Abstract
Agricultural wastes rich in lignocellulosic biomass have been used in the production of poly-γ-glutamic acid (γ-PGA) through separate hydrolysis and fermentation (SHF), but this process is complicated and generates a lot of wastes. In order to find a simpler and greener way to produce γ-PGA using agricultural wastes, this study attempted to establish simultaneous saccharification and fermentation (SSF) with citric acid-pretreated corn straw. The possibility of Bacillus amyloliquefaciens JX-6 using corn straw as substrate to synthesize γ-PGA was validated, and the results showed that increasing the proportion of glucose in the substrate could improve the γ-PGA yield. Based on these preliminary results, the corn straw was pretreated using citric acid. Then, the liquid fraction (xylan-rich) was used for cultivation of seed culture, and the solid fraction (glucan-rich) was used as the substrate for SSF. In a 10-L fermenter, the maximum cumulative γ-PGA concentration in batch and fed-batch SSF were 5.08 ± 0.78 g/L and 10.78 ± 0.32 g/L, respectively. Moreover, the product from SSF without γ-PGA extraction was used as a fertilizer synergist, increasing the yield of pepper by 13.46% (P < 0.05). Our study greatly simplified the production steps of γ-PGA, and each step achieved zero emission as far as possible. The SSF process for γ-PGA production provided a simple and green way for lignocellulose biorefinery and sustainable cultivation in agriculture
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly-γ-glutamic acid (γ-PGA) is a linear biomacromolecule polymerized with D- or L-glutamic acid monomers via γ amide bonds [1]. Owing to novel characteristics, including absorbility, water-solubility, biodegradability and innocuity, γ-PGA has been successfully applied in agriculture, cosmetics industry, sewage treatment and other fields [2]. Especially in agriculture, studies have shown that γ-PGA is an efficient fertilizer synergist and plant regulator. Applying γ-PGA in wheat planting increased the yield by 7.17%, while improving nitrogen utilization efficiency and soil microbe diversity [3]. γ-PGA could promote plant nitrogen metabolism by changing the Ca2+/CaM signaling pathway, thereby improving the growth of Chinese cabbage [4]. γ-PGA was also a good cryoprotectant, which could relieve the growth inhibition of rape seedlings under cold stress [5].
γ-PGA is mainly prepared via fermentation using various Bacillus strains, while glucose and glycerol are the mostly common used carbon sources [6, 7]. However, glucose and glycerol are expensive, which lead to a relatively high price of γ-PGA and hinder its large-scale application. To reduce the production cost of γ-PGA, it is necessary to find a more economical carbon source. Lignocellulosic biomass is rich in carbohydrate and reserves in the forms of wastes or by-products in agriculture and forestry such as corn straw [8] In China, large amount of corn straws are generated every year, leading to a huge potential environmental burden [9]. As renewable and cheap material, recent studies have attempted to use lignocellulosic biomass for γ-PGA production by separate hydrolysis and fermentation (SHF). In SHF, the lignocellulosic biomass was first converted to monosaccharides followed by submerged fermentation using the hydrolysate as the carbon source. After a two-step hydrolysis using sulfuric acid and enzyme, the rice straw was transformed to a carbon source for γ-PGA fermentation by Bacillus subtilis NX-2, and the yield reached 73.0 ± 0.5 g/L [10]. Corncob fibers were treated with dilute sodium hydroxide and hydrochloric acid sequentially, the hydrolysate was applied in γ-PGA production by Bacillus subtilis HB-1, resulting in a 24.92 g/L cumulative production [11]. The corncob hydrolysate was also used by Bacillus amyloliquefaciens C1, and the final γ-PGA yield was 6.56 ± 0.27 g/L [12].
Although SHF has been successfully employed during production, sulfuric acid, hydrochloric acid, and sodium hydroxide were used in the hydrolysis of lignocellulosic biomass [10,11,12]. This would produce a lot of harmful inhibitors to microorganisms such as furfural and 5–hydroxymethyl furfural (5–HMF) [13], leading to a great consumption of clean water to wash and calcium hydroxide or lime for detoxification [10,11,12]. These operations increased the complexity of the process and consumed massive labor, while generating a lot of wastewater and insoluble matters that might pollute the environment. So, the technologies for simple and green conversion of lignocellulose into γ-PGA are urgently needed. Simultaneous saccharification and fermentation (SSF), which integrates hydrolysis and fermentation in one reactor, is a relatively simple process to utilize lignocellulosic biomass as main feedstock [14]. During SSF, sugars are released from lignocellulosic biomass and simultaneously consumed by the microorganism for fermentation, reducing the number of reactors occupied, as well as saving a lot of labor and time inputs [15, 16]. Currently, SSF has been successfully applied in the fermentation of various chemical products, such as bio-ethanol [16], lactic acid [17], succinic acid [18], and butanediol [19]. Moreover, in the pretreatment of lignocellulosic biomass, the use of organic carboxylic acids produced less fermentation inhibitors [20, 21], and the pretreated substrates could also be directly used for SSF without detoxification [22]. This would further simplify the fermentation process, while reducing the generation of sewage and waste.
To overcome the disadvantages of complex steps and pollutant emission in SHF, we established an SSF process for γ-PGA production using corn straw pretreated by citric acid in this study. The SSF process integrated γ-PGA fermentation and corn straw hydrolysis, which extremely simplified the process of γ-PGA production from lignocellulose-rich agricultural waste. The corn straw pretreated by citric acid was fermented directly, which avoided the waste generation from detoxification. Moreover, SSF products could increase the yield of pepper, showing the synergistic effect of fertilizer. So, our results provided a new way for the clean bioconversion and green recycling of corn straw.
Materials and methods
Microorganism
Bacillus amyloliquefaciens JX-6 was used for γ-PGA fermentation. The strain was isolated from the field in Chengdu (Sichuan Province, China) and deposited at China General Microbiological Culture Collection Center with the accession number of CGMCC No. 13715. Our previous study showed that the strain JX-6 could use agricultural wastes (corn stalk and soybean meal) to produce γ-PGA through solid-state fermentation, and the maximum yield was 116.88 ± 5.05 g/kg [23].
Flask experiments
To obtain the seed culture, B. amyloliquefaciens JX-6 suspension was inoculated into a 500-mL Erlenmeyer flask. The flask was filled with 100 mL sterilized LB broth and incubated at 200 rpm for 15 h at 32 °C.
The subsequent γ-PGA fermentations in this study were all performed in a two-stage cultivation: (1) For cell growth, each 500-mL Erlenmeyer flask was filled with 100 ml sterilized fermentation medium (in g/L: (NH4)2SO4, 10; K2HPO4, 1; FeCl3·6H2O, 0.02; MnSO4·H2O, 0.1; MgSO4·7H2O, 0.5; CaCl2, 0.2; xylose/glucose, or glucose, or xylose, 50; pH 6.5–7.5) and inoculated with 5% seed culture (v/v), followed by incubated at 240 rpm for 24 h at 32 °C; (2) For γ-PGA production, 20 mL precursor solution (L-glutamic acid and citric acid) was supplemented at 24 h, the final concentration of L-glutamic acid was 15 g/L and the citric acid was 5 g/L. Subsequently, the mixture was cultured for an additional 48 h.
Corn straw pretreatment
Corn straw collected from field (Shifang, Sichuan Province, China) was milled into 40 mesh particle and dried at 60 °C. The fiber determination instrument CXC-06 (Xinrui Instruments Co., Shanghai, China) was used to analyze the organic composition of straw powder. The straw powder was soaked in a 1.5% citric acid solution (w/v). The initial ratio of liquid-to-solid was 10:1 (w/w). This straw powder slurry was autoclaved at 121 °C for 3 h. Subsequently, the slurry was centrifugated at 4800 rpm for 15 min. The solid fraction was used as substrate for SSF, and the liquid fraction was used for seed culture cultivation. The composition of solid fraction was also analyzed by CXC-06, and the liquid fraction was tested by HPLC (Shimadzu, Kyoto, Japan).
SSF and fed-batch SSF
In all SSF experiments, 10 g/L (NH4)2SO4 and 5 g/L peptone were dissolved in the liquid fraction from the pretreatment to make seed medium. Each 500-mL Erlenmeyer flask was filled with 100 mL seed medium and inoculated with 2% of B. amyloliquefaciens JX-6 suspension. Then, the flasks were incubated at 200 rpm for 24 h at 32 °C.
Solid fraction from the pretreated corn straw was suspended in a neutral cellulase (EC 3.2.1.4, 284.00 FPU/g, Heshibi Biotechnology Co. Ltd., Ningxia Province, China) solution. The total water insoluble solids (WIS) content of this slurry was 20% (w/v), while the titer of enzyme was 40 FPU/(g WIS).
A 10 L bioreactor (Yangge, Shanghai, China) was used for the batch SSF experiments. According to previous SSF studies [18, 24], and considering that γ-PGA is a highly energy-consuming biological process, the SSF operating parameters were set as follows. The total WIS content of SSF was set as 8%, 10%, and 12%, while total enzyme loading was 40 FPU/(g WIS), and the working volume was 4 L. The medium (in g/L: (NH4)2SO4, 10; K2HPO4, 1; MnSO4·H2O, 0.1; MgSO4·7H2O, 0.5; FeCl3·6H2O, 0.02; CaCl2, 0.2) was autoclaved and supplemented with different amounts of enzyme and substrate slurry. The batch SSF was performed in three stages: (1) The pretreated corn straw powder was enzymatically pre-hydrolyzed at pH 5.5 for 10 h at 50 °C with agitation at 200 rpm. (2) 5% of seed culture was inoculated into the bioreactor after pre-hydrolysis, and this point was recorded as time point 0 h. The temperature of batch SSF was 32 °C, and the aeration rate was 2 vvm, as well as the agitation speed was 400 rpm. The pH was maintained at 6.5–7.0 by automatic addition of 2 mol/L HCl or 2 mol/L NaOH. This stage lasted for 24 h. (3) For γ-PGA production, 500 mL precursor solution (L-glutamic acid and citric acid) was supplemented at 24 h for subsequently 48 h of γ-PGA fermentation.
The fed-batch SSF was initially started with 10% (w/v) WIS content in 4 L of working volume. After addition of precursor solution, 580 mL of substrate and enzyme mixture was manually added at 24 h, 48 h and 72 h. The final working volume was up to 6.25 L and WIS content was 12% (w/v).
Testing of synergistic effects of fertilizer and fermentation broth
To investigate the possible synergistic effects of fed-batch SSF on fertilizer, the experiment of pepper cultivation was conducted in greenhouse. The experiment lasted from June 10 to September 25 under natural conditions. Each plastic pot was filled with 4 kg of perlites and humus (the ratio was 9:1 v/v). The pot experiment included two different treatments: (1) In the γ-PGA group, the soil matrix (perlites and humus) was added with 200 mL of fed-batch SSF products after ultrasonication; (2) In the control group, the soil matrix was mixed with the 200 mL pure medium of fed-batch SSF, which was not inoculated with B. amyloliquefaciens JX-6. After transplanting, the pepper seedlings in two groups were fertilized with 100 mL identical nutrient solution every two days (0.31 mM KNO3, 0.25 mM MgSO4·7H2O, 0.5 mM Ca(NO3)2·4H2O, 0.5 mM KH2PO4, 0.5 mM NH4NO3, 0.5 μM Fe·NaEDTA(pH5.5), 1.75 μM MnCl2·4H2O, 0.06 μM CuSO4·5H2O, 0.125 μM ZnSO4·7H2O, 8.75 μM H3BO3, 0.025 μM (NH4)2MoO4, 0.00125 μM CoCl2·6H2O). The experiment was carried out in four replicates. The date of first flowering, first harvest and final production were recorded. In each group, the yield, water content and length of peppers were determined.
Analysis methods
During the shake-flask experiments with mixed sugars and all SSF experiments, bacterial samples were collected every 8 h to monitor the cell density, γ-PGA concentration, residual sugars and L-glutamic acid in the broth.
The fermentation samples were serially diluted using sterilized water. Then, 100 μL aliquots were plated on LB plates in triplicates. After 18 h incubation at 37 °C, the number of colonies on each plate was counted to calculate the cell density.
To precipitate γ-PGA, the samples were centrifuged at 10,000 rpm for 20 min, and the supernatant after centrifugation was mixed with 4 volumes of absolute alcohol. Then, the mixture was spun down at 12,000 rpm for 10 min to collect γ-PGA. Subsequently, the γ-PGA was dissolved in ultra-pure water and measured by HPLC as previous description [25].
Residual sugars (glucose and xylose) were measured by HPLC (LC-20A, Shimadzu Co, Japan) with a InertSustain® NH2 column and an RI-10A refractive-index detector. The mobile phase was 75% (v/v) acetonitrile solution and the flow rate was 1.0 mL min − 1. The biosensor SBA-40D (Shandong Academy of Sciences, China) was used to determine the concentration of residual L-glutamic acid. All experiments were carried out in triplicates. The one-way ANOVA function of the software SPSS 19.0 was used to compare the valuess of different groups.
Results
Utilization of different sugars for γ-PGA production with B. amyloliquefaciens JX-6
Corn straw is rich in lignocellulose which mainly composed of cellulose, hemicellulose and lignin. The major components of cellulose and hemicellulose are corresponding glucan and xylan, whereas, the content of other sugars are very low. To explore the possibility of using corn straw for γ-PGA production by B. amyloliquefaciens JX-6, glucose and xylose, as well as their mixture were used to perform a flask fermentation test. The mixed sugar consisted of glucose and xylose in a ratio of 37:20, which was identical with the ratio of cellulose and hemicellulose in corn straw. As shown in Table 1, all three sugars could be used as carbon sources for cell growth and γ-PGA synthesis. The highest yield of γ-PGA was obtained in fermentation with glucose, which was 19.13 ± 1.18 g/L, followed by mixed sugar group (16.03 ± 0.85 g/L). Additionally, Glucose was most conducive to cell growth and the final cell density in the fermentation broth was (7.5 ± 1.15) 1010 cfu/mL, which was higher than that in sugar mixture group ((6.87 ± 0.44) 1010 cfu/mL). Given the lowest γ-PGA yield (15.30 ± 0.63 g/L) and cell density ((3.40 ± 1.01) 1010 cfu/mL), we could infer that xylose was not suitable for B. amyloliquefaciens JX-6 to synthesize γ-PGA.
Fermentation profile of B. amyloliquefaciens JX-6 using mixed sugars to produce γ-PGA
In order to further understand the characteristics of γ-PGA fermentation with corn straw, the fermentation process of B. amyloliquefaciens JX-6 using mixed sugar was continuously monitored and sampled every 8 h (Fig. 1). The fermentation profile indicated that the consumption of glucose and xylose was not synchronous. Compared with xylose, glucose was preferentially utilized by B. amyloliquefaciens JX-6. The glucose in the fermentation broth was depleted at 48 h, and the average consumption rate was 0.62 ± 0.02 g/(L·h), while xylose was finally exhausted at 72 h and the average consumption rate was only 0.23 ± 0.04 g/(L·h). After precursor supplementation at 24 h, the γ-PGA began to accumulate, and the final γ-PGA concentration reached 16.03 ± 0.85 g/L. Before glucose depletion, γ-PGA was rapidly synthesized, and the γ-PGA average synthesis rate was 0.63 ± 0.02 g/(L·h) during 24–48 h. However, xylose was the only carbon source after 48 h, which was not favored by JX-6. Thus, the production of γ-PGA was almost stagnated, and the average synthesis rate was only 0.04 ± 0.01 g/(L·h). In addition, the cell density in fermentation broth reached the peak value of (7.45 ± 0.16) 1010 cfu/mL at 24 h, then dropped to the valley with glucose consumption. As the γ-PGA synthesis stalled after 48 h, the titer of bacteria re-elevated and the final cell concentration was (6.88 ± 1.44) 1010 cfu/mL.
Batch fermentation of γ-PGA by B. amyloliquefaciens JX-6 using xylose/glucose mixture. γ-PGA fermentation was performed in two-stage cultivation steps: bacteria reproduction was sustained for 24 h, followed by γ-PGA production after supplementation of precursor solutions (citric acid and L-glutamic acid). The results were derived from the average of three independent tests
Effect of glucose/xylose ratio on γ-PGA fermentation
In SSF, the pretreatment of lignocellulosic biomass is essential to improve the production efficiency. However, the contents of glucan and xylan in substrate severely varied under different pretreatment. To find a suitable pretreatment method, the optimum ratio of glucose and xylose was investigated. Flask-fermentation experiments were performed using 50 g/L of glucose/xylose mixtures at five different ratios (9/1, 7/3, 5/5, 3/7, 1/9), and the result was shown in Fig. 2. The yield of γ-PGA under a 1: 9 ratio of glucose/xylose mixture was 12.89 ± 0.40 g/L, which was the lowest among the five groups. As the proportion of glucose in the fermentation medium increased, the final γ-PGA concentration gradually improved. When the ratio of glucose/xylose in the mixed sugar reduced to 9: 1, the yield of γ-PGA was 16.52 ± 0.32 g/L, which was 28.16% higher than the 1: 9 group. The change of glucose/xylose ratio in the mixed sugar has no identical influence on final cell density. In the groups with a high proportion of xylose, the final cell densities were (7.46 ± 1.50) 1010 cfu/mL and (7.25 ± 0.85) 1010 cfu/mL at the glucose/xylose ratios of 1: 9 and 3: 7, respectively. As the further increase of glucose, the final cell densities slightly decreased, which values at the glucose/xylose ratios of 5:5, 7: 3 and 9: 1 were (4.47 ± 0.63) 1010 cfu/mL, (5.31 ± 0.64) 1010 cfu/mL, and (4.45 ± 1.23) 1010 cfu/mL, respectively.
Pretreatment of corn straw with citric acid
Based on the previous results, citric acid was selected for high-pressure steam pretreatment of corn straw. Corn straw was mainly composed of 36.77% cellulose, 19.52% hemicellulose and 15.06% lignin. After citric acid pretreatment, the cellulose and lignin content in pretreated straw (the solid fraction from the pretreatment) separately increased to 52.45% and 23.32%, while the hemicellulose decreased to 5.84% (Table 2). Compared with corn straw, the ratio of cellulose (rich in glucan) to hemicellulose (rich in xylan) was elevated to 52.45:5.84, which was close to 9:1. After filtration, the liquid fraction from the pretreatment contains 10.82 g/L xylose, 1.13 g/L glucose and residual 8.20 g/L citric acid, the hemicellulose of corn straw was degraded into monosaccharides and released.
γ-PGA production by batch SSF of different substrate contents
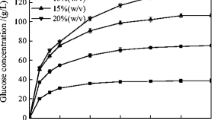
In a 10 L bioreactor, the batch SSF process for γ-PGA production was established and the effects of different contents of water insoluble solids (WIS) were studied. The seed culture was cultivated with the liquid fraction from corn straw pretreatment as the carbon source, and the solid part from pretreated straw was used as substrate for SSF. The batch SSF was performed in three stages: the pre-hydrolysis of substrate, the cell growth, and the γ-PGA production. The consumption of free sugars, the synthesis of γ-PGA and the growth of cells were all different for batch SSF under various WIS contents (8%, 10% and 12%) (Fig. 3). After the pre-hydrolysis stage, the concentration of free glucose in the fermentation broth increased coordinately with the WIS content (31.63 ± 0.21 g/L, 43.66 ± 0.26 g/L and 50.28 ± 0.79 g/L, respectively), whereas, the xylose concentrations were very low (less than 2 g/L). During the subsequent cell growth process, the free glucose was quickly consumed with the increase of cell titer, while the xylose concentration remained constant. In the fermentation broth with 8% and 10% WIS, the free glucose could not be detected after 32 h, while the negative result in 12% WIS group was observed at 48 h. Moreover, the xylose depletion time point was delayed as the WIS content increased (at 48 h, 56 h and 72 h, respectively). The highest γ-PGA yield, 5.08 ± 0.78 g/L, was attained by batch SSF under 10% WIS content. In the 8% and 12% WIS content group, the corresponding eventual γ-PGA concentrations were 2.35 ± 0.11 g/L and 3.57 ± 0.57 g/L, respectively (Fig. 3b). In all batch SSF experiments, the duration of continuous γ-PGA synthesis was relatively short, and the yield of γ-PGA did not increase after 40 h. The maximum cell density (7.00 × 1010 cfu/mL) was also attained in 10% WIS content at 32 h. The peak cell density of 8% and 12% WIS content were 6.30 × 1010 cfu/mL and 5.30 × 1010 cfu/mL, exhibited at 24 h and 32 h, respectively (Fig. 3c). A decrease in cell density during late fermentation stage was observed in SSF under all WIS contents, and the final cell density of three groups had little difference.
γ-PGA production by fed-batch SSF
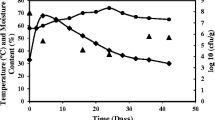
To improve the γ-PGA yield of SSF, the fed-batch SSF process was established based on the result of batch SSF. The start of fed-batch SSF was identical with the batch trial under 10% WIS content, then the slurry of the substrate and enzyme was supplemented at 24, 48 and 72 h after inoculation, while the final WIS content in the reactor reached 12% (Fig. 4). Similarly, free glucose in the fed-batch SSF broth was also consumed quickly and could not be detected after 32 h. The free xylose in the fermentation broth was depleted at 88 h, which was 32 h delayed compared with batch SSF under 10% WIS content. The eventual concentration of γ-PGA was 10. 78 ± 0.32 g/L, which was 2.14 folds than batch SSF. In fed-batch SSF, the yield of γ-PGA over total substrate was 0.10 ± 0.02 g/(g substrate), which was 3 folds higher than the value in batch SSF (0.03 ± 0.0012 g/(g substrate)). During the fed-batch SSF, γ-PGA was continuously synthesized between 24 and 96 h. The maximum recorded cell density was 9.45 × 1010 cfu/mL at 88 h, which was greater than the batch SSF. Particularly, no significant cell density decrease was observed in the later stage of fed-batch SSF.
γ-PGA production by fed-batch SSF using citric acid-pretreated corn straw. The fed-batch SSF was started at 10% and completed at 12% substrate content. 580 mL of substrate/enzyme mixture was manually fed at the 24th, 48th and 72nd hour. The results were derived from the average of three independent tests
Fertilizer synergistic effect of fermentation product from fed-batch SSF
To study the fertilizer synergistic effect of fermentation product from fed-batch SSF, the mixture sterilized by ultrasonication was applied to the pot experiment of pepper cultivation directly. The results showed that the average yield of the γ-PGA applied group was 65.31 ± 0.31 g/pot, which was increased by 13.46% compared with the control (P < 0.05, Table 3). The fermentation broth could also promote the average length of peppers. The length of peppers in the γ-PGA group was 14.8 ± 0.61 cm, while in the control group was only 14.0 ± 0.67 cm. There was a little difference in water content of peppers between two groups, the values of the γ-PGA group and the control group were 83.75 ± 0.59% and 82.60 ± 1.01%, respectively. In two groups, the first flowering date of pepper was same (July 8th). However, the peppers after γ-PGA application possessed a prolonged picking period: the first harvesting date of the γ-PGA group was July 17th, two days earlier than the control group, while the final harvesting date was September 20th, which was nine days later than the control group.
Discussion
To reduce the production cost of γ-PGA, lignocellulosic biomass has been used for γ-PGA fermentation [10,11,12]. Previous researches were mainly carried out through SHF, which was complicated and not environmentally friendly [10, 11]. In this study, the fermentation characteristics of B. amyloliquefaciens JX-6 using glucose/xylose mixtures to produce γ-PGA were explored. Subsequently, a SSF process was successfully established for γ-PGA production using citric acid pretreated corn straw as the main carbon source. The fermentation product from SSF without γ-PGA extraction was directly applied as a fertilizer synergist, which successfully improved the yield of the peppers. Our study established the SSF process of γ-PGA production using corn straw for the first time, which provided a simple and clean bioconversion pathway for agricultural wastes rich in lignocellulose.
The proportions of the main components in lignocellulose are different among diverse plants [26]. Unlike rice straw, the hydrolysate of corn straw mainly harbored xylose and glucose, and other sugars were barely detected [10, 26, 27]. Thus, the mixture of xylose and glucose were used to mimic corn straw for γ-PGA fermentation. The results indicated that B. amyloliquefaciens JX-6 had the ability of using corn straw as the main carbon source for γ-PGA fermentation. Similar to B. amyloliquefaciens C1 and B. subtilis NX-2, B. amyloliquefaciens JX-6 preferred glucose than xylose during γ-PGA synthesis using mixed sugars [10, 12]. This phenomenon is common in most microorganisms, especially in Bacillus species, which is mainly caused by carbon catabolite repression (CCR) [28, 29]. In Bacillus species, CCR exhibits as the bias to use a preferred sugar (usually glucose) when multiple carbon sources exist, causing a lag in the consumption of other carbon sources (such as xylose) [30]. This was primarily the result of gene expression regulated by metabolite profiles of the preferred sugar or its intermediate metabolites, thereby affecting the metabolism of other carbon sources [12, 30, 31]. Also due to CCR, the γ-PGA fermentation using pure glucose was more efficient than using glucose/xylose mixtures [32].
Lignocellulosic biomass has a complex structure, which hinders the access of cellulase and reduces the efficiency of hydrolysis [8]. Therefore, it is necessary to pretreat the lignocellulosic biomass to destroy its structure and improve the release of sugar in SSF [15]. Our results of fermentation using mixed sugars at different glucose/xylose ratios revealed that the hemicellulose (xylan) in the lignocellulosic biomass should be removed as much as possible to increase the proportion of glucan in the substrate of SSF. Finally, we chose citric acid for the high-temperature steaming pretreatment of corn straw. Citric acid has relatively strong acidity with the pKa1 of 3.13, which can promote the degradation of hemicellulose [8]. This could avoid CCR cleverly, resulting in a favorable environment for strain B. amyloliquefaciens JX-6. Besides, using organic acid in pretreatment would generate fewer inhibiting factors compared to inorganic acid, such as sulfuric acid [20, 21], and citric acid itself was not toxic to microorganisms. So, the pretreated corn straw could be directly used in fermentation without detoxification, which reduced a large number of pollutants and massive labor and time input due to the detoxification of the hydrolysates [22]. In addition, the liquid fraction from pretreatment, which containing xylose and residual citric acid, was used for seed cultivation of SSF to avoid waste of resources and pollution to the environment. Previous studies have shown that this operation was conducive to make full use of all the sugars in the lignocellulosic biomass as well as domesticate the strains to improve their resistance to inhibitors in the lignocellulosic biomass [33]. In short, compared to sulfuric acid and sodium hydroxide were used in SHF, the use of citric acid for corn straw pretreatment make our process more concise and environmentally friendly.
As described, batch SSF was successfully established with 8%, 10% and 12% WIS contents in a 10 L bioreactor. Due to the CCR, B. amyloliquefaciens JX-6 preferred glucose in the substrate. Therefore, the initial consumption of xylose meant that glucose was not sufficient, and the xylose exhaustion indicated that the substrate could no longer provide fermentable sugars. In the SSFs with 8% and 10% WIS contents, xylose was depleted at 56 h and 48 h, respectively, indicating that the substrates in these two groups were unsuitable to support long-term fermentation. However, when the WIS content elevated to 12% in our trial, both γ-PGA yield and cell density decreased. This was probably attributed to a higher fermentation broth viscosity [34] and inhibitor titer [35] as the increase of substrate content, thus inhibiting bacteria growth and metabolism. The fed-batch SSF was also established. Compared with batch SSF, fed-batch SSF is a better choice for γ-PGA production under high substrate content, γ-PGA synthesis maintained long period (about 72 h) and the cell density did not significantly decrease. These results indicated that the supplementation of enzymes and substrates correspondingly solved the shortage of substrates and the inhibition caused by high WIS contents in batch SSF. Moreover, we could infer that the viscosity of the fermentation broth is maintained at a lower level due to the feeding of new WIS multiple times [36]. Also, the inhibitory effect from the hydrolysis of lignocellulosic biomass is reduced as a result of both gradual adaption of the bacteria and biological detoxification [24]. Compared with traditional SHF, the SSF process is a simpler way to produce γ-PGA. The process of using rice straw to synthesize γ-PGA using Bacillus subtilis NX-2 includes six steps [10], while the SHF with corncob as carbon source using Bacillus subtilis HB-1 consists of seven steps [11]. In our study, the SSF process integrated fermentation and hydrolysis. This extremely simplified strategy only required three steps: pretreatment, filtration, and fermentation. This results in the conservation of time, equipment, energy and labor, as well as reducing the cost of γ-PGA production [15, 16].
Similar to previous studies [37], the fermentation product from our fed-batch SSF can promote the yield and quality (length) of peppers, which indicate that corn straw can be used to prepare high-quality fertilizer synergists through the SSF process. In it, γ-PGA could increase fertilizer utilization, improve plant metabolic pathways, and promote soil microorganisms [3,4,5], while the corn straw could also enhance soil properties [38]. The traditional strategy to improve the efficiency of fertilizers is adding urease and nitrification inhibitors or coating the fertilizers [39, 40]. However, these two fertilizer synergists may pollute the environment: the inhibitors are toxic to animals and some microorganisms [41], and the polymers used for coating are generally not biodegradable [42]. In contrast, the γ-PGA in the SSF fermentation broth is nontoxic and biodegradable, which can be an environmental friendly fertilizer synergist. In China, corn straw is one of the largest agricultural wastes causing great pressure on the environment [43, 44], the effectively disposal of these straws is an urgent problem. Studies showed that composting technology was the most effective way to use straw as a resource [9], but the economic profit of organic fertilizer was limited and could not attract much attention [45]. The price of ordinary organic fertilizer prepared from straw and manure is 650 RMB/t, while the price of agricultural γ-PGA fermentation broth is as high as 9,000 RMB/t (https://www.1688.com/). It is calculated that 0.12 t of straw, 0.017 t of cellulase, 0.023 t of citric acid, 0.015 t of L-glutamic acid, and 0.01 t of (NH4)2SO4 are required to produce 1 t of SSF fermentation broth, and the total material investment cost is about 1037 RMB/t. Now, the abuse of chemical fertilizers in Chinese agriculture has caused many soil and ecological problems, and there is a need for green and efficient fertilizer synergists [46]. Our study provided a new technology to produce high-value fertilizer synergists from agricultural waste, which was helpful to the recycling of straws and the development of sustainable agriculture.
However, the yield of γ-PGA obtained by our SSF was lower than that of reported SHF [10, 11]. The production efficiency of SSF can be improved by some optimization measures, such as screening effective pretreatment methods for lignocellulosic biomass [18, 47], selecting suitable substrate content [18], developing a reasonable feeding strategy [24, 36], and so on. In the next work, we will optimize the production conditions of SSF to increase production, and try to establish a larger production process.
Conclusion
In this study, a SSF using corn straw pretreated by citric acid to synthesize γ-PGA was established, and the products were proved to have fertilizer synergistic effect. Compared with the traditional SHF process, SSF greatly simplified the production steps, and each step achieved zero emission as far as possible. Based on our results, SSF with corn straw could be a promising strategy to produce γ-PGA, which also be a simple and green way for lignocellulose biorefinery and sustainable cultivation in agriculture. However, the γ-PGA yield of SSF process was slightly lower, we will optimize the production conditions to increase production and try to establish a large-scale production process in next work.
References
Ogunleye A, Bhat A, Irorere VU, Hill D, Williams C, Radecka I (2015) Poly-γ-glutamic acid: production, properties and applications. Microbiology 161:1–17
Bajaj I, Singhal R (2011) Poly (glutamic acid)-an emerging biopolymer of commercial interest. Bioresour Technol 102:5551–5561
Xu Z, Wan C, Xu X, Feng X, Xu H (2013) Effect of poly (γ-glutamic acid) on wheat productivity, nitrogen use efficiency and soil microbes. J Soil Sci Plant Nutr 13:744–755
Xu Z, Lei P, Feng X, Xu X, Liang J, Chi B, Xu H (2014) Calcium involved in the poly(γ-glutamic acid)-mediated promotion of Chinese cabbage nitrogen metabolism. Plant Physiol Biochem 80:144–152
Lei P, Xu Z, Ding Y, Tang B, Zhang Y, Li H, Feng X, Xu H (2015) Effect of poly(γ-glutamic acid) on the physiological responses and calcium signaling of rape seedlings (Brassica napus L.) under cold stress. J Agric Food Chem 63:10399–10406
Ashiuchi M (2013) Microbial production and chemical transformation of poly-γ-glutamate. Microb Biotechnol 6:664–674
Sirisansaneeyakul S, Cao MF, Kongklom N, Chuensangjun C, Shi ZP, Chisti Y (2017) Microbial production of poly-γ-glutamic acid. World J Microb Biot. https://doi.org/10.1007/s11274-017-2338-y
Jorgensen H, Kristensen JB, Felby C (2007) Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuel Bioprod Bior 1:119–134
Hong JL, Ren LJ, Hong JM, Xu CQ (2016) Environmental impact assessment of corn straw utilization in China. J Clean Prod 112:1700–1708
Tang B, Lei P, Xu ZQ, Jiang YX, Xu Z, Liang JF, Feng XH, Xu H (2015) Highly efficient rice straw utilization for poly-(γ-glutamic acid) production by Bacillus subtilis NX-2. Bioresour Technol 193:370–376
Zhu F, Cai J, Zheng Q, Zhu XC, Cen PL, Xu ZN (2014) A novel approach for poly-γ-glutamic acid production using xylose and corncob fibres hydrolysate in Bacillus subtillis HB-1. Chem Technol Biotechnol 89:616–622
Sun JD, Tang C, Zhou J, Wei P, Wang YJ, An W, Yan ZY, Yong XY (2021) Production of poly-γ-glutamic acid (γ-PGA) from xylose-glucose mixtures by Bacillus amyloliquefaciens C1. 3 Biotech 11(2):1–10. https://doi.org/10.1007/s13205-021-02661-7
Rajan K, Carrier DJ (2014) Effect of dilute acid pretreatment conditions and washing on the production of inhibitors and on recovery of sugars during wheat straw enzymatic hydrolysis. Biomass Bioenerg 62:222–227
Takagi M, Abe S, Suzuki S, Emert G, Yata N (1977) A method for production of alcohol directly from cellulose using cellulase and yeast. In: Ghose T (ed) Proceedings of the bioconversion symposium. IIT, New Delhi
Saggi SK, Dey P (2016) An overview of simultaneous saccharification and fermentation of starchy and lignocellulosic biomass for bio-ethanol production. Biofuels. https://doi.org/10.1080/17597269.2016.1193837
Pinaki D, Lhakpa W, Joginder S (2015) Simultaneous saccharification and fermentation (SSF), an efficient process for bio-ethanol production: an overview. Biosci Biotechnol Res Asia 12:87–100
van der Pol EC, Eggink G, Weusthuis RA (2016) Production of L(+)-lactic acid from acid pretreated sugarcane bagasse using Bacillus coagulans DSM2314 in a simultaneous saccharification and fermentation strategy. Biotechnol Biofuels. https://doi.org/10.1186/s13068-016-0646-3
Zheng P, Fang L, Xu Y, Dong JJ, Ni Y, Sun ZH (2010) Succinic acid production from corn stover by simultaneous saccharification and fermentation using Actinobacillus succinogenes. Bioresour Technol 101:7889–7894
Peng X, Zhang C, Tian Y, Guo X, Liu Y, Xiao D (2014) Corncob residue pretreatment for 2,3-Butanediol production by simultaneous saccharification and fermentation. In: Zhang TC, Ouyang P, Kaplan S, Skarnes B (eds) Proceedings of the 2012 International Conference on Applied Biotechnology (ICAB 2012). Springer, Berlin, Heidelberg
Barisik G, Isci A, Kutlu N, Bagder Elmaci S, Akay B (2016) Optimization of organic acid pretreatment of wheat straw. Biotechnol Prog 32:1487–1493
Lee JW, Jeffries TW (2011) Efficiencies of acid catalysts in the hydrolysis of lignocellulosic biomass over a range of combined severity factors. Bioresource Technol 102:5884–5890
Kundu C, Lee HJ, Lee JW (2015) Enhanced bioethanol production from yellow poplar by deacetylation and oxalic acid pretreatment without detoxification. Bioresource Technol 178:28–35
Fang JN, Huan CC, Liu Y, Xu LS, Yan ZY (2020) Bioconversion of agricultural waste into poly-γ-glutamic acid in solid-state bioreactors at different scales. Waste Manage 102:939–948
Olofsson K, Palmqvist B, Lidén G (2010) Improving simultaneous saccharification and co-fermentation of pretreated wheat straw using both enzyme and substrate feeding. Biotechnol Biofuels 3:17
Yong X, Raza W, Yu G, Ran W, Shen Q, Yang X (2011) Optimization of the production of poly-γ-glutamic acid by Bacillus amyloliquefaciens C1 in solid-state fermentation using dairy manure compost and monosodium glutamate production residues as basic substrates. Bioresour Technol 102:7548–7554
Bura R, Chandra R, Saddler J (2009) Influence of xylan on the enzymatic hydrolysis of steam-pretreated corn stover and hybrid poplar. Biotechnol Progr 25:315–322
Yan L, Zhang H, Chen J, Lin Z, Jin Q, Jia H, Huang H (2009) Dilute sulfuric acid cycle spray flow-through pretreatment of corn stover for enhancement of sugar recovery. Bioresour Technol 100:1803–1808
Gorke B, Stulke J (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624
Liu N, Santala S, Stephanopoulos G (2020) Mixed carbon substrates: a necessary nuisance or a missed opportunity? Curr Opin Biotech 62:15–21
Stulke J, Hillen W (2000) Regulation of carbon catabolism in Bacillus species. Annu Rev Microbiol 54:849–880
Stulke J, Hillen W (1999) Carbon catabolite repression in bacteria. Curr Opin Microbiol 2:195–201
Guragain YN, Vadlani PV (2017) 2,3-Butanediol production using Klebsiella oxytoca ATCC 8724: evaluation of biomass derived sugars and fed-batch fermentation process. Process Biochem. https://doi.org/10.1016/j.procbio.2017.05.001
Alkasrawi M, Rudolf A, Lidén G, Zacchi G (2006) Influence of strain and cultivation procedure on the performance of simultaneous saccharification and fermentation of steam pretreated spruce. Enzyme Microb Technol 38:279–286
Dasari RK, Dunaway K, Berson RE (2009) A scraped surface bioreactor for enzymatic saccharification of pretreated corn stover slurries. Energy Fuels 23:492–497
Almeida JR, Modig T, Petersson A, Hähn-Hägerdal B, Lidén G, Gorwa-Grauslund MF (2010) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J chem Technol Biotechnol 82:340–349
Hodge DB, Karim MN, Schell DJ, Mcmillan JD (2009) Model-based fed-batch for high-solids enzymatic cellulose hydrolysis. Appl Biochem Biotechnol 152:88–107
Wang QJ, Chen SW, Zhang JB, Sun M, Liu ZD, Yu ZI (2008) Co-producing lipopeptides and poly-γ-glutamic acid by solid-state fermentation of Bacillus subtilis using soybean and sweet potato residues and its biocontrol and fertilizer synergistic effects. Bioresource Technol 99:3318–3323
Shaukat AA, Tian X, Hussain Q, Singh U (2012) Carbon dioxide emission from maize straw incubated with soil under various moisture and nitrogen levels. J Chem Soc Pakistan 34:22–27
Khalil MI, Gutser R, Schmidhalter U (2009) Effects of urease and nitrification inhibitors added to urea on nitrous oxide emissions from a loess soil. J Plant Nutr Soil Sc 172:651–660
Han WY, Ma LF, Shi YZ, Ruan JY, Kemmitt SJ (2008) Nitrogen release dynamics and transformation of slow release fertiliser products and their effects on tea yield and quality. J Sci Food Agr 88:839–846
Iizumi T, Mizumoto M, Nakamura K (1998) A bioluminescence assay using Nitrosomonas europaea for rapid and sensitive detection of nitrification inhibitors. Appl Environ Microb 64:3656–3662
Majeed Z, Ramli NK, Mansor N, Man Z (2015) A comprehensive review on biodegradable polymers and their blends used in controlled-release fertilizer processes. Rev Chem Eng 31:69–96
Yan XY, Ohara T, Akimoto H (2006) Bottom-up estimate of biomass burning in mainland China. Atmos Environ 40:5262–5273
Zhang HF, Hu DW, Chen JM, Ye XN, Wang SX, Hao JM, Wang L, Zhang RY, An ZS (2011) Particle size distribution and polycyclic aromatic hydrocarbons emissions from agricultural crop residue burning. Environ Sci Technol 45:5477–5482
Lim SL, Lee LH, Wu TY (2016) Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: recent overview, greenhouse gases emissions and economic analysis. J Clean Prod 111:262–278
He R, Shao CF, Shi RG, Zhang ZY, Zhao R (2020) Development trend and driving factors of agricultural chemical fertilizer efficiency in China. Sustainability-Basel 12(11):4607. https://doi.org/10.3390/su12114607
Widmer W, Zhou WY, Grohmann K (2010) Pretreatment effects on orange processing waste for making ethanol by simultaneous saccharification and fermentation. Bioresource Technol 101:5242–5249
Acknowledgements
This work was supported by CAS “Light of West China” Program (2019XBZG_XBQNXZ_A_001; 2019XBZG_JCTD_ZDSYS_001), the Grant from Department of Science and Technology of Sichuan Province (2020ZYD023), the Major Science and Technology Projects of Sichuan Province (No. 2018SZDZX0024), and Talent special support from the Organization Department of Sichuan Provincial Party Committee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ji, G., Xu, L., Lyu, Q. et al. Poly-γ-glutamic acid production by simultaneous saccharification and fermentation using corn straw and its fertilizer synergistic effect evaluation. Bioprocess Biosyst Eng 44, 2181–2191 (2021). https://doi.org/10.1007/s00449-021-02593-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02593-9