc for their contribution to the rheological studies. This work is dedicated to Ayse Yarsuvat for her unforgettable support to the IBSB Research Group.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Küçükaşik, F., Kazak, H., Güney, D. et al. Molasses as fermentation substrate for levan production by Halomonas sp.. Appl Microbiol Biotechnol 89, 1729–1740 (2011). https://doi.org/10.1007/s00253-010-3055-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-3055-8