Abstract
The amylopullulanase of Geobacillus thermoleovorans NP33 (apu105) is Ca2+-independent with a molecular mass of 105 kDa and optimum activity at 80 °C and pH 7.0. The apu105 is extremely thermostable with T 1/2 of 7.8 h at 90 °C and hydrolyzes starch, pullulan, and malto-oligosaccharides, but not panose and cyclodextrins. The low K m values of apu105 (starch, pullulan, amylose, and amylopectin) indicates higher affinity of apu105 than others. The action of the enzyme on mixed substrates (starch and pullulan) confirmed the presence of only one active site for cleaving both α-1,4- and α-1,6- glycosidic linkages. The raw starches are efficiently hydrolyzed into glucose, maltose, and malto-oligosaccharides. Two-step starch saccharification involving pretreatment with apu105 followed by glucoamylase enhanced glucose yield. The supplementation of whole wheat dough with apu105 markedly enhanced the loaf volume, shelf-life, and the texture of bread. The enzyme is compatible with detergents and useful in desizing of cotton fabrics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amylopullulanase (E.C. 3.2.1.1/41) is a debranching and endo-acting enzyme that cleaves both α-1,4- and α-1,6-glycosidic linkages in starch, amylose, amylopectin, and glycogen, and α-1,6- linkages in pullulan. This belongs to the family of glycoside hydrolases (GHs) organized in the sequence-based classification of carbohydrate active enzymes. Amylopullulanases are divided into two subgroups based on the number of active sites for the hydrolysis of α-1,4- and α-1,6-glycosidic bonds [1]. Amylopullulanases from thermophilic anaerobes possess a single active site [2–4], while aerobic microbes produce amylopullulanases that contain either one [5, 6] or two active sites for cleaving α-1,4- and α-1,6-glycosidic linkages [7, 8]. Amylopullulanases possessing two active sites, each being specific for one bond type, are often referred to as α-amylase pullulanases [7].
The bifunctional amylopullulanases find application in starch saccharification for the production of maltose and maltotriose syrups [9]. Amylopullulanases can replace other amylolytic enzymes like α-amylases, β-amylases, and pullulanases that are presently employed in the starch conversion [10], and thus, can act alone in both liquefaction and saccharification of starch. The enzyme should, however, be thermostable and Ca2+-independent. The α-amylases that are currently used in the food industry require Ca2+ for their activity and/or stability, and thus, are Ca2+-dependent. The Ca2+ must be removed from the product streams because Ca2+ inhibits glucose isomerase used in the final step of isomerization of glucose to fructose and can also lead to the formation of calcium oxalate, which clogs heat exchangers and pipes over time [11, 12]. Furthermore, the removal of Ca2+ from the product streams by ion exchangers adds to the cost of the process.
The thermostable amylopullulanases have a potential application in the baking industry as an antistaling agent. Bread staling is a term used to refer to the undesirable changes that occur upon storage of bread, like the increased crumb firmness associated with the loss of crispness of the crust and decreased moisture content of the crumb as well as loss of bread flavor. Staling, which occurs because of the retrogradation of amylopectin, can be retarded by shortening the amylopectin chain length [13]. The amylopullulanases tend to decrease the stickiness of the bread associated with the production of branched maltodextrins by the α-amylases used in bread making [13]. With the growing demand for fiber-rich nutritional foods, the whole wheat bread is getting increasingly popular. Whole wheat bread is made from the bran, germ, and endosperm (starch) of the whole wheat. While white bread uses only the endosperm and is deficient in fiber, vitamins, minerals, and phytonutrients of the whole wheat.
Desizing refers to the process of pretreatment of cotton fabrics and cotton blends so as to prepare the fabric for dyeing and subsequent stages of processing. It is performed as a first step in the pretreatment of cotton fabrics or after singeing. The cotton fabrics contain different metal ions, usually Ca2+ and Mg2+ in substantial amounts. Most of the currently used α-amylases in desizing of cotton fabrics are calcium-dependent and, therefore, would reduce the desizing efficiency [14]. The calcium-independent enzymes can, therefore, act as efficient desizing agents. The enzymes can also be employed for enhancing the wash performance of the commercial detergents [1] by degrading the residues of starchy food.
This extremely thermophilic bacterium Geobacillus thermoleovorans NP33 has been reported earlier to produce an amylopullulanases of 45 kDa [15] and another with a molecular mass of 182 kDa [16], suggesting that the bacterium possesses multiple amylopullulanases. In this investigation, still another highly thermostable amylopullulanase with a molecular mass of 105 kDa (apu105) is being reported for the first time from this bacterium. The enzyme has been purified and characterized, and has been shown to be useful in the saccharification of soluble and raw starches, whole wheat bread making, desizing of cotton fabrics, and detergent formulation.
Materials and Methods
Bacterial Strain
The extremely thermophilic bacterium used in this investigation was isolated from a hot water spring sample collected from Waimangu volcanic valley, New Zealand, and was identified using polyphasic approach as G. thermoleovorans and designated as G. thermoleovorans NP33 [17]. The 16S rDNA sequence has been deposited at NCBI GenBank (accession no. JQ343209), and the culture has been deposited at Microbial Type Culture Collection Centre, Institute of Microbial Technology, Chandigarh, India (MTCC 4219). The bacterial strain has been routinely cultivated in a synthetic complex medium at 70 °C and 200 rev min−1 in shake flasks [15].
Enzyme Assays
The α-amylase and pullulanase activities were determined by quantitating reducing sugars liberated during enzyme-substrate reaction using dinitrosalicylic acid (DNSA) reagent [18]. The enzyme reaction was performed at 80 °C for 20 min using soluble starch/pullulan as substrate for determining α-amylase and pullulanase activities, respectively. One unit of the enzyme is defined as the amount of the enzyme that liberates 1 μmol of reducing sugars as maltose per minute under the assay conditions. As the digestion product of enzyme hydrolysis was mainly composed of maltose, a maltose standard curve was constructed with different concentrations of maltose. The zones of clearance on starch agar [0.5 % starch with 1.5 % agar dissolved in sodium phosphate buffer (100 mM, pH 7.0)] and pullulan-azure agar plates [0.5 % red pullulan (Megazyme) with 1.5 % agar dissolved in sodium phosphate buffer (100 mM, pH 7.0)] were used for the qualitative detection of α-amylase and pullulanase activities. The concentration of protein was determined according to Lowry et al. [19].
Purification of apu105
G. thermoleovorans NP33 was cultivated in a complex synthetic medium (g l−1: 30.6 starch, 4.2 yeast extract, 1.0 (NH4)2SO4, 0.3 MgSO4, 1.0 NaCl, 2.3 K2HPO4, and 1.0 maltose, pH 7.0) in 2 l flasks containing 400 ml liquid medium for 20 h at 70 °C and 200 rev min−1 in an incubator shaker [15]. The cells were harvested by centrifugation at 18,320×g for 30 min at 4 °C, and the culture supernatant was used for enzyme purification.
Proteins were precipitated by the addition of acetone (70 % saturation) to the culture filtrate with constant stirring. The precipitate was collected by centrifugation at 21,650×g for 30 min and made acetone free at 4 °C overnight. The precipitate was dissolved in 50 mM Tris–HCl (pH 8.5) and dialyzed overnight against this buffer at room temperature and filtered using a 0.2-μm membrane. The concentrated enzyme solution was applied onto ResourceTM Q column (Pharmacia Biotech) [1.6 × 5 cm] previously equilibrated with 50 mM Tris–HCl (pH 8.5) in a fast performance liquid chromatography (FPLC) system [AKTA primeTM PLUS, GE Healthcare, Bio-Sciences, Uppsala, Sweden]. The protein was eluted using a linear gradient of 0–1,000 mM NaCl at a flow rate of 0.5 ml min−1. The fractions were checked for both α-amylase and pullulanase activities, and loaded on SephacrylTM S-200 high resolution (Pharmacia) column (16/60) previously equilibrated with 50 mM Tris–HCl (pH 8.5) with a flow rate of 0.4 ml min−1 and analyzed on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (12 %) for the purity. The purified fraction with both α-amylase and pullulanase activities was pooled and dialyzed against 100 mM sodium phosphate buffer (pH 7.0) and concentrated using centricon tubes with 10 kDa cutoff membrane.
Molecular Mass Determination of the Purified Protein by Gel Filtration
The molecular mass of the purified enzyme was determined by using gel filtration chromatography column [SephacrylTM S-200 high resolution (Pharmacia) column, 16/60] previously equilibrated with the sodium phosphate buffer (100 mM, pH 7.0). The fractions (1 ml) were eluted at 18 ml/h. Cytochrome c (12.4 kDa), carbonic anhydrous (29 kDa), bovine serum albumin (66 kDa), yeast alcohol dehydrogenase (150 kDa), and sweet potato β-amylase (200 kDa) were used as molecular weight standards for determining the molecular mass of the enzyme on a semi-log graph by plotting V e/V o on x-axis and molecular weight on y-axis.
Identification of Amylolytic and Pullulanolytic Activities by Zymography
The α-amylase and pullulanase activities of amylopullulanase were identified through native polyacrylamide gel electrophoresis and SDS-PAGE by incorporating substrate [soluble starch (Sigma)/red pullulan (Megazyme)] in the gel at 5 mg ml−1. After electrophoresis, the gel was transferred to 100 mM sodium phosphate buffer (pH 7.0) and incubated at 80 °C for 4 h. The clear zone of starch hydrolysis was visualized by flooding the gel with Lugol’s iodine, while the pullulanase activity was detected by the yellowish white band against the red background on polyacrylamide gel.
Isoelectric Focusing and Peptide Finger Printing
Isoelectric focusing of the pure enzyme was carried out on a polyacrylamide gel (6 %) using 2 % (w/v) ampholyte (pH range, 3.0–10.0; Bio-Rad) under native conditions. Two microliters of the purified apu105 (6 μg) was loaded on the gel in a separate lane to that of the pI ladder. After electrophoresis, the gel was focused on a MiniIEF cell (Bio-Rad, USA) at 4 °C according to the manufacturer’s instructions. The gel was then stained with Coomassie Brilliant Blue to visualize the bands.
The peptide mass spectrometric analysis of the pure protein eluted from SDS-PAGE gel was performed by MALDI-ToF-MS/MS (Agilent 1100 series) at the Proteomics Facility, National Institute of Plant Genome Research (NIPGR), New Delhi. The peptide sequences generated were analyzed using the Mascot search algorithm data of NCBI and searched in the Swiss Prot databases.
Substrate Specificity and Kinetics of apu105
The substrate specificity of the enzyme was assessed by incorporating different soluble and raw substrates (starch, pullulan, amylose, amylopectin, glycogen and cyclodextrins, and raw wheat, rice, water chestnut and corn starches, 0.5 %) in the reaction mixtures. The enzyme reaction was performed at 80 °C for 20 min in 100 mM sodium phosphate buffer (pH 7.0) and the enzyme activities were determined.
The enzyme kinetics studies were performed by determining the velocities of the enzyme reactions at 15 different concentrations (0.25 to 7 mg ml−1) of soluble starch, pullulan, amylose, and amylopectin. The apparent Michaelis constant (K m) and the maximal velocity (V max) of the enzyme activities were calculated by fitting the initial velocities and substrate concentrations into the Michaelis-Menten equation using Lineweaver-Burk and Hanes-Woolf plots.
The enzyme reactions were also performed by mixing varied concentrations of pullulan (0.5 to 3.5 mg ml−1 with an increment of 0.5 mg ml−1) to a fixed concentration of starch (0.5 or 1.75 mg ml−1) in order to determine whether the enzyme has a single active site or dual active sites for cleaving α-1,4- and α-1,6-glycosidic linkages. The initial velocities were estimated for combinations of starch and pullulan, and were plotted against the total substrate concentration ([S]/V vs [S] plot or Hanes-Woolf plot).
Thermodynamics of Soluble Starch and Pullulan Hydrolysis
Thermodynamic parameters for soluble starch and pullulan hydrolysis were determined by rearranging the Eyring’s absolute rate equation from the transition state theory [20]:
where h is the Planck’s constant(6.626 × 10−34 J s), k b is the Boltzmann constant (1.381 × 10−23 J K−1), R is the gas constant (8.314 J K−1), T is temperature in Kelvin, ΔH is the change in enthalpy of activation, and ΔS is the entropy of enzyme activation.
The activation energy (E a) required for starch and pullulan hydrolysis was determined by Arrhenius plot [21]. The enthalpy change (ΔH), entropy change (ΔS), and free energy change (ΔG) values were calculated using the following equations:
Effect of pH and Temperature on Enzyme Activity and Stability, and Determination of Temperature Quotient (Q 10)
The substrates (starch and pullulan, 0.5 %) were separately dissolved in buffers of varying pH values [sodium acetate buffer (100 mM, pH 4.0–5.0), sodium phosphate buffer (100 mM, pH 6.0–8.0), and glycine-NaOH buffer (100 mM, pH 9.0)] and were used in reaction mixtures with the suitably diluted enzyme solutions prepared in the desired buffers to measure the optimum pH for α-amylase and pullulanase activities, respectively. The pH stability of the enzyme was determined by incubating the enzyme dissolved in buffers of varying pH values (3–9, 100 mM) for 2 h at 80 °C and assaying the residual enzyme activity. The optimum temperature for α-amylase and pullulanase activities of apu105 was assessed by assaying the enzymes at various temperatures (30–100 °C) in 100 mM sodium phosphate buffer, pH 7.0. The assay was performed using soluble starch and pullulan (0.5 %) as substrates for α-amylase and pullulanase activities, respectively.
The effect of temperature on the reaction rate was expressed in terms of temperature quotient (Q 10), the factor by which the rate of reaction increases by 10 °C rise in temperature. Q 10 was determined according to [22].
Thermal stability of apu105 was determined by incubating the enzyme (100 mM sodium phosphate buffer, pH 7.0) at different temperatures (70–100 °C) over a period of 24 h. The samples withdrawn periodically were used in α-amylase and pullulanase assays at 80 °C and the residual enzyme activities were determined.
The residual activity is directly related to the deactivation rate constant (K d), which follows first order kinetics.
where K d is the deactivation rate constant and t is the time. K d is determined from the plot of ln[E t/E o] vs t, where K d is the slope of the plot.
Half-life (T 1/2) of the enzyme is calculated from K d values using the equation,
Effect of Metal Ions, Inhibitors, and Surfactants on the Activity of apu105
Cations in the form of chloride/sulfate salts (CaCl2, CuSO4, CoCl2, HgCl2, BaCl2, MnCl2, PbCl2, FeSO4, ZnCl2) and different modulators like ethylenediamine tetraacetic acid (EDTA), ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), ethyl-3-(3-dimethyl aminopropyl)carbodiimide (EDAC), Woodward’s reagent K (WRK), dithiothreitol (DTT), phenyl methyl sulfonyl fluoride (PMSF), iodoacetate (IAA), N-bromosuccinimide (N-BS), N-ethylmaleimide (NEM), cyclodextrins (α, β, and γ) at 1 and 5 mM, as well as various ionic and non-ionic detergents, SDS (1 %), and Triton X-100 (0.1–0.2 %) were incorporated into the reaction mixtures and incubated for 30 min in 100 mM sodium phosphate buffer at 30 °C. The residual α-amylase and pullulanase activities of apu105 were determined under optimal conditions.
End Product Analysis
The hydrolysis products of the enzyme-substrate reaction (0.25 mg enzyme and 0.5 % substrate) were assessed on thin layer chromatography (TLC). The reaction was carried out by incubating the reaction mixture at 80 °C for 1 h. The substrates used were starch (5 mg ml−1), pullulan (5 mg ml−1), panose (10 mg ml−1), maltose (10 mg ml−1), maltotriose (10 mg ml−1), maltotetraose (10 mg ml−1), maltopentaose (10 mg ml−1), amylopectin (5 mg ml−1), amylose (5 mg ml−1), and glycogen (10 mg ml−1) dissolved in sodium phosphate buffer (100 mM, pH 7.0). The reaction was stopped by incubating at −20 °C. Glucose, maltose, maltotriose, maltotetraose, maltopentaose, and panose (10 μg each) were used as standards. The TLC plates [silica gel 60 plates (Merck, Darmstadt, Germany)] were developed in butanol/ethanol/water (5:3:2) solvent system and air-dried at room temperature for overnight. Thereafter, the plates were sprayed with aniline diphenylamine reagent and incubated in an oven at 100 °C for an hour. The individual sugars were identified as blue spots against white background.
Applicability of Enzyme in Starch Saccharification
The applicability of apu105 in starch saccharification was analyzed by subjecting different starches (raw and soluble starches, 20 % w/v) prepared in sodium phosphate buffer (100 mM, pH 7.0) for gelatinization at 105 °C for 10 min. Thereafter, the starch slurry was cooled to 80 °C and incubated with apu105 (20 U g−1 of starch) at 80 °C for 2 h. Aliquots were drawn at intervals of an hour and the reducing sugars liberated were determined. The percent saccharification of starch was obtained using the formula:
where the factor 0.95 normalizes the conversion for the weight gain caused by the addition of water molecule [23].
The percent starch saccharification for raw starches was also determined by using glucoamylase alone (10 U g−1 of starch) and in combination with α-amylase (20 U g−1 of starch) and apu105 (20 U g−1 of starch) after starch gelatinization at 105 °C for 10 min. Glucoamylase was produced by cultivating Thermomucor indicae-seudaticae in sucrose/yeast extract broth at 40 °C and 250 rpm for 40 h [24] and α-amylase was obtained from the extracellular broth of G. thermoleovorans NP54 culture grown in starch/yeast extract/tryptone medium for 14 h at 70 °C and 200 rpm [25]. The pretreatment of starch with α-amylase and apu105 was performed for 3 h at their respective temperature optima (100 and 80 °C) prior to starch saccharification by glucoamylase at 60 °C. The aliquots were drawn at desired intervals and the percent starch saccharification was calculated.
Applicability in Whole Wheat Bread Making
The dough was prepared by mechanically mixing wheat flour (300 g), dry yeast (10 g), NaCl (4.0 g, w/v), 5.0 U g−1 of amylopullulanase, and 60 % (w/v) water for 30 min. The dough was kept undisturbed for 1 h (proofing), followed by fermentation for 1 h, and baked at 240 °C for 20 min before cutting into shapes. The process was performed in the presence of apu105 of G. thermoleovorans NP33 (test) and with the commercial α-amylase (control). The breads made were then checked for reducing sugars, protein and moisture contents, softness, and shelf-life.
The reducing sugars liberated by the action of apu105 on wheat flour in the bread were detected on TLC. The control and test breads were suspended in deionized water (1 mg ml−1) for 20 min, and the supernatants after centrifugation were analyzed for reducing sugars on silica gel 60 plates (Merck, Darmstadt, Germany) and quantitatively assayed using DNSA reagent.
Applicability in Desizing of Cotton Fabrics
The enzyme dosage, incubation time, temperature, and pH on desizing of the fabrics were determined by measuring the weight loss of the fabric and desizing efficiency in terms of the starch content before and after the enzyme treatment.
The loss in weight of the cotton fabric (5 × 5 cm) after the enzyme treatment was determined according to the Eq. (8) [1]:
where W 1 and W 2 are the weights of the cotton fabric before and after the enzyme treatment/desizing, respectively. Prior to the measurement of dry weight of the treated fabric, these are thoroughly washed, dried at 105 °C to constant weight, and cooled.
The starch content of the cotton fabric before and after the enzyme treatment was determined according to [26]. The desizing efficiency was determined using Eq. (9):
where W 0 and W 1 are the amount of starch for control and desized fabric, respectively.
Applicability as a Detergent Additive
The stability of the enzyme with commercially available detergents was assessed by incubating the enzyme (0.5 mg) with detergents (1 %) [Ariel and Tide (Procter and Gamble, India), Ghari (Rohit Surfactants Private Ltd., India), and Surf Excel, Rin, and Wheel (Hindustan Unilever Ltd., India)] for different time intervals (0.5 to 3 h) at 80 °C and determining the residual enzyme activity. Prior to use, the detergents were boiled for 30 min to inactivate the enzymes present in them.
Wash performance analysis of the apu105 purified enzyme was assessed on white cotton cloth (8 × 8 cm) which was stained with starchy food overnight. Separate sets of experiments were conducted to evaluate its applicability as a detergent additive: (a) flask containing distilled water (100 ml) and stained cloth; (b) flask containing distilled water (100 ml), stained cloth, and Rin detergent (1 % w/v); (c) flask containing distilled water (9.9 ml), stained cloth, and 0.1 ml apu105 (100 units ml−1); and (d) flask containing distilled water (9.9 ml), stained cloth, Rin detergent (1 % w/v), and 0.1 ml apu105. The experiments were carried out at 45 °C for 30 min in an incubation shaker (100 rev min−1), and thereafter, the cloth pieces were rinsed with cold water and air-dried. The wash performance was determined by measuring relative reflectance of dried cloth pieces using Reflectance meter (Model No. UEC 1018, Universal Cooperation, India) against the untreated control.
All experiments were carried out in triplicate and the average values with standard deviation are presented.
Results and Discussion
Purification of Amylopullulanase, Isoelectric Point, and MALDI-ToF Analysis
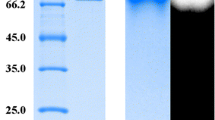
The α-amylase and pullulanase activities of the apu105 were highest in the supernatants obtained after cultivating the bacterium for 20 h at 70 °C (pH 7.0). The crude enzyme was purified by acetone precipitation followed by anion exchange and gel filtration chromatography. The fraction containing apu105 was eluted as a single peak by anion exchange chromatography and was devoid of almost all protein contaminants (Fig. 1). A 26.8- and 35.7-fold purification with 23.88 and 19.07 % yield was attained after anion exchange and gel filtration chromatography, respectively (Table 1a, b). The enzyme fraction displayed homogeneity on SDS-PAGE with a molecular mass of around 105 kDa (Fig. 2a, b). Zymogram analysis helped in identifying α-amylase and pullulanase activities of apu105 (Fig. 2b, c). The molecular mass of the enzyme determined by Sephacryl S-200HR (16/60) gel filtration is also the same, and thus the enzyme is a monomer (Fig. S1). The molecular mass of apu105 is close to that reported from Geobacillus stearothermophilus strain L14 [27] and Lactobacillus amylophilus GV6 [28]. Large variations in the molecular masses of amylopullulanases from different microbial sources have been reported: Anoxybacillus sp. SK3-4 (225 kDa) [29], Thermococcus hydrothermalis (128 kDa) [30], Staphylothermus marinus (75.3 kDa) [31], and Streptomyces erumpens (45 kDa) [32]. The bacterium G. thermoleovorans NP33 has been shown to possess an amylopullulanase encoding gene (gt-apu) that codes for a 182-kDa protein (gt-apu), which was cloned and expressed in Escherichia coli [16]. The strain has also been reported to produce a hyperthermostable amylopullulanase of 45 kDa [15].
Anion exchange chromatography on a ResourceTM Q column (Pharmacia Biotech) [1.6 × 5 cm] previously equilibrated with 50 mM Tris–HCl (pH 8.5) in a fast performance liquid chromatography (FPLC) system [AKTA primeTM PLUS, GE Healthcare, Bio-Sciences, Uppsala, Sweden]. The enzyme was eluted with NaCl gradient at a flow rate of 0.5 ml/min. Both the α-amylase and pullulanase activity was detected in the eluted fractions (only the α-amylase activity is shown in the figure)
a Analysis of the purification of apu105 on SDS-PAGE (12 %). Lanes: 1, standard molecular weight markers; 2, crude extract; 3, acetone precipitate (70 %); 4, purified protein. b Zymogram of the α-amylase activity of apu105 using soluble starch as substrate in the gel. c Zymogram of the pullulanase activity of apu105 using red pullulan (Megazyme) as substrate in the gel
The isoelectric point (pI) of apu105 is 4.8, which is similar to the amylopullulanase of Bacillus sp. KSM-1378 [1] and more than that of Bacillus DSM 405 (pI, 4.3) [6]. The sequence peptides generated by MALDI-ToF-MS/MS analysis did not show identity with any of the known amylopullulanase/α-amylase pullulanase/α-amylase, but with the hypothetical protein of Geobacillus sp. C56-T3 (sequence coverage 35 % and an ion score of 50 in MASCOT search) [Table S1, Fig. S2]. This protein has a pI of 5.3 and molecular mass of 112.457 kDa, which are higher than those of apu105.
Effect of pH and Temperature on the Activity and Stability of apu105
The α-amylase and pullulanase activities of apu105 are stable in the pH range between 6.0 and 9.0 with the optimum at pH 7.0 (Fig. 3a–c) and activities declined below and above the pH optimum. The α-amylase and pullulanase activities were reduced by 70 % upon pre-incubation of the enzyme at pH 5.0 for 2 h, while 25 % loss at pH 9.0. The enzyme is optimally active at 80 °C (Fig. 3d, e). In contrast, the high molecular mass gt-apu is optimally active at 60 °C and pH 7.0 [16]. The enzyme exhibits high thermostability with 80 % residual activity after 4 h exposure to 90 °C, while 50 % loss in both α-amylase and pullulanase activities after 27 min of exposure to 100 °C (Table S2). Owing to its stability over acidic to alkaline pH and high temperatures, the enzyme finds application in starch saccharification, baking, and detergent industries [1, 10, 33]. Pseudo-first order plots were used to study the extent of thermal inactivation (Fig. 4a, b). The deactivation rate constant increased with the rise in temperature, suggesting deactivation of the enzyme, and thus, depicting the decrease in half-life of the enzyme (Table S2). At 70 °C, the enzyme has T 1/2 of 21.7 h, while the enzyme exhibits T 1/2 of 19.8 and 2.8 h at 80 and 90 °C, respectively. T 1/2 declined sharply at 100 °C (0.4 h). The half-life values are higher than those reported for other thermostable amylopullulanases of bacterial origin [27, 34].
a Effect of pH on the α-amylase activity of apu105. b Effect of pH on the pullulanase activity of apu105. c Effect of pH on the stability of the α-amylase/pullulanase of apu105. d Effect of temperature on the α-amylase activity of apu105. e Effect of temperature on the pullulanase activity of apu105. Similar relative activity for the effect of pH and temperature on the activity was observed for both the α-amylase and pullulanase of apu105
a Arrhenius plot for the determination of activation energy (E a) for the hydrolysis of soluble starch by apu105. b Arrhenius plot for the determination of activation energy (E a) for the hydrolysis of pullulan E a = − slope × R, where R (gas constant) = 8.314 J K−1 mol−1. Data presented are average values of triplicates
Substrate Specificity, Enzyme Kinetics, and Thermodynamics of Starch and Pullulan Hydrolysis
The enzyme exhibits significant activity on soluble starch, pullulan, and amylose, while amylopectin was hydrolyzed at a moderate rate and glycogen the least (Table 2). The enzyme did not hydrolyze cyclodextrins (α, β, γ), as reported for other amylopullulanases [27, 35]. Li et al. [31] have recently reported that an amylopullulanase from S. marinus displayed cyclodextrin hydrolyzing activity. The enzyme exhibits significant activity on raw starches (Table 2) as reported for the amylopullulanase from L. amylophilus GV6 [28].
The apu105 followed Michaelis-Menten kinetics with starch as well as pullulan. The lower K m value of apu105 for starch suggested a higher affinity of apu105 towards soluble starch than that for pullulan (K m for soluble starch is 0.142 mg ml−1, while that for pullulan is 0.416 mg ml−1). The V max and K m values for amylose and amylopectin are 19.9 and 14.3 μmol mg−1 min−1 and 0.11 and 0.5 mg ml−1, respectively. The K m values were lower than those reported for amylopullulanases from Bacillus sp. KSM-1378 [1] and Bacillus sp. DSM 405 [6] suggesting high substrate affinity of the enzyme. The catalytic efficiency (K cat/K m) values of the apu105 for soluble starch and pullulan are 260.8 and 87.3, respectively. Kinetic analysis of the enzyme was also carried out in a system with mixed substrates (starch and pullulan). A linear plot was obtained when the initial velocities of apu105 were plotted on [S]/V vs [S] (Fig. S3). The recorded values for the initial velocities fitted into the one-site model of [36]. The apu105 has a single active site for cleaving both α-1,4- and α-1,6-glycosidic linkages in polysaccharides, since V max (starch + pullulan, 27.39 μmol mg−1 min−1) is less than V max (starch) [22.22 μmol mg−1 min−1] + V max (pullulan) [21.78 μmol mg−1 min−1] according to [6]. Amylopullulanases with single active site for both α-amylase and pullulanase have been reported for Bacillus sp. XAL 601 [5], Thermoanaerobacter ethanolicus 39E [2], gt-apu [16], and Pyrococcus furiosus [37].
The Arrhenius plot for the hydrolysis of soluble starch and pullulan reveals the inhibition of enzyme activity at higher temperatures beyond transition point (80 °C) [Fig. 5a, b]. The activation energy (E a) of the enzyme for starch and pullulan hydrolysis at 80 °C are 73.91 and 83.14 kJ mol−1, respectively. The temperature quotient (Q 10) for α-amylase and pullulanase of apu105 is 1.1. The thermodynamic parameters for starch hydrolysis at 80 °C [enthalpy change (ΔH), change in Gibbs free energy (ΔG), and entropy change (ΔS)] for activation of apu105 α-amylase are 70.97 kJ mol−1, 69.62 kJ mol−1, and 0.0038 kJ mol−1 K−1, respectively, while the values for pullulan are 80.20 kJ mol−1, 69.67 kJ mol−1, and 0.029 kJ mol−1 K−1.
a Plot of ln[E t/E 0] vs time (min) for the calculation of deactivation constant (K d) for apu105 α-amylase/pullulanase at 70, 80, and 90 °C. b Plot of ln[E t/E 0] vs time (min) for the calculation of deactivation constant (K d) for apu105 α-amylase/pullulanase at 100 °C. c Arrhenius plot of apu105 α-amylase/pullulanase deactivation
Effect of Metal Ions and Other Modulators
The enzyme activity and stability are not affected by Ca2+, even when used at 5 mM concentration (Table 3). The Ca2+-independent (and acid-stable) enzymes are preferred in the starch saccharification process [11] because the removal of calcium from the product streams by ion exchangers adds to the cost of the process. The Ca2+-independency has also been reported for glucoamylopullulanase from Bacillus subtilis DR8806 [38], Clostridium thermosulfurogenes SVM17 amylopullulanase [39], and gt-apu [16]. Ca2+-dependent amylopullulanases/α-amylase pullulanases have, however, been reported in T. ethanolicus 39E, Pyrococcus furiosus, and Pyrococcus woesei [34]. The enzyme was found moderately stimulated by Zn2+ and Mn2+, while Cu2+ ions strongly inhibited both α-amylase and pullulanase activities, as reported earlier [28, 35]. Among various chemical reagents tested, the α-amylase and pullulanase activities of apu105 were not affected by chelating agents (EDTA and EGTA), suggesting that the enzyme does not require Ca2+ and any other cation for its activity. The effect of N-BS, which is indicative of the role of tryptophan residue(s), is also insignificant on apu105 activity. The lack of enzyme inhibition by sulfhydryl inhibitors like DDT, β-mercaptoethanol, N-ethylmaleimide, and iodoacetate suggests that thiol group is not involved in the enzyme activity. EDAC and Woodward’s reagent K inhibited enzyme activities in a concentration-dependent manner, suggesting the role of carboxyl group(s) in the enzyme hydrolysis (Table 3). The enzyme is highly stable in the presence of the non-ionic (Triton X-100 and Tween 80, 0.1 and 0.2 %) as well as strong anionic (SDS, 1.0 %) surfactants. The stability of apu105 at alkaline pH and in the presence of detergents suggests its applicability as a detergent additive.
Analysis of Hydrolysis Products
The apu105 attacked both α-1,4- and α-1,6-glycosidic linkages of soluble and raw starches, amylose, amylopectin, and glycogen producing glucose, maltose, and maltotriose (not panose) as major products [Fig. 6a, b]. In contrast, the action of amylopullulanases from Bacillus sp. XAL601 [5], T. ethanolicus 39E [34], and G. thermoleovorans NP33 [16] on starch, amylose, amylopectin, and glycogen liberated maltose, maltotriose, and maltotetraose as the end products. Pullulan has also been efficiently hydrolyzed by apu105 forming glucose, maltose, and maltotriose (Fig. 6a). Similar observations have also been recorded for the amylopullulanases from G. stearothermophilus L14 [27] and Anoxybacillus sp. SK3-4 [29]. While the hydrolysis of pullulan by most of the amylopullulanases formed maltotriose as the only product [30, 27, 35, 16]. The enzyme also hydrolyzes maltotriose, maltotetraose, maltopentaose, maltohexaose, and maltoheptaose. Panose and cyclodextrins are not, however, hydrolyzed. The enzyme is, therefore, not an isopullulanase (pullulan hydrolase type II), which is known to hydrolyze panose to form glucose and isomaltose and is also active on cyclodextrins [40]. The apu105 is also not a neopullulanase (pullulan hydrolase type I), which hydrolyzes pullulan to form panose [41].
TLC of hydrolysis products from different saccharides by apu105. a Hydrolysis products of starch and pullulan. Lanes: 1, G1–G5 malto-oligosaccharide markers; 2, panose; 3, starch hydrolysates after 2 h of incubation with apu105; 4, pullulan hydrolysates after 2 h of incubation with apu105; 5, maltotriose hydrolysate; 6, panose hydrolysate; 7, sugars released from the test bread. b Hydrolysis products from different saccharides. Lanes: 1, G1–G5 malto-oligosaccharide markers; 2, panose; 3, amylose/apu105; 4, amylopectin/apu105; 5, glycogen/apu105; 6, raw rice starch/apu105; 7, corn starch/apu105; 8, raw wheat starch/apu105; 9, water chestnut/apu105; 10, tapioca starch/apu105
Application of apu105 in Starch Saccharification and in Making of Whole Wheat Bread
Both soluble and raw starches are efficiently hydrolyzed by apu105 (Table S3). Among raw starches, a high rate of hydrolysis has been attained with corn starch (43 %) as compared to water chestnut (37.1 %), wheat (33.25 %), and rice (31.8 %) starches after 2 h of enzyme-substrate reaction. Furthermore, the raw starches pretreated with apu105 have been saccharified to a greater extent by glucoamylase as compared to the starches saccharified by glucoamylase alone and the α-amylase-pretreated starches (Table 4). This is consistent with the observations reported earlier [15]. Raw wheat and corn starches are saccharified to 84.4 and 92.5 %, respectively, when apu105/α-amylase-pretreated starch was saccharified using the glucoamylase of T. indicae-seudaticae (Table 4). The apu105 finds application in both starch liquefaction and saccharification because of its activity and stability at acidic pH and high temperatures, cleavage of α-1,4- and α-1,6-glycosidic linkages, Ca2+-independence, and raw starch degrading ability.
The bread made by supplementing the dough with apu105 of G. thermoleovorans NP33 (Test) was soft with higher moisture content, reducing sugars, and soluble protein than that made using the commercial fungal α-amylase (control) (Table 5). The analysis of apu105-treated bread revealed that the malto-oligosaccharides are formed as the hydrolysis products (Fig. 6a). Furthermore, the addition of enzyme resulted in a 5-cm increase in the dough rise as compared to that of control as well as improved loaf volume and crumb structure (Fig. 7a, b). The loaf volume is an important parameter for checking bread quality. Whole wheat bread has much smaller loaf volume in comparison with white bread, and therefore, in order to increase loaf volume, additional flour has to be added, which makes it expensive [42]. The shelf-life of bread increased to 5 days as compared to 4 days of the control bread at room temperature due to antistaling effect of the enzyme. Because of the debranching action of the amylopullulanases, the maltodextrins are produced, and therefore, eliminates the increased gumminess of α-amylase-treated bread [13]. The supplementation of wheat flour with apu105 has also ameliorated the texture, softness, and sweetness of the bread. Furthermore, the presence of malto-oligosaccharides in bread would help in improving human health [43].
Test and control bread made with and without the apu105 of G. thermoleovorans NP33. a Rise in crumb size. b Improved texture of bread. The test bread was made using dough prepared by mechanically mixing wheat flour (300 g), dry yeast (10 g), NaCl (4.0 g, w/v), 5.0 U g−1 of amylopullulanase and 60 % (w/v) water for 30 min and the control bread made by supplementing 5.0 U g−1 commercial enzyme (Bacillus licheniformis α-amylase) instead of amylopullulanase. The test bread had increased loaf volume and improved texture compared to the control bread
Applicability of the Enzyme in Desizing of Cotton Fabrics
The effective desizing of cotton fabric was achieved with 9 to 10 U of apu105 in sodium phosphate buffer (pH 7.0) after 40 min of treatment at 80 °C (Fig. 8). The desizing efficiency of the enzyme and the weight loss of the fabric did not show any significant changes with the increase in the amount of the enzyme from 9.0 to 10 IU and incubation time from 40 to 60 min, while the efficiency was lost below and above 80 °C and pH 7.0. The α-amylases which are currently employed for desizing (Rapidase L40, Rucolase HCH and others) have an optimum temperature between 60 to 100 °C and the pH range of 6.5 to 7.5 [44]. Moreover, the desizing efficiency of apu105 is higher at acidic pH than at alkaline pH, thus favoring the simultaneous acidic desizing and acid-demineralization in a single step, which would eliminate the water-soluble salts formed from the metals as a result of acid steeping [14].
Effect of enzyme dosage (a), incubation time (b), temperature (c), and pH (d) on weight loss (open diamond) and desizing efficiency (closed diamond). The loss in weight of the cotton fabric (5 × 5 cm) after the enzyme treatment was determined according to the Eq., Wt % = (W 1 − W 2)/W 1 × 100, where W 1 and W 2 are the weights of the cotton fabric before and after the enzyme treatment/desizing, respectively. The desizing efficiency was determined using the formula, desizing (%) = (W 0 − W 1)/W 0, where W 0 and W 1 are the amount of starch for control and desized fabric, respectively
Application of apu105 as a Detergent Additive
The apu105 exhibits significant stability over a wide range of commercially available detergents (1 %) (Table 6), as the enzyme retained higher than 45 % activity even after 3 h in the presence of detergents. The amylopullulanases have not been tested for their applicability as detergent additive. The wash performance analysis of starchy food stain on cotton fabric showed an increase in reflectance from 66 to 90 % when washed with the combination of enzyme and the detergent as compared to the detergent alone (Fig. 9). Thus, the enzyme is efficient in the removal of starch stain when used as a detergent additive like α-amylase of Bacillus sp. [45].
Removal of starchy food stain at 37 °C by apu105 (analysis of reflectance). (W = water, D = detergent, E = enzyme). Control is the untreated stained cloth. The white cloth without stain is taken as 100 %. Separate sets of experiments were conducted to evaluate the enzymes as a detergent additive: (a) flask with distilled water (100 ml) and stained cloth; (b) flask containing distilled water (100 ml), stained cloth, and Rin detergent (1 % w/v); (c) flask containing distilled water (9.9 ml), stained cloth, and 0.1 ml apu105 (100 units ml−1); and (d) flask containing distilled water (9.9 ml), stained cloth, Rin detergent (1 % w/v), and 0.1 ml apu105. The wash performance was determined by measuring the relative reflectance of dried cloth pieces using Reflectance meter (Model No. UEC 1018, Universal Cooperation, India) against the untreated control
Conclusions
The amylopullulanase (apu105) of G. thermoleovorans NP33 is highly thermostable and Ca2+-independent with a broad range of temperature and pH for activity and optima at 80 °C and pH 7.0. The enzyme cleaves α-1,4- and α-1-6-glycosidic linkages in soluble and raw starches and pullulan into glucose, maltose, and malto-oligosaccharides. The supplementation of whole wheat dough with the enzyme improves the quality of the bread. The malto-oligosaccharides in the bread will act as prebiotics in ameliorating human health. The enzyme finds application in textile desizing and as a detergent additive. In view of multiple applications of apu105, our efforts are underway for cloning and over expressing it heterologously in order to bring down the cost of enzyme production.
References
Ara, K., Igarashi, K., Saeki, K., & Ito, S. (1995). An alkaline amylopullulanase from alkaliphilic Bacillus sp. KSM-1378; kinetic evidence for two independent active sites for the α-1,4 and α-1,6 hydrolytic reactions. Bioscience Biotechnology & Biochemistry, 59, 662–665.
Mathupala, S. P., Lowe, S. E., Podkovyrov, S. M., & Zeikus, J. G. (1993). Sequencing of the amylopullulanase (apu) gene of Thermoanaerobacter ethanolicus 39E and identification of the active site by site-directed mutagenesis. Journal of Biological Chemistry, 268, 16332–16344.
Coleman, R. D., Yang, S. S., & McAlister, M. P. (1987). Cloning of the debranching-enzyme gene from Thermoanaerobium brockii into Escherichia coli and Bacillus subtilis. Journal of Bacteriology, 169, 4302–4307.
Ramesh, M. V., Podkovyrov, S. M., Lowe, S. E., & Zeikus, J. G. (1994). Cloning and sequencing of the Thermoanaerobacterium saccharolyticum B6A-RI apu gene and purification and characterization of the amylopullulanase from Escherichia coli. Applied Environmental Microbiology, 60, 94–101.
Lee, S.-P., Morikawa, M., Takagi, M., & Imanaka, T. (1994). Gene cloning and characterization of α-amylase-pullulanase (AapT) from thermophilic and alkaliphilic Bacillus sp. XAL601. Applied Environmental Microbiology, 160, 3764–3773.
Brunswick, J. M., Kelly, C. T., & Fogarty, W. M. (1999). The amylopullulanase of Bacillus sp. DSM 405. Applied Microbiology and Biotechnology, 51, 170–175.
Kim, C. H., & Kim, Y. S. (1995). Substrate specificity and detailed characterization of a bifunctional amylase-pullulanase enzyme from Bacillus circulans F-2 having two different active sites on one polypeptide. European Journal of Biochemistry, 227, 687–693. PMID: 7532585.
Hatada, Y., Igarashi, K., Ozaki, K., Ara, K., Hitomi, J., & Kobayashi, T. (1996). Amino acid sequence and molecular structure of an alkaline amylopullulanase from Bacillus that hydrolyzes α-1,4 and α-1,6 linkages in polysaccharides at different active sites. Journal of Biological Chemistry, 271, 24075–24083.
Nisha, M., & Satyanarayana, T. (2013). Recombinant bacterial amylopullulanases: developments and perspectives. Bioengineered, 4, 1–13.
Jiao, Y., Wang, S., & Lv, M. (2011). Structural and functional analysis of GH57 family thermostable amylopullulanase—a review. Wei Sheng Wu Xue Bao, 51, 21–28.
Kikani, B. A., & Singh, S. P. (2012). The stability and thermodynamic parameters of a very thermostable and calcium-independent α-amylase from a newly isolated bacterium, Anoxybacillus beppuensis TSSC-1. Process Biochemistry, 47, 1791–1798.
Antranikian, G. (1992). In G. Winkelmann (Ed.), Microbial degradation of natural products (pp. 27–56). Germany: Weinheim: VCH
Boyle, P. J., & Hebeda, R. E. (1990). Antistaling enzyme for baked goods. Food Technology, 44, 129.
Dehabadi, V. A., Opwis, K., & Gutmann, J. (2011). Combination of acid-demineralization and enzymatic desizing of cotton fabrics by using industrial acid stable glucoamylases and α-amylases. Starch-Starke, 63, 760–764.
Satyanarayana, T., Noorwez, S. M., Kumar, S., Rao, J. L., Ezhilvannan, M., & Kaur, P. (2004). Development of an ideal starch saccharification process using amylolytic enzymes from thermophiles. Biochemical Society Transactions, 32, 276–278.
Nisha, M., & Satyanarayana, T. (2013). Characterization of recombinant amylopullulanase (gt-apu) and truncated amylopullulanase (gt-apuT) of the extreme thermophile Geobacillus thermoleovorans NP33 and their action in starch saccharification. Applied Microbiology and Biotechnology, 97, 6279–6292.
Noorwez, S. M., Ezhilvannan, M., & Satyanarayana, T. (2006). Production of a high maltose forming, hyperthermostable and Ca2+ independent amylopullulanase by an extreme thermophile Geobacillus thermoleovorans in submerged fermentation. Indian Journal of Biotechology, 5, 337–345.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Eyring, H., & Stearn, A. E. (1939). The application of the theory of absolute reaction rates to proteins. Chemical Reviews, 24, 253–270.
Siddiqui, K. S., Azhar, M. J., Rashid, M. H., & Rajoka, M. I. (1996). Activity and thermostability of carboxymethylcellulase from Aspergillus niger is strongly influenced by non-covalently attached polysaccharides. World Journal of Microbiology and Biotechnology, 12, 213–216.
Dixon, M., & Webb, E. C. (1979). Enzyme kinetics. In M. Dixon & E. C. Webb (Eds.), Enzymes (Vol. 3, pp. 47–206). New York: Academic.
Mishra, R., & Maheshwari, R. (1996). Amylases of the thermophilic fungus Thermomyces lanuginosus; their purification, properties, action on starch and response to heat. Journal of Biosciences, 21, 653–672.
Kumar, S., Kumar, P., & Satyanarayana, T. (2007). Production of raw starch-saccharifying thermostable and neutral glucoamylase by the thermophilic mold Thermomucor indicae-seudaticae in submerged fermentation. Applied Biochemistry Biotechnology, 142, 221–230.
Rose, R., Rose, C. L., Omi, S. K., Forry, K. R., Durall, D. M., & Bigg, W. L. (1991). Starch determination by perchloric acid vs enzymes: evaluating the accuracy and precision of six colorimetric methods. Journal of Agricultural Food Chemistry, 39, 2–11.
Rao, J. L. U. M., & Satyanarayana, T. (2003). Statistical optimization of a high maltose-forming, hyperthermostable and Ca2+-independent alpha-amylase production by an extreme thermophile Geobacillus thermoleovorans using response surface methodology. Journal of Applied Microbiology, 95, 712–718.
Zareian, S., Khajeh, K., Ranjbar, B., Dabirmanesh, B., Ghollasi, M., & Mollania, N. (2010). Purification and characterization of a novel amylopullulanase that converts pullulan to glucose, maltose, and maltotriose and starch to glucose and maltose. Enzymes Microbiology Technology, 46, 57–63.
Vishnu, C., Naveena, B., Md, J., Altaf Venkateshwar, M., & Reddy, G. (2006). Amylopullulanase—a novel enzyme of L. amylophilus GV6 in direct fermentation of starch to L(+) lactic acid. Enzymes Microbiology Technology, 38, 545–550.
Kahar, U. M., Chan, K. G., Md Salleh, M., Hii, S. M., & Goh, K. M. (2013). A high molecular-mass Anoxybacillus sp. SK3-4 amylopullulanase: characterization and its relationship in carbohydrate utilization. International Journal of Molecular Sciences, 14, 11302–11318.
Erra-Pujada, M., Debeire, P., Duchiron, F., & O’Donohue, M. J. (1999). The type II pullulanase of Thermococcus hydrothermalis: molecular characterization of the gene and expression of the catalytic domain. Journal of Bacteriology, 181, 3284–3287.
Li, X., Li, D., & Park, K.-H. (2013). An extremely thermostable amylopullulanase from Staphylothermus marinus displays both pullulan- and cyclodextrin-degrading activities. Applied Micobiology Biotechnology, 97, 5359–5369.
Kar, S., Roy, R. C., & Mohapatra, U. B. (2012). Purification, characterization and application of thermostable amylopullulanase from Streptomyces erumpens MTCC 7317 under submerged fermentation. Annals of Microbiology, 62, 931–937.
Roy, I., & Gupta, M. N. (2004). Hydrolysis of starch by a mixture of glucoamylase and pullulanase entrapped individually in calcium alginate beads. Enzymes Microbiology Technology, 34, 26–32.
Lin, F. P., & Leu, K. L. (2002). Cloning, expression, and characterization of thermostable region of amylopullulanase gene from Thermoanaerobacter ethanolicus 39E. Applied Biochemistry and Biotechnology, 97, 33–44.
Kim, J. H., Sunako, M., Ono, H., Murooka, Y., Fukusaki, E., & Yamashita, M. (2008). Characterization of gene encoding amylopullulanase from plant-originated lactic acid bacterium, Lactobacillus plantarum L137. Journal of Bioscience and Bioengineering, 106, 449–459.
Hiromi, K., Hamauzu, Z.-I., Takahashi, K., & Ono, S. (1966). Kinetic studies on glucoamylase II. competition between two types of substrate having α-1,4 and α-1,6 glucosidic linkage. Journal of Biochemistry, 59, 411–418.
Brown, S. H., & Kelly, R. M. (1993). Characterization of amylolytic enzymes, having both α-1,4 and α-1,6-hydrolytic activity, from the thermophilic archaea Pyrococcus furiosus and Thermococcus litoralis. Applied Environmental Microbiology, 59, 2614–2621.
Asoodeh, A., & Lagzian, M. (2012). Purification and characterization of a new glucoamylopullulanase from thermotolerant alkaliphilic Bacillus subtilis DR8806 of a hot mineral spring. Process Biochemistry, 47, 806–815.
Mrudula, S., Reddy, G., & Seenayya, G. (2011). Purification and characterization of highly thermostable amylopullulanase from a thermophilic, anaerobic bacterium Clostridium thermosulfurogenes SVM17. Malaysian Journal of Microbiology, 7(2), 97–106.
Duffner, F., Bertoldo, C., Andersen, J. T., Wagner, K., & Antranikian, G. (2000). A new thermoactive pullulanase from Desulfurococcus mucosus: cloning, sequencing, purification, and characterization of the recombinant enzyme after expression in Bacillus subtilis. Journal of Bacteriology, 182, 6331–6338.
Takata, H., Takaha, T., Kuriki, T., Okada, S., Takagi, M., & Imanaka, T. (1994). Properties and active center of the thermostable branching enzyme from Bacillus stearothermophilus. Applied Environmental Microbiology, 60, 3096–3104.
Lai, C. S., Davis, A. B., & Hoseney, R. C. (1989). Functional effect of bran in bread making. Journal of Cereal Chemistry, 66, 217–219.
Kayode, J., Sola, A., Adelani, A., Adeyinka, A., Kolawole, O., & Bashiru, O. (2009). The role of carbohydrate in diabetic nutrition: a review. The Internet Journal of Laboratory Medicine, 3, 2.
Anis, P., Davulcu, A., & Eren, H. A. (2008). Enzymatic pre-treatment of cotton. Part 1: desizing and glucose generation in desizing liquor. Fibre Textile Eastern Europe, 16, 100–103.
Carvalho, R. V. D., Correa, T. L. R., Matos, S. J. C., Mansur, L. R. C., & Martins, M. L. L. (2008). Properties of an amylase from thermophilic Bacillus sp. Brazilian Journal of Microbiology, 39, 102–107.
Acknowledgments
The authors gratefully acknowledge financial assistance from the PURSE grant of the Department of Science Technology-University of Delhi while carrying out the work presented in the manuscript. Authors wish to thank Mr. Vijay Kumar Gupta (Tushar Nutritive Food Industry, New Delhi) for extending help in assessing the applicability of amylopullulanase in bread making.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 41.8 kb)
Rights and permissions
About this article
Cite this article
Nisha, M., Satyanarayana, T. Characterization and Multiple Applications of a Highly Thermostable and Ca2+-Independent Amylopullulanase of the Extreme Thermophile Geobacillus thermoleovorans . Appl Biochem Biotechnol 174, 2594–2615 (2014). https://doi.org/10.1007/s12010-014-1212-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1212-8