Abstract
Waste carbon straw like rice straw (RS) could be consumed as an ideal substrate in anaerobic fermentation for biofuel production. However, this practice is obstructed by the existence of the lignin polymer in RS. Enzyme lysates from individual strains and enzyme lysate mixture from all seven strains were used in to remove the lignin of waste carbon straw (RS). The effect of enzyme lysate on removing lignin and increasing fermentation rate was measured. Maximum delignification of RS was obtained from enzyme mixture of all seven bacterial strain crude samples as compared to individual enzyme lysates of these bacterial strains. A selective lignin reduction of 46.7% was obtained from enzyme mixture-pretreated RS samples; however, the highest individual TL26E enzyme lysate degrades 19.9% of the lignin from the treated RS sample. Scanning electron microscopy (SEM) analysis displayed a visible modification in the organic fiber of the pretreated RS samples compared to the untreated RS. The cumulative biogas was up to 260–280 NmL/gVS from the individual enzyme lysate. However, maximum cumulative biogas of 489.6 NmL/gVS was obtained from enzyme mixture-pretreated RS. Cumulative biogas produced from treated RS was 38% higher than the untreated RS. The study suggests that the synergistic enzymatic treatment could be more effective for waste biomass hydrolysis in bioprocess technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The energy crises and dependency on fuels from petroleum sources focus on the demand for renewable energy from waste biomass. To build an efficient renewable energy and biochemical system, a specific robust hydrolytic inoculum, waste biomass, and safe biochemical process are necessary. It is necessary to isolate, screen, and purify only the efficient microbial strains. The introduction of standardized inoculum and enzyme lysate for each specific bioproduct is likely to have an enormous outcome on the cost of product yield at the industrial level [1].
Food waste and waste lignocellulosic biomass (WLB) are of potential interest for biomaterials and biobased products. Food waste is easily digestible; however, WLB like grasses, waste paper, wood residues, and crop residues are abundant and most of the countries practiced it to burn them in the field. So, a valuable carbon source has vanished. WLB is composed of mainly three basic molecules, lignin, hemicellulose, and cellulose. Lignin crosslinking between the polysaccharides (cellulose and hemicellulose) confers hydrolytic stability and resists degradation and structural recalcitrance. Due to this barrier, the sugars remain entrapped inside lignin. Several pretreatment methods, which include chemical and physical, are employed for waste biomass hydrolysis. These waste biomass pretreatment methods are not suitable because of high cost and inhibitor generation [2]. Without treatment, the final yield of products are less as lignin acts as a barrier to bioenergy production and other industrial fermentation [2]. Thus, an economical pretreatment method will be chosen to provide readily digestible waste sugar carbon from lignocellulosic biomass for energy, biomaterials, and biochemicals and other industrial products [3]. The selection of a suitable pretreatment method is vital but the treatment method should be efficient and must be inexpensive, must increase energy yield, and should not generate any inhibitors [4].
Gaseous anaerobic fermenting microorganisms are reported to breakdown simple sugars into energy and biochemicals. However, the isolation of solvents producing aerobic and anaerobic microorganisms from complex biomass is not known yet [5]. So far, known microorganisms are acetogenic, acidogenic, and methanogenic. These microorganisms produce acetic acid, hydrogen, carbon dioxide, and methane. The acetogenic and acidogenic-specific microorganisms yield acids and VFA production. The final product is methane which is generated mainly from the acids generated in these steps as shown in the equation below [6, 7].
To replace the need for chemical treatment for waste biomass and to purify standardize strains and enzyme is desirable in future bioprocess technology. This study targeted to use enzyme from already isolated ligninolytic Bacillus sp. strains. These strains were capable of growth on lignin waste and straw. Laccase and lignin peroxidase are two important enzymes critical to lignin degradation. Besides other microorganisms, Bacillus sp. is reported with multiple enzymes including laccase and lignin peroxidase [8]. These two enzymes are vigorously involved in the breakdown of aromatic amines, phenols, and many other xenobiotic molecules [9]. In this study, enzyme lysate in separate and enzyme mixture from selected seven best strains of Bacillus sp. were used. We tested the efficiency of enzyme extracts from these strains to break down lignin in rice straws. After the enzyme lysate pretreatment, the compositional, morphological, and fermentation yields were analyzed for possible improvement.
2 Methodology
2.1 Substrate
Substrate, i.e., rice straw (RS) residue, was chopped and collected as dried materials from a local field. The RS residue was packed in polythene bags at room temperature for a 1-week time. The RS was ground to 20-mesh size and kept at room temperature in polyethylene plastic bags.
2.2 Microbial culture selection
In our previously reported study [8], seven strains out of 27 Bacillus sp. strains were selected based on the ability to grow on alkali lignin and synthetic azure B dye. The medium of these strains consisted of 1 g/L alkali lignin dissolved in mineral salt of 1.0 g/L NaCl, 1.0 g/L MgSO4, 1.0 g/L KH2PO4, 0.4 g/L CuSO4, 0.002 g/L MgSO4, and 0.5 g/L CaCl2. The selected best strains with the uppermost actions against azure B are listed in Table 1.
2.3 Enzyme lysate preparation
To prepare enzyme lysates, the Bacillus sp. strains were cultured in 50 mL of media consisting of 1% glucose, 0.5% (NH4)2SO4, 0.5% peptone, 1.0 g/L KH2PO4, 1.0 g/L MgSO4, 1.0 g/L NaCl, 0.5 g/L CaCl2, 0.4 g/L CuSO4, and 0.002 g/L MgSO4 at 37°C for 5 days at 150 rpm. Crude enzyme was harvested from bacterial culture by disrupting the cells through sonication for 5 min followed by centrifugation at 10,000 rpm for 10 min at 4°C.
2.4 Lignin peroxidase (LiP) and laccase (Lac) assay
The bacterial culture was incubated at 150 rpm at 37°C for 7 days on a medium containing lignin (0.01 g/100 mL), peptone (0.05 g/100 mL), and yeast extract (0.05 g/100 mL). The crude enzyme supernatants were assayed for lignin peroxidase (LiP) and laccase (Lac) enzyme activities as described previously in detail [8]. The enzyme activity of LiP and Lac was calculated as
C is for the substrate concentration in the assay.
2.5 Rice straw hydrolysis
Five grams of rice straw (RS) was moistened in 100 mL of phosphate buffer (0.5 M) pH 5 for pretreatment RS hydrolysis at 50°C for 7 days at 150 rpm either enzyme lysates of the bacteria. For the RS hydrolysis by crude enzymes, the lysates from each strain were tested either individually or as a mixture from all seven strains. For the individual lysate, 40 mL of crude enzyme (0.7 mg proteins) from each strain was added to the flask. For the lysate mixture, 5.7 mL of a crude enzyme (0.1 mg proteins from each of the seven strains equal to 0.7 mg proteins) was combined and added to the flask. The flasks were shaken at 50 ± 1°C, pH 5, 150 rpm for 7 days. RS without enzyme addition was kept as a control sample. The weight change was calculated as the difference between the initial and final weight of RS using this formula:
2.6 Proximate analysis for composition
Proximate analysis can be used to assess a sample’s moisture, ash, volatile and solid organic matter (TS, VS), and proportion of total fixed carbon. In order to perform proximate analysis, the untreated rice straws used in our current research were first analyzed by Laboratory Analytical Procedures LAP-001 (23) and LAP-005 (24) [10] to calculate the composition of untreated rice straw. The initial composition of moisture content, ash, volatile matter, lignin, hemicellulose, and cellulose of rice straws was determined. Change in the amount of hemicellulose and cellulose was measured for each tested RS sample according to the National Renewable Energy Laboratory (NREL) method. The moisture was determined by taking 2 g of rice straw and dried at 105°C overnight. Moisture content was calculated using Eq. 1,
where M is the initial sample weight/g, M1 is the sample weight/g + container before drying, and M2 is the sample weight/g + container after drying. Similarly, 2 g dried rice straw was heated to 500°C in a muffle furnace for 3 h, cooled to ambient temperature in a desiccator, and weighed to assess the samples’ ash content. The ash was estimated as such (Eq. 2),
where S is the burn dish weight, S1 is the sample without moisture, and S2 is the weight of sample plus dish after furnace ignition. A clean crucible was heated for 24 h to 105–110°C; the substrate was weighted and dried in the oven set at 70°C. The TS is calculated as Eq. 3,
where T is the dried sample + dish and S is the dish weight. The volatile solids (VS) were measured by heating the substrate to 450°C for 1 h in a desiccator. The dry ash was collected from the muffle furnace, and VS was calculated after cooling the sample.
where V is the substrate weight + dish and D is the substrate weight + dish after ignition. A 300-g dry sample was soaked in 3 mL of 72% sulfuric acid in a pressure tube for lignin measurement, and the sample was then incubated in a water bath at 30°C for 60 min. A tiny glass rod was used to agitate the samples continuously. Eighty-four milliliters of pure water is added to dilute the reaction to 4%. The reaction ceased after full breakdown and cooled to ambient temperature. The soluble lignin is measured from the UV-visible spectrophotometer’s absorbance value, and insoluble lignin is from the weight of the ash after overnight oven-dried rice straw sample and burned at 575 °C. The calculations are done according to the NREL laboratory analytical process [10]. The National Renewable Energy Laboratory’s (NREL) established analytical method (NREL/TP-510-42618) was used for the analysis of rice straw composition of cellulose and hemicellulose sugars (hexose and pentose sugars) by using high-pressure liquid chromatography (HPLC) system for rice straw samples. The compositions of rice straw were determined before and after pretreatment processes. Acid hydrolysis was used to convert the samples’ cellulose and hemicellulose into monomeric sugars. HPLC from Agilent (USA) equipped with a refractive index detector (RID) and an Agilent Hi-Plex-H column that was maintained at 55 °C and 80 °C, respectively, was run to measure monomeric sugars. A flow rate of 0.6 mL/min was used as a mobile phase in HPLC. Glucose and galactose were quantified for cellulose amount, xylose and arabinose for hemicelluloses. The calculations were done using Eqs. 5 and 6.
where GGR is the galactose glucose released (g), dW is dry weight of sample (g), and AXR is the arabinose and xylose released (g), whereas the calculation for the lignin degradation was done as in Eq. 7.
dW is the dry weight of sample (g), ASLf is the acid-soluble lignin in treated rice straw, ASLf is the acid-insoluble lignin in treated rice straw, Wi is the initial weight (g) of the untreated rice straw, ASLi is the acid-soluble lignin in untreated rice straw, and ASLi is the acid-insoluble lignin in untreated rice straw.
The total mass loss as a dried base was measured total solid loss percentage after reaction pretreatment completion. All the samples were stored for scanning and anaerobic fermentation to biogas.
2.7 Scanning electron microscopy (SEM)
The enzyme lysate treated of individual strain and enzyme lysate mixture-treated RS samples were checked for surface destruction using SEM (JSM-7800F PRIME, JEOL USA). The method details for taking the SEM images to note the changes induced by enzyme lysate on RS surface were used as described previously [2].
2.8 Anaerobic digestion assay
For the anaerobic digestion, the rice straw treated with enzyme mixture and enzyme lysate of the respective isolates and untreated RS were selected for evaluation of biogas production. The anaerobic digestion assay (ADA) was performed in 100 mL of anaerobic reactors. In each anaerobic reactor, 1 g untreated RS, control inoculum sludge, individual enzyme lysate-treated RS, and enzyme lysate mixture-treated RS were added. Each anaerobic reactor is added with 2 mL of multivitamin solutions, 8 mL of sodium bicarbonate solution, and 8 mL of potassium bicarbonate (2.5 g/L). For each anaerobic reactor, food/microbe ratio (F/M) of 0.56 g/VS was set as the initial starter inoculum. The anaerobic reactor was degassed using nitrogen gas flushing for 5 min. The ADA anaerobic reactors were brought to pH 8 and kept in triplicate at 37 ± 1°C conditions. The ADA anaerobic reactors were checked daily for biogas production using the water displacement method. The methane and carbon dioxide percentage of each anaerobic reactor was analyzed using GFM406, a multichannel portable gas analyzer. The obtained biogas data was checked using OBA simulator as described previously. This OBA biogas software uses R package to measure the total methane, total biogas produced, rate of biogas in volume, and rate of methane in volume as described previously [4, 11].
3 Results
3.1 Effect of enzyme hydrolysis on rice straw (RS)
The rice straw was composed of 19.8% lignin, 26.4% hemicellulose, 36.2% cellulose, 93.4% TS, and 76.6% VS. These results are consistent with previously reported data of rice straw [12]. Enzyme hydrolysis is supposed to induce changes in the physical, chemical structures and chemical compositions of RS. The isolated and selected bacterial culture strain, when grown on lignin and the lignin analog dye azure B, substantially reduced the lignin and azure B dye solution after 72 h of incubation. In comparison to other strains, TL4, TL6, and TL26 had the highest activity for decolorizing lignin and the azure B dye out of the seven selected cultures. TL4, TL6, and TL26 were greater among the seven Bacillus sp. strains examined for the optimum expression time and activity condition, despite all the strains producing the lignin peroxidase (LiP) and laccase (Lac) being expressed. All of the strains showed suitable LiP and Lac activity at 50 °C, while optimal LiP expression was seen at 48 h of growth and optimal activity was at pH 3. Similar to this, 72 h of growth produced the maximum expression of Lac activity, which peaked at pH 5. The greatest LiP and Lac activity, for TL26, TL6, and TL4, was 2.99 IU/mL, 2.81 IU/mL, 2.74 IU/mL, 2.69 IU/mL, 1.96 IU/mL, and 1.77 IU/mL, respectively (data not shown).
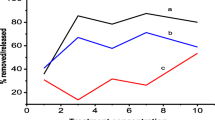
Among the seven Bacillus sp. strains, TL26, TL6, and TL4 had the highest enzyme activity for LiP and Lac; as a result, these three strains were chosen as the highest producers. Rice straw hydrolysis effect with individual enzyme lysate and enzyme mixture of all seven Bacillus sp. strains is shown in Fig. 1. In the testing of lignin degradation from the rice straw for the crude enzyme samples of TL4E, TL6E, TL8E, TL24E, TL26E, TL27E, and TL33E, the crude enzyme sample of TL33E and TL27E showed the highest 10.49% and 8.75% degradation of cellulose contents from the rice straw initial composition as compared to other tested samples (Fig. 1A). Likewise, TL33E and TL27E also produced 5.1% and 4.6% decrease of hemicellulose contents from the rice straw initial composition as compared to other tested samples (Fig. 1C). Interestingly, TL4E, TL6E, and TL26E displayed the maximum lignin degradation of 13.3%, 18.6%, and 19.5%, respectively, decrease of lignin from the rice straw initial composition as compared to other tested samples (Fig. 1B), whereas the enzyme mixture sample of all the seven decreased the cellulose contents from the sample by 42.2% and 24.9% of hemicellulose and 46.7% of lignin (Fig. 1D). This degradation of lignin of rice straw for TL4E, TL63, and TL26E corresponded to the high expression of LiP and Lac. In all the seven enzyme individual test and enzyme lysate mixture treatment, a decrease of lignin was detected; however, the reduction of lignin percentage is more significant than the individual enzyme treatment, whereas a negligible amount of reduction in cellulose and hemicellulose content was observed in case of both individual enzyme lysate and enzyme lysate mixture samples (Fig. 1).
Ligninolytic bacteria have been isolated from different ecosystems, including soil, animal excreta, organic waste, compost deposits, the digestive tracts of insects, and wastewater. These bacteria have the potential to play a significant role in the process of lignin degradation and modification. The bacteria that possess the ability to modify and degrade lignin can be categorized into Actinomycetes, specific members of the Firmicutes phylum, α-Proteobacteria, and γ-Proteobacteria. A list of such bacterial strain is given in Table 2.
3.2 SEM micrographs of rice straw
The surface structure of the untreated RS and pretreated RS with enzyme was compared applying SEM micrographs (Fig. 2). The untreated RS (designated as C) has highly compact, smooth, and homogeneous structure. The homogeneous and smooth structure was degraded after enzyme and bacterial culture treatment; the solid residue of RS lost their highly dense structure. The surface was showing a clear degradation and distortion. The images in Fig. 2, for TL4E, TL6E, and TL26E, clearly show broken fibrils and disrupted bundles in the cell wall complex of each sample. Our results are consisted with previously reported SEM micrograph of RS treated with ligninolytic consortia and ligninolytic enzyme. They also reported similar distortion and degradation after biological pretreatment of RS [14, 15].
3.3 ADA for biogas and methane yield
The biogas potential is often defined as the volume of biogas produced per gram volatile solid (VS) added for the specific substrate. The accumulated final methane production is regarded as the methane potential of the particular substrate. In the current study, the methane (CH4) content of the biogas was 32% for untreated, around 36–38% for enzyme lysate, and 60.5% for enzyme mixture-pretreated rice straw (Table 3).
The daily biogas volume, daily volumetric methane rate, cumulative biogas, and cumulative methane yield were measured from the anaerobic digestion of RS for untreated, individual enzyme lysate, and enzyme mixture of Bacillus sp. strain-pretreated rice straw. The results show that maximum biogas and methane yield both occurred between 6 and 30 days and the highest results were obtained from enzyme mixture compare to enzyme lysate and untreated RS, respectively. Likewise, the daily biogas volume and daily volumetric methane yield were also similarly maximum between 6 and 30 days from enzyme mixture, compared to individual enzyme lysate and untreated RS, respectively. In the biogas potential experiment from rice straw from samples of untreated as control, TL4E, TL6E, TL26E, and enzyme mixture as treated samples were observed and measured as the volume of biogas per gram volatile solid of the rice straw added in the reaction bottle. A 48-h lag phase was detected in the TL4E, TL6E, TL26E, and enzyme mixture-treated samples, whereas 6 days of lag phase in the case of untreated rice straw sample. The methane and biogas yield was gradually increased from the 4th day and continued till two-week time; later, the yield per day starts to decline in the daily biogas yield. The experiments were run till the biogas completely stopped and no further the methane content was detected through GC and no daily biogas pressure observed from the reactor bottle. When biogas stopped, the methane content was dropped in the reactor bottle samples. The experimental values of methane percentage from the enzyme mixture-pretreated rice straw had produced into an expressively high methane % and methane yield than that of individual enzyme lysate and untreated rice straw. The high methane yield could be of the low lignin quantity in the enzyme mixture-treated sample and efficient methanogenesis in the treated as compared to the untreated sample.
The highest daily biogas was 7, 26–28, and 55 mL/day from untreated, enzyme lysate separately (TL4-E, TL6-E, TL26-E), and enzyme mixture of all Bacillus sp. strains, respectively (Fig. 3). Analogous response of daily biogas yield was observed for increasing and dropping down to 10 mL/day at the end of the experiment for the untreated RS and enzyme lysate separately (TL4-E, TL6-E, TL26-E). However, the daily biogas yield was speedily increased and gradually decreased for the pretreated enzyme mixture of all Bacillus sp. strains and reached up to minimum 13 mL/day, respectively, at the end of the experiment. Likewise, the highest daily volumetric methane rate was 4.2, 5–8, and 19.5 mL/gVS from enzyme lysate separately (TL4-E, TL6-E, TL26-E) and enzyme mixture of all Bacillus sp. strains, respectively (Fig. 3).
Provided with standard conditions in the biogas software package (written in R) and using the web-based interface calculation measure with 101.325 kPa and 0 °C for each sample data by default, the web-based interface called OBA calculated the volumetric rate of methane from the daily volume of biogas for each data point. The results obtained showed that the three samples treated with TL4E, TL6E, and TL26E produced a slower methane production compared to the enzyme-mixture sample. During the accumulation and production of methane, the second week of fermentation period in 8–12th day has produced maximum methane yield. It is evident the TL4E, TL6E, and TL26E 4.43 NmL/gVS volumetric rate of methane per day during the fermentation period in the 8–12th day, whereas the enzyme-mixture sample 10.25 NmL/gVS volumetric rate of methane per day fermentation period in the 8–12th day. Although the untreated rice straw sample showed a minute expression of 0.169 NmL/gVS volumetric rate of methane per day fermentation period in the 8–12th day, the methane yield showed that the enzymatic pretreatment, individual enzyme lysate, and enzyme mixture increase the production rate of methane compared to untreated rice straw sample. This may be primarily due to the action of the enzyme laccase and lignin peroxidase pretreatment which caused lignin to become soluble, allowing quick hydrolysis of the cellulose and hemicellulose fractions and enhancing the fermentation process, which eventually increased the methane yield.
The cumulative maximum biogas (Fig. 4) was 265.3, 270–280, and 528.9 NmL/gVS from untreated, individual isolates (TL-4, TL6, TL26), and co-inoculated Bacillus sp. strain-pretreated rice straw, respectively. The cumulative maximum biogas (Fig. 4) was 250.5, 260–286, and 489.9 NmL/gVS from untreated, individual enzyme lysate (TL4E, TL6-E, TL26-E), and enzyme mixture of all Bacillus sp. strains, respectively (Fig. 4). The results of daily biogas, daily volumetric methane rate, and cumulative biogas of the pretreated RS for enzyme mixture of all Bacillus sp. strains were twofold or 50% higher than that of the individual enzyme lysate and untreated RS, respectively, after 50 days of anaerobic digestion. These results indicate that enzyme mixture of Bacillus sp. strains increased 38.1% biogas production.
To observe a significant difference in the overall methane production between the testes samples for TL4E, TL6E, TL26E, and the enzyme mixture. An analysis of variance (ANOVA) in one direction was used to look into the statistical variation in methane production. The results of the variance analysis (ANOVA) and multiple comparisons of the samples of untreated rice straw and treatment tests for TL4E, TL6E, TL26E, and enzyme mixture are shown in Table 3. The substrate samples for the enzyme mixture, TL4E, TL6E, and TL26E treatments on untreated rice straw totaled 94 reaction samples. Table 4 shows that two different tests—the Brown-Forsythe test and Bartlett’s test—were carried out in the one-way ANOVA to determine the significance of the results and methane output. The results are significant according to both the Brown-Forsythe test and Bartlett’s test analyses, which both gave R2 values of 0.2767 and P values of P > 0.001. The majority of the variables for all of the substrates under investigation—rice straw that had not been treated, rice straw that had been treated with TL4E, TL6E, TL26E, and enzyme mixture—were consistently significant in the one-way ANOVA, with a P value of 0.001 being the consistent value. The yields of the treated samples and the untreated sample are substantial and favorably suggest that this impact is attributable to the improved lignin breakdown and cellulose digestibility, both of which promote anaerobic digestion.
4 Discussion
Microorganisms are a favorable source for production of biochemicals and metabolites. In fact, a microbial cell is functioning as a factory, which synthesized different kinds of useful secondary materials [16]. Enzymes are one of the useful secondary molecules synthesized by bacterial culture that can be used for the different industrial purpose in the desired field [17]. These enzymes have wide applications in the formation of detergents, starch processing, food industry, paper and pulp industry, and biofuel production. Genetic manipulation can make enzymes more vital for commercial applications in biorefinery industry. The globe is spending a huge amount of money on the imports of fuel produced from fossil resources. The increasing of population further intensifies the demand for fuel [3]. The researchers are now looking for a method to utilize waste biomass for alternative sustainable energy at cheaper cost [18]. Bacillus strains are known for their ability to break down lignin. These strains can use alkaline lignin as their sole source of carbon or energy and have been found to have at least four different pathways for lignin degradation, including the gentisate pathway, the benzoic acid pathway, and the β-ketoadipate pathway. Whole-genome sequencing has revealed that these strains contain genes that encode enzymes involved in lignin degradation, such as laccases and peroxidases. Bacterial ligninolytic enzymes, including lignin peroxidase, manganese peroxidase, and laccase, facilitate the degradation of lignin. These enzymes can be classified into two groups: lignin-modifying enzymes (LME) and lignin-degrading auxiliary enzymes (LDA). LME enzymes like lignin peroxidase and manganese peroxidase modify the structure of lignin, while LDA enzymes like β-aryl ether and biphenyl degrading enzymes help break down lignin monomers and dimers. The presence of these enzymes in bacteria can work together to enhance the efficiency of lignin degradation. The breakdown of lignin can be achieved through a combination or mixture of different enzymes. Bacillus aryabhattai BY5, for example, is a strain that has been found to degrade lignin and express the simultaneous action of laccase and manganese peroxidase enzymes in the lignin disintegration process [19].
Due to lignin’s complex and heterogeneous nature, the involvement of multiple enzymes is necessary for its degradation. Using a mixture or combination of enzymes can be an effective approach to achieve better lignin degradation compared to using a single enzyme. In a combination of mixed enzymes, laccases oxidize lignin and other phenolic compounds, producing free radicals that break down the lignin polymer. Similarly, peroxidases speed up the oxidation process, generating reactive intermediates that break down the lignin polymer. Manganese peroxidases, on the other hand, facilitate the oxidation of lignin and other phenolic compounds in the lignin polymer. β-Etherases play a significant role in efficiently degrading lignin by cleaving the β-O-4 bond, which is the most abundant linkage in lignin [20]. Therefore, combination of enzymes from robust culture with high enzyme yield could be considered a good choice to be investigated for lignin degradation. Therefore, this study targeted to use the combination of enzyme lysate from robust culture to hydrolyzed rice straw and analyze biogas yield from anaerobic digestion. Lignin and azure B were successfully decolored by the seven powerful bacteria that degrade synthetic dye and lignin. Both lignin peroxidase and laccase, two crucial enzymes involved in lignin’s breakdown, were expressed by each and every one of the strains. The high enzyme activities and lignin degradation of the TL4E, TL6E, and TL26E enzymes suggest their possible use as pretreatment for the hydrolysis of waste biomass. To enhance lignin degradation, more research on optimization is required, particularly in the areas of nutrition and the optimization of conditions for enzymes in mixture dose. TL4E, TL6E, and TL26E enzymes expressed the highest lignin degradation. The results obtained are in line with earlier research that demonstrated lignin breakdown and the production of lignin peroxidase and laccase for Bacillus sp. strain VUS [21] and Bacillus subtilis [22].
The results of rice straw in composition similar for mix microbial pretreatment of corn straw are reported in an earlier study, a 5–6% total reduction in cellulose and hemicellulose contents while 25% reductions in total lignin observed; the study further proved an enhanced biogas production [23]. It is also known that bacterial peroxidase and laccase are capable to modify and degrade lignin. Further, these enzymes predominantly attack the phenolic compound like lignin and then non-phenolic benzylic structures [24]. Bacillus sp. strains CS-1 and CS-2 are reported to degrade alkali lignin, and the strains displayed high laccase activities [12]. In earlier study, it is shown that the degradation ability of lignin consortium from rice straw can be increased using mix microbial within 72 h of incubation [25]. These observations determined that the assessment of advance technology, microbial isolates, and hydrolytic enzymes are necessary tools for production of biofuels from lignocellulosic biomass [26]. SEM analysis after rice straw pretreatment with enzyme lysate is supported with similar degradation by microbial consortium; the micrograph of SEM reported penetration of the outer structure and disruption of rice straw [27].
An analogous study of biological pretreatment of lignocellulosic biomass reported that microbial activity expressively improved structural modification of cellulose in straw and increased its accessibility up to sixfold within the first days of pretreatment [28]. Correspondingly, in an evaluation of biogas production from rice straw after the biological treatment, the main carbohydrate component (cellulose) is easily transformed into biogas in pretreated rice straw as compared to untreated rice straw solid residue and enhancement of cumulative biogas is testified from treated rice straw than untreated sample [29]. This study emphasizes the importance of conducting additional research on the regulation of enzyme activity and the action of these enzymes into new low-molecular-weight compounds. The study highlights the importance of using combination of enzymes, including lignin-degrading enzymes and co-functional enzymes in the future, to achieve efficient separation of lignin and lignocellulosic biomass. This approach can greatly enhance the valorization of lignin biomass, and utilizing this approach has the potential to significantly enhance the valorization of lignin biomass, leading to promising results for fermentation, biogas production, and methane production.
5 Conclusion
In the present study, seven Bacillus sp. strains with optimum ligninolytic were screened for RS hydrolysis. These strains consumed alkali lignin and synthetic dyes (azure B) as a carbon source. The strains were capable of lignin degradation and were producing lignin peroxidase (LiP) and laccase activity. Utilizing their lignin degradation was vital in pretreatment of LB biomass hydrolysis for bioenergy production. Synergistic enzyme reaction exhibits a significant delignification from rice straw waste residue in comparison to untreated rice straw reside. The enzyme mixture delignified rice straw displayed more biomethane yield as compared to untreated and individual enzyme lysate-treated rice straw. The study suggested that synergistic bioprocessing strategy could be an attractive way for pretreatment of waste biomass and fermentation yield.
Data Availability
All the data has been included in the manuscript.
References
Aimen S et al (2020) Fermentation of simple and complex substrates to biohydrogen using pure Bacillus cereus strains. Environ Technol Innov 18:100704
Shah TA, Raheem U, Asifa A, Tabassum R (2018) Effect of alkalis pretreatment on lignocellulosic waste biomass for biogas production. Int J Renew Energy Res 8:1318–1326
Shah TA, Asifa A, Shehbaz A, Rahim U, Romana T (2017) A review on biohydrogen as a prospective renewable energy. Int J Biosci 11:106–130
Shah TA, Tabassum R (2018) Enhancing biogas production from lime soaked corn cob residue. Int J Renew Energy Res 8:761–766
Shah TA et al (2022) Composition and role of lignin in biochemicals. In: Lignin-Chemistry, Structure, and Application. IntechOpen
Parawira W (2012) Enzyme research and applications in biotechnological intensification of biogas production. Crit Rev Biotechnol 32:172–186
Ali S et al (2018) Exploring lignocellulosic biomass for bio-methane potential by anaerobic digestion and its economic feasibility. Energy & Environment 29(5):742–751
Shah TA et al (2019) Biological pretreatment of rice straw by ligninolytic Bacillus sp. strains for enhancing biogas production. Environ Prog Sustain Energy 38:e13036
Shen Z et al (2017) Synergy of lignocelluloses pretreatment by sodium carbonate and bacterium to enhance enzymatic hydrolysis of rice straw. Bioresour Technol 249:154–160
Sluiter A et al (2013) Methods for biomass compositional analysis. National Renewable Energy Laboratory (NREL), Golden, CO
Shah TA et al (2018) Simultaneous pretreatment and biohydrogen production from wheat straw by newly isolated ligninolytic Bacillus Sp. strains with two-stage batch fermentation system. Bioenergy Res 11:835–849
Chang Y-C et al (2014) Isolation of Bacillus sp. strains capable of decomposing alkali lignin and their application in combination with lactic acid bacteria for enhancing cellulase performance. Bioresour Technol 152:429–436
Lee S et al (2019) Bacterial valorization of lignin: strains, enzymes, conversion pathways, biosensors, and perspectives. Front Bioeng Biotechnol 7:209
Ramarajan R, Manohar CS (2017) Biological pretreatment and bioconversion of agricultural wastes, using ligninolytic and cellulolytic fungal consortia. Biorem J 21(2):89–99
Qu P et al (2017) Physicochemical changes in rice straw after composting and its effect on rice-straw-based composites. J Appl Polym Sci 134
Afzal A et al (2019) Phylogenetic analysis of methanogenic archaea by mcrA gene in anaerobic digester. Int J Agric Biol 22:413–419
Afzal A et al Characterization of extracellular protease from Bacillus licheniformis. Int J Biosci 11(4):228–236
Shah TA, Ullah R, Rashida MM (2020) Reprocessing of NaOH black liquor for pre-treatment of agribiomass. Int Res J Agric Sci Vet Med 8:1–10
Xiong Y et al (2020) Characterization of ligninolytic bacteria and analysis of alkali-lignin biodegradation products. Pol J Microbiol 69:339–347
Zhu D et al (2017) Biodegradation of alkaline lignin by Bacillus ligniniphilus L1. Biotechnol Biofuels 10:44
Dawkar VV et al (2009) Peroxidase from Bacillus sp. VUS and its role in the decolorization of textile dyes. Biotechnol Bioprocess Eng 14:361
Santos A et al (2014) New dye-decolorizing peroxidases from Bacillus subtilis and Pseudomonas putida MET94: towards biotechnological applications. Appl Microbiol Biotechnol 98:2053–2065
Zhong W et al (2011) Effect of biological pretreatments in enhancing corn straw biogas production. Bioresour Technol 102:11177–11182
Li J et al (2009) Bacteria and lignin degradation. Front Biol China 4:29–38
Wang W et al (2011) Characterization of a microbial consortium capable of degrading lignocellulose. Bioresour Technol 102:9321–9324
Manisha, Yadav SK (2017) Technological advances and applications of hydrolytic enzymes for valorization of lignocellulosic biomass. Bioresour Technol 245:1727–1739
Matthews S, Kamal EA (2015) Identification of rice straw degrading microbial consortium. J Trop Agric Food Sci 43:119–127
Tian J-H et al (2017) Impact of wet aerobic pretreatments on cellulose accessibility and bacterial communities in rape straw. Bioresour Technol 237:31–38
Ghosh A, Bhattacharyya B (1999) Biomethanation of white rotted and brown rotted rice straw. Bioprocess Biosyst Eng 20:297–302
Acknowledgements
The authors greatly acknowledge and express their gratitude to the Researchers Supporting Project number RSP2023R335, King Saud University, Riyadh, Saudi Arabia.
Funding
The study was a part of HEC-SRGP Project grant number 2613, received by the corresponding author.
Author information
Authors and Affiliations
Contributions
Conceptualization, T.A.S. and T.M.; methodology, T.A.; software, M.A.; validation, A.F.A.; formal analysis, T.A.; investigation, T.A.S. and T.M.; resources, A.S.A. and M.A.; data curation, A.S.; writing—original draft preparation, T.A. and A.S.; writing—review and editing, T.A.S. and T.M.; visualization, T.A.S. and T.M.; supervision, T.A.; project administration, T.A.S. and T.M.; funding acquisition, M.A. and A.F.A. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. All the authors contributed equally.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, T.A., Majeed, T., Rahman, S.u. et al. Synergistic treatment of crude enzymes from Bacillus sp. strains to boost anaerobic fermentation of rice straw. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-05090-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05090-z