Abstract
Multi-strain mixed fermentation can provide a relatively complete lignocellulosic enzyme system compared with single-strain fermentation. This study was firstly to screen strains which have a strong ability to hydrolyse rice straw (RS) enzymatically and enrich true protein (TP). Then, the conditions in the process of SSF, including the optimum inoculum size of mixed strains, inoculation ratio, and different inoculation time of N. crassa 14–8, were optimized. The experimental results showed that the highest TP content could be obtained by using N. crassa 14–8, C. utilis, and P. chrysosporium as mixed strains, and 5 mM Mn2+ and 50 mM veratryl alcohol were used as inducers of lignin peroxidase (LiP) to improve the efficiency of enzymatic hydrolysis. When N. crassa 14–8 was inoculated 1 day later than P. chrysosporium, the total inoculum size was 10%, and the optimum ratio of N. crassa 14–8 to P. chrysosporium was 1:2, the maximum TP yield (8.89%) was obtained, with 123.37% of its increase rate. This work proposed a technique with potential application in large-scale feedstuff protein conversion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice straw (RS) is one of the most abundant renewable lignocellulosic feedstocks with low protein content (2~5%), containing 32~47% cellulose, 19~27% hemicellulose, and 5~24% lignin [15, 27]. RS is a potential source of dietary energy for ruminants. However, due to the presence of a certain proportion of lignin in the cell wall, the crystallinity of cellulose, the degree of polymerization of polysaccharides, the surface area, and the moisture content of the polysaccharides all affect the degradation of RS [28]. Due to these limitations, the digestibility of RS is poor, thus hindering its effective use. In general, the utilization of RS resources is low, and the added value is not high. Therefore, it is the key to establish reasonable agricultural waste utilization mode and adopt effective method to conduct biological conversion of RS.
Bio-pretreatment mainly degrades lignocellulose by microbial fermentation and enzymatic hydrolysis with lower cost and simpler operation while maintaining the palatability of lignocellulose and producing no or only less toxic compounds [37]. Microorganisms usually make use of RS and other lignocellulosic materials as carbon source to effectively degrade the straw by the secretion of lignocellulosic enzyme. In short, biological pretreatment method is a safe, less energy consuming, low cost, relatively mild, environmentally friendly, and efficient pretreatment technology [7]. However, the pretreatment is usually time-consuming, and the enzyme system of lignocellulose degrading strain is incomplete, with few species available for such utilization. Therefore, the breeding of lignocellulose degrading strains and the optimization of the culture conditions are the key of the biological pretreatment method.
As solid state fermentation (SSF) occurs when microorganisms grow on solid materials without the presence of free water, it can only be accomplished by a limited number of microorganisms. Fungi are well adapted to SSF as their hyphae can grow on particle surfaces and penetrate into the interparticle spaces and, therefore, colonize solid substrate [29]. Four different fungi, Neurospora crassa, Candida utilis, Aspergillus oryzae, and Phanerochaete chrysosporium, were chosen for protein enrichment of RS by SSF. N. crassa has been studied for its ability to produce all three cellulase enzymes including endoglucanase, exoglucanase, and β-glucosidase [11, 23], various hemicellulases [24] and ligninolytic enzymes [13] by SSF of agricultural residues. Therefore, it has a relatively complete system of lignocellulose degrading enzymes, which could degrade and utilize lignocellulosic materials such as RS. Moreover, N. crassa grows more rapidly and has strong ability of protein expression and secretion, and its safety has been verified. Therefore, it can be used for SSF. White rot fungi such as P. chrysosporium are known to secrete cellulase, xylanase [10, 12], and especially lignin-degrading enzyme [21], which is capable of completely hydrolyzing and converting lignin into CO2 and H2O [34]. A large number of reports have shown that the degradation rate and enzymatic hydrolysis efficiency of lignin were significantly improved by P. chrysosporium pretreatment [5, 30]. A. oryzae has also been widely used for its production of cellulase as well as some proteases, phytase, amylase, and other enzymes [14], which can participate in the degradation of cellulose, and also has the ability to produce lignin hydrolase. Thus, it also plays a certain role in the degradation of lignin and aromatic compounds, and can be used for SSF to improve the content of protein-like nutrients of products by enzymatic hydrolysis of RS [1]. Co-culturing of A. oryzae and T. reesei in SSF has been shown to obtain the products with low fiber and high protein content [22]. In addition, yeasts have the ability to promote low-value protein by-products to high-protein animal feed. C. utilis is commonly used for the production of protein feed, which could make use of various types of substrates (such as wheat bran) by SSF to obtain microbial feeding-protein rich in nucleic acid, protein, vitamin, and fat nutritional composition [2, 26]. In some studies of the degradation of lignocellulosic waste by microbial fermentation, it was found that the SSF of multiple strains has more advantages than single strain, which can compensate the disadvantages of incomplete enzyme system, thus forming a mutual support and benefit among different fungi, and promoting the full degradation of the RS. Together, the action of multiple enzymes and the interaction among different fungi are necessary to decompose the RS.
SSF of crop residue with the four individual fungi N. crassa, C. utilis, A. oryzae, and P. chrysosporium were previously investigated to produce enzymes or microbial protein, but few literatures were reported in the field of the optimization of fermentation conditions using various combinations of the four fungi to maximize true protein (TP) yield. The objectives of this study were to determine the best combination of fungi for SSF and to improve TP production by optimizing the fermentation conditions of multiple strains.
Materials and Methods
Materials
Rice straw (RS) was dried at 60 °C and chopped to lengths of 1.5~2.0 cm, and the small pieces were sieved (30 mesh) for analytical purposes.
Microorganisms
The filamentous fungi Neurospora crassa 14–8 mutant and Phanerochaete chrysosporium used in this study were preserved in the lab. Candida utilis CGMCC2.1180 and Aspergillus oryzae CGMCC3.2825 were provided by the China General Microbiological Culture Collection Center.
Inoculum Medium and Condition
Potato dextrose agar (PDA) slant was used to cultivate N. crassa 14–8 at 30 °C for 3 days until sufficient sporulation was observed. The spore suspensions were harvested using sterile distilled water from the PDA agar slant, and the spore cell count of about 3.5 × 108 cells/mL using a hemacytometer was used for inoculation purposes. The malt extract agar slant was inoculated with C. utilis and put at 30 °C for 48 h to obtain sufficient colonies. Liquid seed medium of C. utilis was malt extract medium (pH 5.6), the ingredients of which included 130.0 g/L malt extract and 0.1 g/L chloramphenicol; colonies selected from the slant were incubated at 30 °C overnight, and then, 0.5 mL overnight culture was dissolved in a 250-mL flask with 50 mL seed medium and shaken at 30 °C for 12 h arriving the maximum growth rate in an orbital shaking incubator at 170 rpm. The yeast suspensions were later used as seed of SSF; yeast count was determined by a plate counting method with a total of 7.3 × 107 cells/mL. P. chrysosporium was cultivated with PDA slant medium for sufficient sporulation, and then, the spores were inoculated into high-carbon, low-nitrogen medium (pH 5.5) consisting of 10 g/L glucose, 0.5 g/L ammonium tartrate, 2 g/L KH2PO4, 0.5 g/L MgSO4•7H2O, 0.1 g/L CaCl2, and 2 g/L sodium acetate. Then, the strain was cultured at 30 °C for 6 days at 120 rpm. Finally, the spore suspensions were collected by filtration for SSF. The basal medium (pH 5.5) for ligninase production by P. chrysosporium consists of 10 g/L glucose, 0.2 g/L ammonium tartrate, 2 g/L KH2PO4, 0.25 g/L MgSO4•7H2O, 0.1 g/L CaCl2, 0.5 g/L KCl, and 1 mg/L ammonium sulfate. A. oryzae was cultured on Czapek’s agar slant medium (pH 6.0~6.5) which contains 30 g/L sucrose, 3 g/L NaNO3, 0.5 g/L MgSO4•7H2O, 0.5 g/L KCl, 0.01 g/L FeSO4•4H2O, 1 g/L K2HPO4, and 15 g/L agar. The spores were washed by sterile distilled water, and the spore suspensions were collected by filtration. The total number of spores was 5 × 107 cells/mL.
Solid State Fermentation
Solid state fermentation (SSF) was carried out at 30 °C in 250-mL Erlenmeyer flask. The medium used for SSF included 5 g RS and 10 mL basal medium used for ligninase production. Then, the prepared medium was autoclaved at 121 °C for 20 min. After cooling, all the flasks were inoculated with microbial suspension for the mycelium to grow and invade the RS. The moisture was adjusted to 60~70%. All SSF were performed in triplicate.
Screening of Strain Combinations
Four different combinations of fungi were selected for SSF. The strains were inoculated to fermentation media for 18 days at an inoculum volume fraction of 10%. By determining the TP content of the fermentation products of different strains, the combination of highest TP yield was screened, and the test was repeated for three times.
Enzyme Assays
Five grams of the fermented sample was suspended with distilled water at a ratio of 1:20 (w/v) and agitated on a rotary shaker at 200 rpm for 3 h. The content of each flask was filtered using Whatman no. 1 filter paper. The filtrate obtained was centrifuged at 5000 rpm at 4 °C for 20 min. The supernatant was filtered through 0.45-μm filter paper and the filtrate was stored at − 20 °C. The filtrate was taken for the determination of enzyme activity.
Laccase Activity
Laccase activity was determined by the method which is based on the oxidation of 2, 20-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS). The increase in absorbance was measured at 420 nm for 3 min. One unit of laccase activity was defined as the amount of enzyme that oxidized 1 μmol of ABTS per minute [9]. The activities were reported as U/g.
Lignin Peroxidase Activity
Lignin peroxidase (LiP) activity was determined by monitoring the conversion of veratryl alcohol to veratryl aldehyde at 30 °C in the presence of hydrogen peroxide at 310 nm. The final reaction mixture (total volume, 4 mL) contained 3.4 mL of sodium tartrate (250 mM, pH 3.0), 0.1 mL of veratryl alcohol (10 mM), and 0.4 mL of enzyme sample. The reaction was initiated by adding 0.1 mL of H2O2 (10 mM) at 30 ± 1 °C. One unit of enzyme activity was defined as the amount of the enzyme which can produce 1 μmol veratryl aldehyde from the oxidation of veratryl alcohol per minute [6]. The activities were reported as U/g.
Manganese-Dependent Peroxidase Activity
Manganese-dependent peroxidase (MnP) activity was determined according to the method described, which was based on the oxidation of Mn2+ to Mn3+. MnSO4 (0.2113 g) was added into 1 L of sodium succinate (50 mM, pH 4.5). The final reaction mixture contained 3.4 mL of sodium succinate (50 mM, pH 4.5) and 0.4 mL of enzyme sample. The reaction was initiated by adding 0.1 mL of H2O2 (1.6 mM) at 37 °C. The rate of Mn3+-succinate complex formation was monitored by measuring the increase in absorbance at 238 nm. One unit of enzyme activity was defined as the amount of the enzyme which can produce 1 μmol Mn3+ from the oxidation of Mn2+ per minute [18]. The activities were reported as U/g.
Carboxymethyl Cellulase
One unit of enzyme was defined as the amount of enzyme required to release 1 μmol of glucose equivalents from the appropriate carboxymethyl cellulose sodium (1% CMC-Na) per minute under the standard assay conditions. Carboxymethyl cellulase (CMCase) activity was measured using the 3,5,dinitro salicylic acid (DNS) assay method. The increase in absorbance was measured at 540 nm [31]. The activities were reported as U/g.
Filter Paper Enzyme
FPA was determined in 30 min of the reaction a mixture of proper diluted enzyme solution (50 μL) and the substrate of 50-mg Whatman no. 1 filter paper (1 × 6 cm) participated, and its pH value was controlled at 4.8 by adding 1 mL 0.05 M citrate buffer. The mixtures were incubated at 50 °C and the reducing sugars, produced in 30 min of the reaction, were analyzed by DNS method [17]. One unit of FPA was defined as the amount of enzyme needed for releasing 1 μmol of reducing sugars in 1 min. The activities were reported as U/g.
Optimization of Fermentation Conditions
The effects of different inoculum size, inoculation ratio, and inoculation time of N. crassa 14–8 on the production of TP were studied. The whole period of SSF was 13 days.
Effect of Inoculum Size
Different inoculum sizes (5, 10, 15, 20, 25%) were optimized by evaluating the production of TP with mixed strains by SSF.
Effect of Inoculation Ratio
Inoculation proportion: seven inoculation ratios of N. crassa 14–8 to P. chrysosporium (1:1, 1:2, 1:3, 2:1, 2:3, 3:1, 3:2) were applied to study their effects on the TP contents in SSF.
Effect of Inoculation Time
N. crassa 14–8 was, respectively, inoculated 0, 1, 2, 3, and 4 days later than P. chrysosporium at 30 °C. The production of TP was evaluated throughout the fermentation.
Analytical Determinations
Neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were determined in product of SSF by Van Soest method [35] using filter bag technology. The nitrogen content of RS was determined with micro-Kjeldahl method [32], and the protein content was calculated from measured total nitrogen using a conversion factor of 6.25. True protein (TP) content was calculated as above. RS after fermentation were dried to constant weight at 40 °C, and loss of weight was calculated based on the initial and final dry weight [33]. All analyses were performed triplicate and mean values were worked out. Results obtained from the experiment were subjected to statistical analysis using SPSS 19.0. Statistically significant difference was determined with two-way ANOVA and was declared at p < 0.05.
Results and Discussion
Effect of Different Strain Combinations on TP Production
In combination with different strains, C. utilis can consume the reducing sugar produced by the enzymatic hydrolysis of RS and accelerate the rate of enzymatic reaction. Moreover, it can promote rapid synthesis of feeding-protein. Therefore, C. utilis was added into each group, followed by inoculation of other strains for mixed compounding, and TP content of the product of each combination after SSF was determined. The results are shown in Table 1. The yield of TP was the highest (8.08%, p < 0.05) when N. crassa 14–8, P. chrysosporium, and C. utilis were mixed with the ratio of 1:1:5. N. crassa 14–8 grows rapidly, thus producing more reducing sugars such as glucose in a relatively short period by the enzymatic degradation of RS. P. chrysosporium grows slowly and can be used to restore part of the sugar for its own growth and to produce enzyme to speed up the degradation of RS as well as TP production. At the same time, C. utilis can also make use of the reducing sugar for synthesis of TP. Relatively high TP production was obtained when three strains were used for co-fermentation. A. oryzae grows slowly, the optimum temperature for fermentation is different from that of N. crassa 14–8, and the ability to produce ligninase is also weaker than that of P. chrysosporium. Therefore, when the inoculation ratios of A. oryzae:C. utilis and N. crassa 14–8:A. oryzae:C. utilis were 1:5 and 1:1:5, respectively, the TP content was lower (p < 0.05) than that of the combination of N. crassa 14–8, P. chrysosporium, and C. utilis with the inoculation ratio of 1:1:5, and the growth of N. crassa 14–8, P. chrysosporium, and C. utilis was significantly better than that of the other three groups (p < 0.05). This result is consistent with the conclusion of the previous research that the utilization of co-culture was advantageous over a single strain [4], and multi-strain fermentation has been widely used to produce microbial protein in SSF [22, 36].
Effects of Inducers on Ligninase by P. chrysosporium
Based on the results of mixed fermentation, it was indicated that P. chrysosporium plays an important role in multi-strain fermentation. Therefore, in order to utilize the advantages of ligninase production by P. chrysosporium, this research studied the effects of four kinds of inducers including CuSO4, guaiacol, MnSO4, and veratryl alcohol on ligninase production by P. chrysosporium. The effects of different concentrations of CuSO4 (0, 1, 2, 3, 4, 5, 6 mM) and guaiacol (0, 1, 5, 10, 20, 30, 50 mM) on the activities of three ligninases during different fermentation stages were determined and analyzed. The activity of any ligninase was not detected, and LiP activity was detected when the inducers of MnSO4 and veratryl alcohol were added.
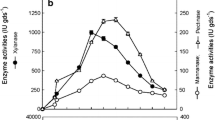
The results of LiP production induced by different Mn2+ concentrations are shown in Fig. 1a. Compared with the LiP activity without Mn2+, the activity of LiP was improved after adding MnSO4, indicating that Mn2+ could regulate the activity of LiP in lignin degradation. At high Mn levels, MnP production was induced, and LiP was inhibited; at low Mn levels, MnP was inhibited, but LiP secretion was induced [25]. When the concentration of Mn2+ was 5 mM, the activity of LiP was the highest at 1.57 U/g. During the whole fermentation period, less LiP was produced by Mn2+ with less than or higher than 5 mM, and with the extension of the fermentation time, the secretion of LiP decreased gradually due to the consumption of nutrient and the change of fermentation environment. Therefore, the selection of 5 mM Mn2+ as an inducer enhances the enzymatic hydrolysis efficiently.
Veratryl alcohol is a natural fungal secondary metabolite that acts as a redox medium to stimulate the LiP catalytic oxidation of the substrate [19], which is also a commonly used inducer for the synthesis of LiP. Studies have shown that the use of P. chrysosporium in the SSF by adding veratryl alcohol as an inducer can increase the yield of LiP [3, 16]. The effects of different concentrations (0, 10, 20, 30, 40, 50, 60 mM) of veratryl alcohol on LiP production were studied. The experimental results are shown in Fig. 1b. The results showed that the activity of LiP with addition of veratryl alcohol during 6 to 10 days of SSF was higher than that of the control, which indicated that veratryl alcohol could play a certain role in promoting the production of LiP. The optimal dose of veratryl alcohol was 50 mM, with the activity of LiP as 0.91 U/g. Then, the enzyme activity decreased continuously, but it was still higher than that of other inducer at different concentrations. Therefore, 50 mM veratryl alcohol can be added into the medium to enhance LiP production and used for the subsequent optimization of fermentation conditions.
Optimization of Process Parameters by Mixed Strains
To optimize the process of SSF by mixed strains with different combinations, experiments were performed, in which three factors such as inoculum size, inoculation ratio, and different inoculation time of N. crassa 14–8 were studied.
Effect of Inoculum Size
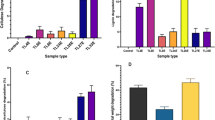
The effects of different inoculum size on TP content were studied in Fig. 2a. The results showed that when the total inoculum size was 10%, the TP yield was the highest (8.15%) by SSF. At higher inoculum sizes, the various strains grow vigorously, and compete for nutrients in the culture medium, thus limiting the rapid propagation of fungi, and affecting the TP production. Therefore, in the mixed fermentation of multiple strains, it is necessary to fully consider the problem of inoculum size [8]. The appropriate inoculum size of strains can not only improve the degradation rate of RS but also can effectively accelerate the co-growth of strains to increase the TP production. Inoculum size as an important factor was investigated for maximum crude protein production by C. utilis, with 10% as the optimal one [20], which was used for subsequent studies.
Effect of Inoculation Ratio
N. crassa 14–8 and P. chrysosporium were used for SSF with seven proportions (1:1, 1:2, 1:3, 2:1, 2:3, 3:1, 3:2); then, C. utilis was added into the solid fermentation medium, with the total inoculum size as 10%. By measuring the TP yield with different inoculation ratios of strains, the results are shown in Fig. 2b. When N. crassa 14–8 and P. chrysosporium were added to the solid fermentation medium at a ratio of 1:1, TP content was 6.58%. When the amount of P. chrysosporium increased and the ratio was adjusted to 1:2, the yield of TP significantly increased to 8.43%. However, when N. crassa 14–8 was added at the same amount, the TP production significantly decreased, with the increase of P. chrysosporium. This result indicated that P. chrysosporium together with N. crassa 14–8 can play a strong degradation capacity of RS, but it grows slowly and takes a long time to produce lignin-degrading enzyme. N. crassa 14–8 grows rapidly for rich spores, which can quickly exert its effect of enzymatic hydrolysis and produce reducing sugar for rapid growth and reproduction of P. chrysosporium, so the ratio of P. chrysosporium should be higher than N. crassa 14–8. However, the excess addition of P. chrysosporium will hinder the normal growth of N. crassa 14–8, thus not providing glucose and other nutrients for P. chrysosporium, while its own growth is also seriously affected. As a result, the TP production of product is low. In contrast, when N. crassa 14–8 is excessive, due to its large number of propagation, the mycelium is spread throughout the culture medium, which hinders the normal growth of P. chrysosporium as well as the effective degradation of RS. In view of this, it is necessary to select the appropriate inoculation ratio of the mixed strains used for SSF, so that the multi-strain can exert the ability of lignocellulosic degradation in a synergic way, and the TP content of fermentation products can be further improved.
Effect of Inoculation Time of N. crassa 14–8
As shown in Fig. 2c, the highest TP production was obtained when N. crassa 14–8 was inoculated 1 day later than P. chrysosporium (8.89%), and the relatively low TP production was obtained when N. crassa 14–8 and P. chrysosporium were inoculated at the same time (7.12%). When N. crassa 14–8 was inoculated too late, it was not possible to provide available reducing sugar for P. chrysosporium, thus affecting the rapid growth of P. chrysosporium and the production of ligninase; also, the fermentation cycle of N. crassa 14–8 was shortened. Thus, it cannot degrade RS sufficiently, resulting in low TP content.
Analysis of Composition and Enzyme Activity After Multi-Strain SSF
The content of TP, crude protein (CP), and lignocellulose of the fermentation product was determined using the optimal combination of strains under the optimized fermentation conditions. The results are shown in Table 2. It was found that some cellulose and lignin were degraded. The degradation rate of cellulose and lignin was 15.46 and 33.57%, respectively, while the hemicellulose was almost not degraded. It was noted that the addition of P. chrysosporium caused enzymatic hydrolysis of part of the lignin due to its ability to produce ligninase. Three fungi species, consisting of P. chrysosporium, N. crassa 14–8, and C. utilis, synergistically degrade RS. In addition, the weight loss rate of RS after SSF was 26.58%, and the content of TP and CP increased by 123.37 and 70.31%, respectively (p < 0.05).
The activity of CMCase and filter paper enzyme (FPAase) during the whole fermentation period was determined (Fig. 3). The results showed that the amount of enzymes produced by the mixed strains was relatively low at the beginning of SSF. During the fermentation process, the activity of CMCase and FPAase increased gradually. The highest activity of CMCase was 135.34 U/g on the 8th day, while the activity of FPAase reached the maximum value of 15.16 U/g on the 12th day. The results showed that N. crassa 14–8, C. utilis, and P. chrysosporium co-cultured for SSF can produce a certain amount of cellulase for hydrolysis of RS and promote the production of TP.
Conclusions
In this work, the production of TP by multi-strain was studied in detail. The optimal combination of fungi was determined by the mixed strain screening test, that is, including three strains of N. crassa 14–8, C. utilis, and P. chrysosporium. Meanwhile, the effects of different inducers on the ligninase produced by P. chrysosporium were studied. In addition, the conditions of mixed strains for SSF were studied, including the inoculum size of mixed strains, inoculation ratio, and different inoculation time of Neurospora crassa 14–8. N. crassa 14–8 was inoculated 1 day later than P. chrysosporium with a total inoculum size of 10%, and the inoculation ratio of N. crassa 14–8 to P. chrysosporium was 1:2. Under optimum conditions, the maximum TP yield can be obtained. Through the analysis of the product after SSF, it was found that the multi-strain fermentation system can effectively hydrolyze lignocellulose, promote the accumulation of TP, and improve the quality of feeding-protein.
References
Abu, O. A., Tewe, O. O., Losel, D. M., & Onifade, A. A. (2000). Changes in lipid, fatty acids and protein composition of sweet potato (Ipomoea batatas) after solid-state fungal fermentation. Bioresource Technology, 72(2), 189–192.
Aggelopoulos, T., Katsieris, K., Bekatorou, A., Pandey, A., Banat, I. M., & Koutinas, A. A. (2014). Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production. Food Chemistry, 145, 710–716.
Asgher, M., Ahmed, N., & Iqbal, H. M. N. (2011). Hyperproductivity of extracellular enzymes from indigenous white rot fungi (P. chrysosporium IBL-03) by utilizing agro-wastes. Bioresources, 6, 4454–4467.
Bader, J., Mast-Gerlach, E., Popovi, M. K., Bajpai, R., & Stahl, U. (2010). Relevance of microbial coculture fermentations in biotechnology. Journal of Applied Microbiology, 109(2), 371–387.
Bak, J. S., Ko, J. K., Choi, I. G., Park, Y. C., Seo, J. H., & Kim, K. H. (2009). Fungal pretreatment of lignocellulose by Phanerochaete chrysosporium to produce ethanol from rice straw. Biotechnology and Bioengineering, 104(3), 471–482.
Bonugli-Santos, R. C., Durrant, L. R., Silva, M. D., & Sette, L. D. (2010). Production of laccase, manganese peroxidase and lignin peroxidase by Brazilian marine-derived fungi. Enzyme and Microbial Technology, 46(1), 32–37.
Chen, S. L. (2010). Biological pretreatment of lignocellulosics: Potential, progress and challenges. Biofuels, 1(1), 177–199.
Danesh, A., Mamo, G., & Mattiasson, B. (2011). Production of haloduracin by Bacillus halodurans using solid-state fermentation. Biotechnology Letters, 33(7), 1339–1344.
Dias, A. A., & Rui, M. B. (2003). In vivo and laccase-catalysed decolourization of xenobiotic azo dyes by a basidiomycetous fungus: Characterization of its ligninolytic system. World Journal of Microbiology and Biotechnology, 19(9), 969–975.
Dobozi, M. S., Szakács, G., & Bruschi, C. V. (1992). Xylanase activity of Phanerochaete chrysosporium. Applied and Environmental Microbiology, 58(11), 3466–3471.
Eberhart, B. M., Beck, R. S., & Goolsby, K. M. (1977). Cellulase of Neurospora crassa. Journal of Bacteriology, 130(1), 181–186.
Elshafei, A. M. (1990). Cellulase and hemicellulase formation by fungi using corn Stover as the substrate. Biological Wastes, 32(3), 209–218.
Froehner, S. C., & Eriksson, K. E. (1974). Induction of Neurospora crassa laccase with protein synthesis inhibitors. Journal of Bacteriology, 120(1), 450–457.
Garrido, S. M., Kitamoto, N., Watanabe, A., Shintani, T., & Gomi, K. (2012). Functional analysis of FarA transcription factor in the regulation of the genes encoding lipolytic enzymes and hydrophobic surface binding protein for the degradation of biodegradable plastics in Aspergillus oryzae. Journal of Bioscience and Bioengineering, 113(5), 549–555.
Garrote, G., Dominguez, H., & Parajo, J. C. (2002). Autohydrolysis of corncob: Study of non-isothermal operation for xylooligosaccharide production. Journal of Food Engineering, 52(3), 211–218.
Gassara, F., Brar, S. K., Tyagi, R. D., John, R. P., Verma, M., & Valero, J. R. (2011). Parameter optimization for production of ligninolytic enzymes using agro-industrial wastes by response surface method. Biotechnology and Bioprocess Engineering, 16(2), 343–351.
Ghose, T. K. (2009). Measurement of cellulase activities. Pure and Applied Chemistry, 59, 257–268.
Huang, D. L., Zeng, G. M., Feng, C. L., Shuang, H., Zhao, M. H., Cui, L., Yu, Z., Jiang, X. Y., & Liu, H. L. (2010). Mycelial growth and solid-state fermentation of lignocellulosic waste by white-rot fungus Phanerochaete chrysosporium under lead stress. Chemosphere, 81(9), 1091–1097.
Huang, X., Wang, D., Liu, C., Hu, M., Qu, Y., & Gao, P. (2003). The roles of veratryl alcohol and nonionic surfactant in the oxidation of phenolic compounds by lignin peroxidase. Biochemical and Biophysical Research Communications, 311(2), 491–494.
Irfan, M., Nazir, M. I., Nadeem, M., Gulsher, M., Syed, Q., & Baig, S. (2011). Optimization of process parameters for the production of single cell biomass of Candida utilis in solid state fermentation. American-Eurasian Journal of Agricultural and Environmental Sciences, 10, 264–270.
Kirk, T. K., & Farrell, R. L. (1987). Enzymatic "combustion": The microbial degradation of lignin. Annual Review of Microbiology, 41(1), 465–501.
Lio, J. Y., & Wang, T. (2012). 75.Solid-state fermentation of soybean and corn processing coproducts for potential feed improvement. Journal of Agricultural and Food Chemistry, 60(31), 7702–7709.
Mahadevan, P. R., & Eberhart, B. (1964). The Beta-glucosidase system of Neurospora Crassa. Ii. Purification and characterization of aryl beta-glucosidase. Archives of Biochemistry and Biophysics, 108(1), 22–29.
Mishra, C., Keskar, S., & Rao, M. (1984). Production and properties of extracellular Endoxylanase from Neurospora crassa. Applied and Environmental Microbiology, 48(1), 224–228.
Perez, J., & Jeffries, T. W. (1993). Role of organic acid chelators in manganese regulation of lignin degradation by Phanerochaete chrysosporium. Applied Biochemistry and Biotechnology, 39-40(1), 227–238.
Rajoka, M. I., Kiani, M. A. T., Khan, S., Awan, M. S., & Hashmi, A. S. (2004). Production of single cell protein from rice polishings using Candida utilis. World Journal of Microbiology and Biotechnology, 20(3), 297–301.
Saha, B. C. (2003). Hemicellulose bioconversion. Journal of Industrial Microbiology and Biotechnology, 30(5), 279–291.
Sanchez, C. (2009). Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnology Advances, 27(2), 185–194.
Santos, M. M. D., & Rosa, A. S. D. (2004). Thermal denaturation: Is solid-state fermentation really a good technology for the production of enzymes? Bioresource Technology, 93(3), 261–268.
Shi, J., Sharma-Shivappa, R. R., Chinn, M., & Howell, N. (2009). Effect of microbial pretreatment on enzymatic hydrolysis and fermentation of cotton stalks for ethanol production. Biomass and Bioenergy, 33(1), 88–96.
Singh, P., Sulaiman, O., Hashim, R., Peng, L. C., & Singh, R. P. (2013). Evaluating biopulping as an alternative application on oil palm trunk using the white-rot fungus Trametes versicolor. International Biodeterioration and Biodegradation, 82, 96–103.
SKC, C. (2010). Protein Analysis (2nd ed.). USA: Aspen Publishers Inc.
Talaeipour, M., Hemmasi, A. H., Kasmani, J. E., Mirshokraie, S. A., & Khademieslam, H. (2010). Effects of fungal treatment on structural and chemical features of hornbeam chips. Bioresources, 5, 477–487.
Ten, H. R., & Teunissen, P. J. (2001). Oxidative mechanisms involved in lignin degradation by white-rot fungi. Chemical Reviews, 101, 3397.
Van Soest, P. J. (1963). Use of detergents in the analysis of fibrous feeds. 1. Preparation of fiber residues of low nitrogen content. Journal of the Association of Official Agricultural Chemists, 46, 825–829.
Xiao, L., Yang, L. Y., Zhang, Y., Gu, Y. F., Jiang, L. J., & Qin, B. Q. (2009). Solid state fermentation of aquatic macrophytes for crude protein extraction. Ecological Engineering, 35(11), 1668–1676.
Zhao, L., Cao, G. L., Wang, A. J., Ren, H. Y., Dong, D., Liu, Z. N., Guan, X. Y., Xu, C. J., & Ren, N. Q. (2012). Fungal pretreatment of cornstalk with Phanerochaete chrysosporium for enhancing enzymatic saccharification and hydrogen production. Bioresource Technology, 114, 365–369.
Acknowledgements
This work was supported by the Key R&D Program of Jiangsu Province (Modern Agriculture), China (BE2017355); the Agricultural Sci-Tech Self-Innovation Program of Jiangsu Province, CX(17)3044, China; the Open Funding Project of National Key Laboratory of Biochemical Engineering; and Jiangsu Special Research and Development Grant for Northern Jiangsu Area, China (SZ-YC2017001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, J., Chen, H., Wu, B. et al. Protein Production Through Microbial Conversion of Rice Straw by Multi-Strain Fermentation. Appl Biochem Biotechnol 187, 253–265 (2019). https://doi.org/10.1007/s12010-018-2792-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2792-5