Abstract
Amylases represent a versatile group of catalysts that are used for the saccharification of starch because they can hydrolyze the glycosidic bonds of starch molecules to release glucose, maltose, and short-chain oligosaccharides. The amylolytic complex of the thermophilic filamentous fungus Humicola brevis var. thermoidea (AmyHb) was produced, biochemically characterized, and compared with the commercial amylase Termamyl. In addition, the biotechnological application of AmyHb in starch saccharification was investigated. The highest production was achieved using a wheat bran medium at 50 °C for 5–6 days in solid-state fermentation (849.6 ± 18.2 U·g−1) without the addition of inducers. Optimum amylolytic activity occurred at pH 5.0 at 60 °C, and stability was maintained between pH 5.0 and 6.0, with thermal stability at 50–60 °C, especially in the presence of Ca2+. These results were superior to those found with Termamyl. Both enzymes were strongly inhibited by Hg2+, Cu2+, and Ag+; however, AmyHb displayed increased activity in the presence of Mn2+ and Na+. In addition, AmyHb showed greater tolerance to a wide range of ethanol concentrations. AmyHb appears to be a complex consisting of glucoamylase and α-amylase, based on its substrate specificity and TLC. The hydrolysis tests on cornstarch flour showed that the cocktail of AmyHb50% + Termamyl50% significantly increased the release of glucose and total reducing sugars (36.6%) when compared to the enzymes alone. AmyHb exhibited promising physicochemical properties and good performance with commercial amylase; therefore, this complex is a biotechnological alternative candidate for the bioprocessing of starch sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amylases (E.C 3.2.1.-) are widely used industrial enzymes with a history dating back to the early nineteenth century. They currently constitute 30% of the global enzyme market and are used to cleave the glycosidic bonds present in starch, thereby releasing glucose, maltose, and short-chain oligosaccharides (Paul et al. 2021; Far et al. 2020). Starch is a polysaccharide that is widely utilized in various industries (Iuga and Mironeasa 2020; Seung 2020) and is composed of D-glucose units connected by glycosidic bonds to form amylose and amylopectin. Amylose consists of up to 6000 D-glucose residues that are linearly polymerized with α-1,4 glycosidic bonds, whereas amylopectin is a branched polymer consisting of short chains containing 10–60 D-glucose residues that are interconnected with the same bonds. In addition, amylopectin has side chains of 15–45 D-glucose residues attached to the principal chain by α-1,6 bonds (Macneill et al. 2017; Oh et al. 2020). Using amylases as starch biocatalysts has several advantages, including high specificity, bioeconomic support, environmental damage reduction, and decreased chemical catalyst dependence (Läufer 2017; Troiano et al. 2020; Paul et al. 2021; Mesbah 2022; Mondal et al. 2022).
According to the CAZy database of active carbohydrate enzymes (http://www.cazy.org/), most amylases belong to the glycoside hydrolase (GH) family 13, which is known as the α-amylase family. However, the activity of this enzyme has been observed in other families, including GH14, GH15, GH 57, GH 119, and GH 126. This classification system mainly considers the amino acid sequence and three-dimensional structure of the protein (Møller et al. 2016; Gangadharan 2020; Cripwell et al 2021).
Amylases can be divided into four main groups according to their mode of action: endoamylases, exoamylases, debranching enzymes, and transferases (Castro et al. 2011; Gangadharan et al. 2020). The substrate-specific nature of some amylases causes the positioning of the starch-binding site (SBD) to often determine the family to which the amylase belongs. In the CAZy classification, the starch-binding site (SBD) is characterized next to the carbohydrate-binding module (CBM). The CBM corresponds to noncatalytic auxiliary domains and is associated with the affinity of the enzyme for insoluble crude starch (Cripwell et al 2021; Gangadharan 2020). Currently, SBDs in 15 CBM families are located in the C- or N-terminal region of the protein. Of particular note are the CBM20 microbial amylases, which exhibit a high affinity for raw starch from two SBD sites in the C-terminal region (Ngo et al. 2019; Paul et al. 2021).
Although several organisms have the ability to produce amylases, fungi offer greater industrial potential than bacteria and yeasts because of their superior production systems. Fungi thrive in media consisting of low-cost agricultural byproducts, employ a simple enzyme extraction process, and exhibit greater pH and temperature stabilities than the other organisms (Meyer et al., 2020; Balakrishnan et al. 2021). Furthermore, the enzyme complexes produced by these fungi exhibit beneficial traits for industrial purposes, including thermotolerance and stability at various pH levels (Pandey et al. 2000; Sauer et al. 2000; Norouzian et al. 2006; Mesbah 2022).
The genus Humicola belongs to the family Chaetomiaceae and the first species described were H. fuscoatra and H. grisea (Traaen 1914). Currently, the genus officially consists of 24 species found in habitats such as compost, decomposition material, and soil (Ibrahim et al. 2021). Many produce extracellular enzymes of industrial importance, such as cellulases, xylanases, and amylases. In particular, thermophilic fungi of this genus, which produce thermoenzymes, are promising sources for meeting biotechnological demands (Ibrahim et al. 2021), including species such as H. insolens, which has an excellent production of alkaline and thermo-resistant cellulases, and the fungus Humicola brevis var. thermoidea, which contains an alkali-halo-tolerant and thermostable endo-xylanase (Masui et al. 2012; Fan et al. 2021; Almeida et al. 2022).
Previous research has demonstrated that H. brevis var. thermoidea is proficient in producing hydrolases like xylanase and β-glucosidase during solid-state fermentation (SSF) using wheat bran as carbon source to cultivation (Masui et al. 2012; Almeida et al. 2022). SSF offers several advantages including cost-effectiveness, simple equipment requirements, utilization of inexpensive agricultural residues, minimal water consumption, and reduced catabolic repression. The furan production during agricultural waste treatment with steam and high pressure for sterilization do not appear to affect the enzyme production in H. brevis (Balkan and Ertan 2010; Saxena and Singh 2011; Jilani and Olson 2023). Additionally, wheat bran is an abundant and cost-effective agricultural residue that contains starch, proteins, lipids, cellulose, and hemicellulose, as essential micronutrients for microbial growth and enzyme production (Onipe et al. 2015; Chen et al. 2023).
This study aimed to produce and characterize the amylolytic complex produced by the fungus H. brevis var. thermoidea (AmyHb) and compare it to the commercial enzyme Termamyl. In addition, the possible potential of this enzyme in the biofuel industry was investigated through its ability to produce fermentable sugars and oligomers by the saccharification of cornstarch.
Materials and methods
Microorganisms and maintenance of fungi
The H. brevis var. thermoidea were obtained from the American Type Culture Collection (ATCC 28402). The strain was maintained using slant tubes with potato dextrose agar medium (Oxoid, GBR) at 40 °C with 70% humidity for 15 days (Masui et al. 2012).
Production of AmyHb by solid-state fermentation (SSF)
The surface of the culture medium was gently scraped with 10 mL of autoclaved distilled water to obtain a solution containing the strain. The fungus was inoculated into the substrate by transferring 2 mL of the suspension into a carbon source.
Optimization of AmyHb production using SSF
Amylase production was optimized using the one-factor-at-time (OFAT) method. The best carbon source (5 g carbon source and 10 mL distilled water), which consisted of wheat bran, corn grits, corn meal, corn straw, corn cobs, barley, and soluble starch, was placed in a 250 mL Erlenmeyer flask. Additional carbon inducers (corn grits, corn meal, barley, and soluble starch) and nitrogen inducers (casein, peptone, yeast extract, and urea) were added to the flask (1%, w/w).
The optimal temperature and duration of growth of the fungus to express AmyHb were subsequently assessed. The best culture growth temperature for enzyme production was analyzed using samples at 40, 50, 55, 60, and 70 °C. The daily enzymatic activity of the samples was measured for 10 days to verify the time required for optimal amylolytic production. Initially, all media were cultured using SSF, and amylolytic activity was measured periodically to obtain the best enzyme production profile.
Production of the crude extract rich in amylase
Under optimal conditions for amylase production, the culture medium was suspended in 30 mL of ice-cold distilled water and filtered through a synthetic sieve. The filtered solution was then centrifuged at 10,000 × g for 10 min (min) at 4 °C. The pellet was discarded and the supernatant was used as the crude extract for enzyme activity assays.
Enzyme and protein assays
The amylolytic activities of AmyHb and the commercial enzyme Termamyl were measured by releasing reducing sugars using 3,5-dinitrosalicylic acid (DNS) according to Miller (1959), with glucose as the standard. A 96-well plate was prepared as follows: 10 µL of enzyme solution, 40 µL of distilled water, and 50 µL of 50 mmol·L−1 sodium acetate buffer at pH 5.0 with 0.5% (w/v) commercial starch, at 60 ºC. After 10 min of incubation, the reaction was stopped by adding DNS at a 1:1 ratio, followed by heating in boiling water. An aliquot of 100 μL was taken for reading on a microplate at 540 nm using a Spectramax 384 Plus spectrophotometer (Molecular Devices, USA).
Control points were created by replacing the active enzymes with heat-denatured enzymes to estimate the spontaneous hydrolysis of the substrate. One unit of enzyme activity (U) was defined as the amount of enzyme capable of releasing 1.0 μmol of reducing sugar per minute. One unit of specific activity (U·mg−1) was determined as the number of units of activity per mass of protein. A unit of enzyme production in SSF (U·g−1) was defined as the ratio of the number of units of enzyme activity per gram of carbon source.
The protein concentration was determined using bovine serum albumin as the standard (Read and Northcote 1981).
Effect of pH on AmyHb activity and stability
The influence of pH on amylolytic activity was determined by individual incubation of 0.12 µg AmyHb and Termamyl® (Novozymes, DNK) in 50 µL of a solution containing 1% (w/v) commercial starch diluted in distilled water and 40 µL of 100 mmol·L−1 glycine-citrate–phosphate buffer with pH values between 3.0 and 10.0, for 10 min at 60 ºC (Ruller et al. 2014). Enzyme pH stability was determined by incubating 1.2 µg of AmyHb and Termamyl in 900 µL of 100 mmol·L−1 glycine-citrate–phosphate buffer with pH values between 3.0 and 10.0, for 24 h (h) at 25 ºC (Masui et al. 2012). A 10 µL aliquot of each solution (0.12 µg) was used to determine enzymatic activity as described above.
Effect of temperature on AmyHb activity and stability
The effect of temperature on enzymatic activity was determined in a medium reaction containing 0.12 µg of AmyHb and Termamyl at temperatures between 25 and 80 ºC. Thermostability was evaluated by pre-incubating 1.2 µg of AmyHb and Termamyl in 900 µL of distilled water at 50, 60, and 70 ºC. To improve the thermostability of the enzyme, different aliquots containing MnCl2 or CaCl2 were prepared at a concentration of 10 mmol·L−1. For each temperature, aliquots of 100 µL were taken at time intervals between 1 and 24 h and cooled in an ice bath for 1 min. A 10 µL aliquot containing 0.12 µg of enzyme was used to determine enzymatic activity as described above.
Effect of salts and EDTA on enzyme activity
The effect of various salts (KCl, LiCl, NaCl, SrCl2, MnCl2, CaCl2, CoCl2, HgCl2, ZnCl2, FeCl3, AgNO3, CuSO4, NiSO4, and MgSO4) and EDTA were evaluated by adding 5.0–10 mmol·L−1 of these substances to the enzymatic reaction. The enzymatic activities were then evaluated using the optimal conditions determined from the previous tests (at 60 °C and pH 5.0).
Effect of ethanol on enzyme activity and stability
The effect of increased ethanol concentration on enzymatic activity was determined using a reaction containing 0.12 µg of AmyHb and Termamyl in the presence of an ethanol concentration gradient of 0–50% (v/v). The reactions were then performed under the optimal conditions obtained from the previous tests. Stability tests using increasing concentrations of ethanol were conducted individually by pre-incubating 1.2 µg of AmyHb and Termamyl in a solution containing water and 0 to 50% ethanol (v/v) for 24 h at 25 ºC. An aliquot of each solution (0.12 µg) was used to determine the enzymatic activity.
Effect of different starch substrates on enzyme activity
Enzymatic hydrolysis was conducted using various starch sources, including rice, wheat flour, cornstarch, potato amylopectin, corn amylopectin, and potato amylose, along with disaccharides containing glucose, maltose, and sucrose (Sigma-Aldrich, USA). The substrates were prepared at 1% concentration, and the starch sources were boiled to facilitate gelatinization. The reactions were prepared under the previously determined optimal conditions.
Thin-layer chromatography (TLC)
The hydrolysis products of commercial starch produced by AmyHb were determined by thin-layer chromatography (TLC) using silica plates (Merck, DEU). Enzymatic hydrolysis was performed by incubating 0.12 µg of AmyHb, 40 µL of distilled water, and 50 µL of 50 mmol·L−1 sodium acetate buffer at pH 5.0 containing 0.5% (w/v) commercial starch, at 60 °C. The amylolytic activity of the samples was stopped using trichloroacetic acid (TCA) from Sigma-Aldrich after 0.5, 1.0, 2.0, and 24 h of reaction.
Ten microliters each of the hydrolyzed sample and glucose and maltose (1% w/v) standard were applied to the plate. The plate was then placed in a glass container containing a running solution of ethyl acetate, acetic acid, formic acid, and distilled water at a ratio of 9:3:1:4 (v/v). After each run, the chromatograms were dried at 25 °C for 24 h. The plates were then sprayed with a solution of 0.2% (w/v) orcinol diluted in a 9:1 ethanol and sulfuric acid mixture. Finally, the plates were heated in an oven at 100 °C until the bands became visible.
Enzymatic saccharification of cornstarch
The amylolytic complex AmyHb was enzymatically used to hydrolyze cornstarch based on findings from various studies (Jain and Katyal 2018; Li et al. 2014; Pervez et al. 2014). The enzymatic hydrolysis assay was conducted in microtubes containing 5% (w/v) commercial corn flour (Yoki™, BRA) and 1 mL distilled water. This solution was boiled for 10 min to gelatinize the starch and then cooled in an ice bath. Enzymes, including AmyHb (6.0 mg/g of substrate), Termamyl (6.0 mg/g of substrate), and a cocktail of AmyHb50% + Termamyl50% (3.0 + 3.0 mg/g of substrate), were added to a final volume of 2 mL. The pH was adjusted to 5.0 with 50 mmol·L−1 sodium acetate buffer. Controls were similarly prepared without the enzymes. The reactions occurred in a thermomixer bath (Thermo Scientific, USA) at 60ºC and 300 rpm for 24 h.
Determination of amylolytic activity of the hydrolyzed cornstarch
To test the hydrolysis of cornstarch, the amylolytic activities of AmyHb, Termamyl, and the cocktail (AmyHb50% + Termamyl50%) were determined by the enzymatic method using peroxidase/glucose-oxidase (Bergmeyer and Gawehn 1974), and reducing sugars were determined using DNS. The results were expressed as the concentration of glucose (g·L−1), and the percentage of saccharification was calculated using the equations below:
The results were expressed as total reducing sugars (TRS) using the equation below:
Analysis of data
All experiments were performed in quintuplicate (n = 5) and results were expressed as mean ± standard deviation. The data were analyzed using One-way Analysis of Variance (ANOVA) followed by the comparison of means using the Dunnett test (non-parametric: one-way ANOVA, mixed model) for biochemical of characterization and the Tukey test (non-parametric: one-way ANOVA, mixed model) for hydrolysis through Graphpad Prism 9 software (Graphpad Software, USA). The significance level was set at p < 0.05.
Results and discussion
Optimization of AmyHb production by solid-state fermentation
Wheat bran was considered the optimal carbon source for producing AmyHb (Table 1). This results from the rich nutritional composition of non-starch carbohydrates (55–60%), starch (14–25%), protein (13–18%), minerals (3–8%), and fat (3–4%) (Katileviciute et al. 2019). This source has facilitated the efficient secretion of amylase from the fungi Penicillium chrysogenum (687 U·mg-1), P. griseofulvum (652 U·mg-1) (Ertan et al. 2014), Thermomyces lanuginosus (535 U·g-1) (Kunamneni et al. 2005), Lichtheimia ramosa (320.7 U·g-1), Gongronella butleri (63.25 U·g-1) (Cavalheiro et al. 2017), Thermoascus aurantiacus (44.2 U·g-1) (Oliveira et al. 2016), and P. purpurogenum (23.5 U·g-1) (El-Naggar and El-Hersh 2011).
In addition, other types of lignocellulosic biomasses have been used for amylase production in SSF. For example, A. oryzae has been applied to soy husks and beer grains (Melnichuk et al. 2020; Francis et al. 2002), Monasus sanguineus to beetroot (Tallapragada et al. 2017), Trichoderma pseudokoningii to orange peel (Abdulaal 2018), Monasus anka and A. kawachii to barley and rice (Yoshizaki et al. 2010), T. virens to watermelon peel (Abdel-Mageed et al. 2022), Rhizopus delemar to apple bagasse (Pathania et al. 2018), A. glaums, A. oryzae and P. purpurogenum to rice and maize cobs (El-Naggar and El-Hersh 2011); Phanerochaete chrysosporium to fruit skins (Olorunnisola et al. 2018), and Rhizopus stolonifer, A. niger, and Phanerochaete chrysosporium to cassava skins (Pothiraj and Eyini, 2007).
Supplementation of 5 g of wheat bran (5 g per 10 mL distilled water) with other carbon sources (1% w/w) did not improve AmyHb production (Table 2). However, the addition of 1% (w/w) carbon sources increased amylase production in T. lanuginosus when supplemented with wheat bran (Kunamneni et al. 2005). Similarly, the addition of nitrogen sources (1%, w/w) to 5 g of wheat bran (5 g per 10 mL distilled water) did not enhance AmyHb production (Table 2), consistent with findings in A. terreus for amylase production (Sethi et al. 2016). Moreover, P. citrinum exhibited decreased amylase production in nitrogen-supplemented conditions, particularly with the addition of yeast extract, which was also observed in H. brevis (Shruthi et al. 2020).
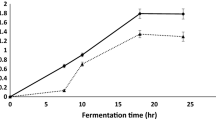
When testing the effect of temperature and cultivation time, the highest expression of amylase occurred at 50 °C (861.4 ± 19.3 U·g−1) with maximum production at 5 days (864.8 ± 13.8 U·g−1) (Fig. 1). Similar results were obtained for amylase production by T. lanuginosus in wheat bran at 50 °C after 5 d (261 U·g−1) (Kunamneni et al. 2005), T. aurantiacus (44.2 U·g−1), and the mesophilic fungus L. ramosa (320.4 U·g−1) in wheat bran for 5 days (Oliveira et al. 2016). As for fungi of the same genus, a report indicated the production of a glucoamylase by a Humicola sp. (10.44 U·mg−1), at 37 °C for 3 days in submerged fermentation composed of Vogel medium, in which glucose was replaced by wheat bran (Riaz et al. 2007).
The results show that wheat bran adequately supports microbial growth for producing enzymes by providing sufficient carbon and nitrogen (3.3%) for different enzyme´s production (Chick et al. 1947; Javed et al. 2012). Additionally, H. brevis growth at temperatures close to 50 °C validates its thermophilic nature, suggesting the probable expression of amylases with thermo-stable properties. The pH impact on enzyme expression wasn’t assessed due to the simplicity of the wheat bran and water culture, which makes pH observation difficult. Consequently, the final pH (6.45) reflects interactions among enzymatic complexes, metabolites, and the fungal medium.
Effect of pH on enzyme activity and stability
An analysis of the effect of pH on the enzymatic activity of AmyHb and Termamyl revealed that 100% activity occurred at pH values of 5.0 and 5.5 for the two compounds, respectively (Fig. 2A). The optimum pH obtained using AmyHb was similar to that found for the amylases of other Humicola species, such as the glucoamylase of H. grisea var. thermoidea (Tosi et al. 1993). Similar results were reported for amylases from the fungi G. butleri (Cavalheiro et al 2017) and M. sanguineus when grown by SSF (Tallapragada et al. 2017). In addition, optimal activity levels at pH 5.0 have been described for fungal species such as Thamnidium elegans, Cordyceps farinosa, Rhizomucor pusillus (Roth et al. 2019), A. wentii (Lago et al. 2021), and T. pinophilus when using SSF (Xian et al. 2015).
Effect pH and temperature on AmyHb and Termamyl. Effect of optimal pH (A). pH stability (B) and Optimal temperature (C). (●) AmyHb; (▲) Termamyl. The enzyme activity considered 100% were 24.2 ± 0.5 U.mg−1 and 484.8 ± 31.9 U.mg−1, respectively. The values represent the mean ± SD (n = 5) of enzyme activities
AmyHb maintained a relative activity of over 80% at pH values between 4.5 and 6.0. This is consistent with the acidic nature of most fungal amylases, which have pH values ranging from 4.0 to 7.0. These results are promising for starch saccharification techniques, as they have a pH range of 3.0–5.0. These amylases do not require the neutralization of starch paste, as is required when fuel ethanol is produced from corn (Cripwell et al. 2021; Mesbah 2022). Additionally, for probiotic supplementation, the amylases must remain active at neutral or acidic pH levels typically encountered in animal digestion (El-Saadony et al. 2021). The presence of a microenvironment acid can enhance the hydrolysis of starch in lower pH values and it can promote a better access of amylases into starch, increasing the hydrolysis of substrate (Wang and Copeland 2015).
In contrast, Termamyl showed relative activity of over 80% at pH levels of 5.5 and 7.5; therefore, it had greater activity levels at a basic pH than an acidic pH when compared to AmyHb. Termamyl is an α-amylase produced by Bacillus licheniformis and one of the most widely used commercial amylases.
The pH stability test revealed that AmyHb remained stable when incubated at pH values between 5.0 and 6.0 over a 24 h period at 25 °C (Fig. 2B). Under the same conditions, AmyHb had relative activities of approximately 70% at pH 4.0, 6.5, 7.0, and 7.5 and 60% at pH 4.0, 8.0, and 9.0. For amylases produced with wheat bran by SSF, the amylase activity of A. fumigatus decreased at high pH levels when incubated at 40 °C for 30 min (Singh et al. 2014). However, the three amylolytic isoforms of the fungus A. awamori KT-11 remained active over wide pH ranges (AmylI 4.5–8.5, AmylII 3.5–7.5, and AmylIII 4.0–7.0) for 15 h at 4 °C (Anindyawati et al. 1998). Similarly, the purified glucoamylase from a Humicola sp. had an optimum pH of 4.7 and was stable over a pH range of 3.5 to 5.9 (Riaz et al. 2007).
The stability of Termamyl was maintained at pH 5.5 when incubated for 24 h at 25 °C, but activity levels declined by approximately 10% at a pH of 5.0 and 30% at pHs of 6.0 to 7.0. Amylases on the market are often unstable under certain acidic/basic conditions and this limits their application. Therefore, mutant strains of microorganisms that express amylase are produced, such as that derived from Termamyl, which has greater stability at pH 4.5 (Gangadharan et al. 2020).
Effect of salts and EDTA on AmyHb and Termamyl activities
The effects of various salts and EDTA on the AmyHb and Termamyl enzymes are shown in Table 3. The relative activity of AmyHb was stimulated by NaCl and MnCl2 by approximately 30% at a concentration of 5 mmol·L−1. In addition, MnCl2 increased the enzyme activity by 47.1% at a concentration of 10 mmol·L−1, whereas NaCl stimulated it by 9.4% at the same concentration. Similar results were obtained for Mn2+ and Ca2+ with fungal amylases from T. lanuginosus (Petrova et al. 2000; Nguyen et al. 2002) and Aureobasidium pullulans (Li et al. 2007).
Termamyl showed only a slight increase in its amylolytic activity in the presence of 5 mmol·L−1 of KCl (18.0%), SrCl2 (13.0%), or MgSO4 (7.8%). Some Bacillus species appear to be capable of replacing Ca2+ with Sr2+ (Gupta et al. 2003). In contrast, the activities of AmyHb and the commercial amylase were strongly inhibited by CuSO4 and HgCl2 and completely inhibited by AgNO3. These results corroborate those reported by Tosi et al. (1993) with the amylase from Humicola grisea var. thermoidea. Furthermore, the glucoamylase activity of N. crassa exo-1 was shown to increase by 50–60% in the presence of Mn2+ and decrease by 50–65% in the presence of Cu2+ (Spinelli et al. 1996).
Moreover, the addition of EDTA more strongly inhibited Termamyl than AmyHb. This level of inhibition generated by EDTA has also been reported for amylases from A. niger (Wang et al. 2018), Rhizomucor miehei (Wang et al. 2020), and Penicillium citrinum (Carvalho et al. 2014). However, EDTA had no reported influence on amylase activity in A. brasiliensis, R. oryzae (Almeida et al. 2017), A. terreus (Sethi et al. 2016), and Humicola grisea var. thermoidea (Tosi et al. 1993). Hence, Termamyl exhibits notable inhibition when exposed to EDTA, while its activity increases in the presence of ions like KCl, SrCl2, and MgSO4, indicating a greater reliance on ions at its catalytic site. Conversely, EDTA has minimal inhibitory effects on AmyHb, implying a lower dependence on ions for its catalytic function. Nonetheless, certain ions such as Mn2+ and Na+ positively modulate AmyHb, improving its performance, which holds potential benefits for industrial applications (Raza and Rehman 2016).
Effect of temperature and thermal stability on enzyme activity
AmyHb showed optimum activity at 60 °C, 90% activity at 55 and 50 °C, and 70% at 45, 65, and 70 °C (Fig. 2C). These values were similar to those of other amylases expressed by thermophilic fungi, which ranged from 50 to 80 °C (Mesbah 2022; Tomasik and Horton 2012). In contrast, Termamyl had optimum activity levels at 70–75 °C. Although Bacillus licheniformis is a mesophilic microorganism, it can produce α-amylases with optimum activity levels at 50–100 °C (Muras et al. 2021). The glucoamylase expressed by H. grisea var. thermoidea with SSF displayed peak activity at temperatures of 60–55 °C (Tosi et al. 1993; Campos and Felix 1995). Similarly, Paecilomyces variotti (Michelin et al. 2010) and A. terreus (Sethi et al. 2016) have been shown to produce an amylase at an optimum temperature of 60 °C.
In terms of thermostability, AmyHb retained 100% of its activity for up to 8 h. After 24 h, the enzyme activity decreased by approximately 75% at 50 °C. This result was superior to that of the commercial enzyme, which maintained 90% activity over 2 h and had a half-life of approximately 8 h at the same temperature (Fig. 3A and B). At 60 °C, AmyHb was stable for up to 1 h, showed a decrease in stability at 2 h, and had a t1/2 value of close to 8 h, while Termamyl reached the t1/2 value in the first hour of incubation. In addition, AmyHb maintained approximately 25% of its stability for 4 h, while the commercial enzyme only retained 15% of its activity for 8 h, at 70 °C.
Thermostability of AmyHb and Termamyl in absence and presence of ions. Thermostability of AmyHb and Termamyl in absence of ions (A) and (B), in the presence of Mn 2+ (C) and (D), and the presence of Ca2+ (E) and (F). Temperatures of 50 ºC (●);60 ºC (■);70 ºC (▲). The enzyme activity considered 100% were 25.7 ± 1.8 U.mg−1 and 481.3 ± 28.9 U.mg−1, to Amy H and termamyl, respectively. The values represent the mean ± SD (n = 5) of enzyme activities
Almeida et al. (2017) reported that the amylase produced by A. brasiliensis remained stable for 120 min at 50 °C and 100 min at 60 °C, but showed a t1/2 of 32 min at 70 °C. In addition, the same authors reported that the amylase of R. oryzae only retained residual activity at 50 °C, with a half-life of 12 min. In addition, among the nine recombinant α-amylases from A. niger, four (AmyA, AmyD, AmyF, and AmyM) were completely stable at temperatures below 40 °C, two (AmyC and AmyE) maintained more than 60% residual activity between 40 °C and 50 °C for 1 h, and two (AmyG and AmyH) maintained residual activity above 40% at 70 °C (Wang et al. 2018). The findings from AmyHb demonstrate superior thermal stability compared to Termamyl and enhanced starch hydrolysis capabilities compared to other amylase in literature.
Enzymes from the Bacillus and Aspergillus genera are commonly used for production because of their stability at high temperatures. Although this thermostability is largely due to ion supplementation (Sindhu et al. 2017; Parashar and Satyanarayana 2018), the literature states that most amylases produced by microorganisms are ion-dependent or more stable when these ions are present (Paul et al. 2021). Termamyl is only able to maintain its enzymatic activity at temperatures close to 100 °C when incubated in the presence of Ca2+ (Lim et al. 2020; Muras et al. 2021).
The AmyHb and Termamyl amylolytic enzymes were incubated in the presence of Mn2+ and Ca2+ to enhance their thermal tolerance. Results showed that although Mn2+ did not improve the thermostability of AmyHb at 50–60 °C, a 10% enhancement in residual activity was observed at 70 °C, maintained for up to 24 h (Fig. 3C and D). Mn2+ also showed limited efficacy in stabilizing Termamyl at 50–70 °C, with slight improvement at 60 °C. Termamyl was inactivated with or without Mn2+ presence, regardless of temperature (Fig. 3B and C).
In contrast, Ca2+ improved the stability for both AmyHb and Termamyl at all temperatures tested (Fig. 3E and F). Both enzymes remained stable for 24 h at 50 °C and 2 h at 60 °C and retained approximately 60% residual activity over 24 h at the same temperature. At 70 °C, AmyHb maintained 65% of its residual activity for 24 h, while Termamyl stability lasted only 1 h, continuously decreasing until it reached 30% residual activity at 24 h.
The findings indicate that Mn2+ has minimal thermal stabilizing effect on both enzymes examined. In contrast, Ca2+ emerged as the most effective stabilizer for both amylases, consistent with the literature (Paul et al. 2021). Moreover, the thermal stability of AmyHb, with or without ions, was consistently higher than that of Termamyl across the tested temperatures and time.
Effect of ethanol on amylolytic activity and stability
AmyHb activity was stimulated by the presence of up to 10% ethanol (Fig. 4A) and remained stable until the 40–50% ethanol level, at which it was inhibited by close to 20%. In contrast, Termamyl was inhibited at all the ethanol concentrations tested. For the stability tests, both enzymes remained stable when incubated in 1 to 50% ethanol for 24 h (Fig. 4B).
Effect of ethanol on the activity and stability from AmyHb and Termamyl. Effect of ethanol (A) and ethanol stability (B). (●) AmyHb; (■) Termamyl®. The enzyme activity considered 100% were 23.7 ± 0.9 U.mg−1 and 481.1 ± 27.2 U.mg−1, respectively. The values represent the mean ± SD (n = 5) of enzyme activities
Research on the impact of organic solvents on fungal amylase activity is limited, but some suggest that enzyme tolerance to these compounds may be related to stability in salts such as NaCl. In high-salinity environments, organic solvents decrease activity by displacing water (Amoonzegar et al. 2019; Mesbah 2022). Additionally, organic compounds can stimulate enzymatic hydrolysis by binding to active sites and increasing hydrophobic interactions among non-polar amino acids, enhancing resistance to unfolding and thermal denaturation, resulting in improved stability (Hasan et al. 2024). Conversely, Silva et al. (2009) propose that the glycosylation of eukaryotic enzymes protects their catalytic sites from conformational changes induced by organic solvents.
These results are promising for future studies of recombinant microorganisms in the ethanol industry. In recent decades, consolidated bioprocessing (CBP) has become an efficient and economical approach for producing fuels derived from lignocellulosic and starchy biomasses. This technology is advantageous because it uses a single organism for enzyme production, substrate hydrolysis, glucose fermentation, and ethanol production (Cripwell et al. 2021; Haan et al. 2021).
Effect of hydrolysis potential on different substrates
The substrate specificity of AmyHb and Termamyl was determined by hydrolyzing various starch sources (Table 4). AmyHb hydrolyzed the starches at the following levels: potato amylose (99.8%), potato amylopectin (84.1%), cornstarch (82.3%), wheat starch (78.6%), maltose (59.7%), rice starch (47.1%), and corn amylopectin (41.2%). The percentages for Termamyl were as follows: cornstarch (89.1%), potato amylose (86.9%), wheat starch (81.7%), potato amylopectin (74.1%), rice starch (62.9%), corn amylopectin (56.0%), and finally maltose (6.0%).
The results found for AmyHb corroborate those of the α-amylase from Thermomyces lanuginosus, which effectively hydrolyzed potato starch (Petrova et al. 2000). Similarly, Xian et al. (2015) found that the α-amylase from Talaromyces pinophilus had a high preference for amylose (105.9%), followed by potato starch (97.5%), wheat starch (94.4%), cornstarch (93.2%), rice starch (94.0%), and amylopectin (68.29).
Similarly, it was observed that the A. tritici glucoamylase had a lower specific activity (30.14 ± 0.51 U·mg−1) for soluble potato starch than the other sources tested (Xian and Feng 2017), while P. oxalicum glucoamylase had a greater preference for soluble starch (81.2 ± 0.6 U·mg−1), raw rice starch (24.3 ± 0.5 U·mg−1), raw corn starch (24.3 ± 0.4 U·mg−1), raw cassava starch (11.5 ± 0.2 U·mg−1), raw potato starch (10.8 ± 0.3 U·mg−1), raw buckwheat starch (6.9 ± 0.3 U·mg−1) and raw sweet potato starch (2.9 ± 0.2 U·mg−1) (Xu et al. 2016). The α-amylase from A. oryzae showed the highest percentages with hydrolyzed rice starch (67%), followed by wheat starch (60%), potato starch (60%), cornstarch (60%), and amylopectin (56%) (Dey and Banerjee 2015).
The hydrolysis of maltose was greater with AmyHb than with Termamyl because α-amylases find it difficult to hydrolyze short glucose chains. Glucoamylases have a greater affinity for these chains, which suggests that the amylolytic complex of H. brevis contains a glucoamylase (Castro et al. 2011; Paul et al. 2021). However, the specificity of certain substrates depends on both the amylase class and the producing microorganism.
Thin-layer chromatography (TLC)
The TLC analysis showed that the hydrolysis of commercial starch by AmyHb produced mainly glucose and maltose, although short-chain oligosaccharides were also released during the tested time intervals (Fig. 5). Previous studies conducted with TLC revealed that glucoamylases predominantly release glucose, whereas α-amylases release maltotriose and maltopentaose (Lago et al. 2021; Michelin et al. 2010). In many of these studies, the exclusive release of glucose was considered conclusive evidence that the enzyme was a glucoamylase, similar to what was reported for the amylolytic enzyme from A. niveus (Silva et al. 2009).
The TLC tests showed that the crude extracts containing amylolytic enzymes from G. butleri and A. brasiliensis in different starch sources were found in hydrolyzed starch and released glucose and small amounts of oligosaccharides at higher concentrations, indicating greater secretion of glucoamylase (Cavalheiro et al. 2017). Similar results were found with R. oryzae, whereby the crude extract secreted glucoamylase and also minimum quantities of α-amylase (Almeida et al. 2017).
The TLC analysis and the results obtained previously suggest that the enzyme extract of H. brevis has predominantly saccharifying activity (exoamylases), because it releases glucose and maltose, and reduces the dextrinizing potential (endoamylases) as smaller oligosaccharide chains appear. Their results allied with the Ca2+ suggest the presence of α-amylase working together with glucoamylase on the amylolytic complex of H. brevis. However, further experiments are required to confirm whether AmyHb corresponds to two different classes of amylases.
Cornstarch saccharification tests
For the cornstarch hydrolysis tests, commercial corn flour was used as the substrate due to its processing similarity to corn used in the bioethanol industry. In both cases, the entire clean corn kernel is dry-milled, thereby allowing it to retain all or part of the original corn germ and fiber and reduce the particle size of the kernel (Gwirtz and Garcia-Casal 2013; Serna-Saldivar and Carrillo 2019).
The hydrolysis tests of corn flour with AmyHb at pH 5.0 at 60 °C showed the release of 9.4 g·L−1 of glucose and 15.0 g·L−1 of total reducing sugars (TRS) in 24 h, with a saccharification rate of 36.6% (Fig. 6A, B and C, circles). Under the same conditions, Termamyl released 10.0 g·L−1 of glucose and 16.1 g·L−1 of TRS over 24 h and had a saccharification rate of 37.4% (Figs. 6A, B and C, squares). These values were slightly better than those presented by AmyHb, and, together with the results of the previous substrate specificity tests, suggest a greater affinity of the commercial enzyme for corn amylopectin, since corn flour has a composition of 23–25% amylose and 75–77% amylopectin (Kumar and Singh 2019).
Hydrolysis of corn starch by AmyHb, Termamyl® and Cocktail AmyHb50% + Termamyl®50%). Reducing sugars (A), Glucose (B) and Saccharification (C). The amount of carbohydrate equivalent to 100% was 41 g.L.−1. (●) AmyHb; (■) Termamyl®; (▲) Cocktail (AmyHb50% + Termamyl®50%). The values represent the means ± SD (n = 5) of the enzymatic activities. Significant difference by Tukey’s test (p < 0.05)
Starch saccharification experiments using different microbial amylases vary greatly in terms of the amount of enzyme and substrate used, as well as the starch source, time, pH, and hydrolysis temperature. Jain and Katyal (2018) found that the hydrolysis of cornstarch by the crude extract of A. niger (1.5 mL) containing glucoamylase released 13.9 g·L−1 of TRS in 2 h with a saccharification efficiency of 52.6% at a temperature of 60 °C and pH 5.5. Pervez et al. (2014) reported a saccharification percentage of 60.0%, with 40.0 g·L−1 of glucose after 90 min of hydrolysis of cassava starch by 30 mL of α-amylase and amyloglucosidase purified from A. fumigatus at 60 °C.
Interestingly, the cocktail (AmyHb50% + Termamyl50%; Figs. 6A, B and C, triangles) showed the best hydrolysis results from the first hour onwards, with an increasing release of glucose up to 17.2 g·L−1 and 20.9 g·L−1 of TRS in 24 h, with a saccharification percentage of 51.9% (Figs. 6A, B and C, triangles). These results indicate a good synergism between AmyHb and Termamyl, which suggests that the AmyHb amylolytic complex in the crude extract is a promising enzyme for industrial use, especially in bioethanol production.
Conclusion
The present study showed that H. brevis var. thermoidea presented a high production of the amylolytic complex by SSF in a wheat bran medium at 50 °C for 5–6 days without the need for inducers. Furthermore, AmyHb presented optimal activity at pH 5.0 and 60 °C, with stability at a pH of 5.0 to 6.0 and high thermotolerance at 50 °C for 24 h. At 60 °C and 70 °C, the thermostability was greater in the presence of Ca2+; however, the results were superior to those observed with Termamyl.
AmyHb had increased activity in the presence of Na+ and Mn+2, and the addition of EDTA inhibited Termamyl to a greater extent than it inhibited AmyHb. Both the H. brevis amylolytic complex and Termamyl were stable in different concentrations of ethanol for 24 h.
The cornstarch saccharification tests showed that the cocktail (AmyHb50% + Termamyl®50%) had the best hydrolysis results, with a saccharification percentage of 51.9% in 24 h, indicating good synergism between AmyHb complex and Termamyl. These results suggest that the AmyHb amylolytic complex found in crude extracts is a promising enzyme for bioprocessing starch sources. In addition, the high stability of the enzyme in ethanol and its strong hydrolysis performance indicate that AmyHb has a possible potential for use in studies involving recombinant microorganisms in the ethanol fuel industry. Additionally, the formation of glucose, maltose, and smaller oligosaccharide chains was verified by TLC through the hydrolysis of commercial starch by AmyHb and ions, which suggested greater saccharifying and reduced dextrinizing activities of the enzyme suggesting the presence of glucoamylases with α-amylases on amylolytic complex of H. brevis.
References
Abdel-Mageed HM, Barakat AZ, Bassuiny RI, Elsayed AM, Salah HA, Abdel-Aty AM, Mohamed SA (2022) Biotechnology approach using watermelon rind for optimization of α-amylase enzyme production from Trichoderma virens using response surface methodology under solid-state fermentation. Folia Microbiol 67:253–264. https://doi.org/10.1007/s12223-021-00929-2
Abdulaal WH (2018) Purification and characterization of α-amylase from Trichoderma pseudokoningii. BMC Biochem 19:4. https://doi.org/10.1186/s12858-018-0094-8
Almeida PZ, Pereira MG, Carvalho CC, Heinen PR, Ziotti LS, Messias JM, Jorge JA, Polizeli MLTM (2017) Bioprospection and characterization of the amylolytic activity by filamentous fungi from Brazilian atlantic forest. Biota Neotrop 17:e20170337. https://doi.org/10.1590/1676-0611-BN-2017-0337
Almeida AP, Vargas IP, Marciano CL, Zanoelo FF, Giannesi GC, Polizeli MLTM, Jorge JA, Furriel RPM, Ruller R, Masui DC (2022) Investigation of biochemical and biotechnological potential of a thermo-halo-alkali-tolerant endo-xylanase (GH11) from Humicola brevis var. thermoidea for lignocellulosic valorization of sugarcane biomass. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2022.102424
Amoozegar MA, Safarpour A, Noghabi KA, Bakhtiary T, Ventosa A (2019) Halophiles and their vast potential in biofuel production. Front Microbiol 10:1895. https://doi.org/10.3389/fmicb.2019.01895
Anindyawati T, Melliawati R, Ito K, Iizuka M, Minamiura N (1998) Three different types of α-amylases from Aspergillus awamori KT-11: their purifications, properties, and specificities. Biosci Biotechnol Biochem 62:351–1357. https://doi.org/10.1271/bbb.62.1351
Balakrishnan M, Jeevarathinam G, Kumar SKS, Muniraj I, Uthandi S (2021) Optimization and scale-up of α-amylase production by Aspergillus oryzae using solid-state fermentation of edible oil cakes. BMC Biotechnol 21:33. https://doi.org/10.1186/s12896-021-00686-7
Balkan B, Ertan F (2010) The production of a new fungal α-amylase degraded the raw starch by means of solid-state fermentation. Prep Biochem Biotechnol 40:213–228. https://doi.org/10.1080/10826068.2010.488549
Bergmeyer HU, Gawehn K (1974) Methods of enzymatic analysis, 2ed. Elsevier 1:800
Campos L, Felix CR (1995) Purification and characterization of a glucoamylase from Humicola grisea. Appl Environ Microbiol 61:2436–2438. https://doi.org/10.1128/aem.61.6.2436-2438.1995
Carvalho CC, Ziotti LS, Pereira MG, Cruz AF, Jorge JA, Polizeli MLTM (2014) Production and functional properties of free and immobilized glucoamylases of Penicillium citrinum. J J Biotechnol Bioeng 1:007
Castro AM, Castilho LR, Freire DMG (2011) An overview on advances of amylases production and their use in the production of bioethanol by conventional and non-conventional processes. Biomass Convers Biorefin 1:245–255. https://doi.org/10.1007/s13399-011-0023-1
Cavalheiro GF, Sanguine IS, Santos FRS, Costa AC, Fernandes M, Paz MF, Fonseca GG, Leite RSR (2017) Catalytic properties of amylolytic enzymes produced by Gongronella butleri using agroindustrial residues on solid-state fermentation. Biomed Res Int. https://doi.org/10.1155/2017/7507523
Chen Z, Mense AL, Bewer LR, Shi YC (2023) Wheat bran layers: composition, structure, fractionation, and potential uses in food. Crit Rev Food Sci Nutr 10(1080/10408398):2171962
Chick H, Cutting MEM, Martin CJ, Slack EB (1947) Observations on the digestibility and nutritive value of the nitrogenous constituents of wheat bran. Br J Nutr 1:161–182. https://doi.org/10.1079/BJN19470026
Cripwell RA, van Zyl WH, Viljoen-Bloom M (2021) Fungal Biotechnology: Fungal Amylases and Their Applications. In: Zaragoza Ó, Casadevall A (eds) Encyclopedia of Mycology. Elsevier, Oxford, pp 326–336
Dey TB, Banerjee R (2015) Purification, biochemical characterization and application of α-amylase produced by Aspergillus oryzae IFO-30103. Biocatal Agric Biotechnol 4:83–90. https://doi.org/10.1016/j.bcab.2014.10.002
El Saadony MT, Mahmoud Alagawany M, Patra AK, Kar I, Tiwari R, Dawood MAO, Dhama K, Hany MR, Abdel-Latif HMR (2021) The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol 117:36–52. https://doi.org/10.1016/j.fsi.2021.07.007
El-Naggar NE, El-Hersh MS (2011) Organic acids associated with saccharification of cellulosic wastes during solid-state fermentation. J Microbiol 49:58–65. https://doi.org/10.1007/s12275-011-0288-x
Ertan F, Balkan B, Yarkın Z (2014) Determination of the effects of initial glucose on the production of α-amylase from Penicillium sp. under solid-state and submerged fermentation. Biotechnol Equip 28:96–101. https://doi.org/10.1080/13102818.2014.901670
Fan C, Zhang W, Su X, Ji W, Luo H, Zhang Y, Liu B, Yao B, Huang H, Xu X (2021) CRISPR/Cas9-mediated genome editing directed by a 5S rRNA-tRNA(Gly) hybrid promoter in the thermophilic filamentous fungus Humicola insolens. Biotechnol Biofuels 14:206. https://doi.org/10.1186/s13068-021-02057-y
Far BE, Ahmad Y, Khosroshahi AY, Dilmaghani A (2020) Microbial alpha-amylase production: progress, challenges and perspectives. Adv Pharm Bull 10:350–358. https://doi.org/10.34172/apb.2020.043
Francis F, Sabua A, Nampoothiri KM, Szakacs G (2002) Pandey A (2002) synthesis of alpha-amylase by Aspergillus oryzae in solid-state fermentation. J Basic Microbiol 42(5):320–326. https://doi.org/10.1002/1521-4028(200210)42:5%3c320::AID-JOBM320%3e3.0.CO;2-6
Gangadharan D, Jose A, Nampoothiri KMJ (2020) A. recapitulation of stability diversity of microbial α-amylases. J Amylase 4:11–23. https://doi.org/10.1515/amylase-2020-0002
Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Microbial a-amylases: a biotechnological perspective. Process Biochem 38:1599–1616. https://doi.org/10.1016/S0032-9592(03)00053-0
Gwirtz JA, Garcia-Casal MN (2013) Processing maize flour and corn meal food products. Ann NY Acad Sci 1312:66–75. https://doi.org/10.1111/nyas.12299
Haan R, Rose SH, Cripwell RA, Trollope KM, Myburgh MW, Bloom MV, van Zyl WH (2021) Heterologous production of cellulose- and starch-degrading hydrolases to expand Saccharomyces cerevisiae substrate utilization: Lessons learnt. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2021.107859
Hasan K, Baroroh U, Madhani IN, Muscifa ZS, Novianti MT, Abidin M, Yusuf M, Subroto T (2024) Enzymatic performance of Aspergillus oryzae α-Amylase in the presence of organic solvents: activity, stability, and bioinformatic studies. Bioinform Biol Insights 23:18. https://doi.org/10.1177/11779322241234767
Ibrahim SR, Mohamed SG, MohamedGajcm AAE (2021) Natural products of the fungal genus humicola: diversity, biological activity, and industrial importance. Curr Microbiol 78:2488–2509. https://doi.org/10.1007/s00284-021-02533-6
Iuga M, Mironeasa S (2020) A review of the hydrothermal treatments impact on starch based systems properties. Crit Rev Food Sci Nutr 60:3890–3915. https://doi.org/10.1080/10408398.2019.1664978
Jain D, Katyall P (2018) Optimization of gluco-amylase production from Aspergillus spp for its use in saccharifcation of liquefed corn starch. 3 Biotech. https://doi.org/10.1007/s13205-018-1131-4
Javed MM, Zahoor S, Shafaat S, Mehmooda I, Gul A, Rasheed H, Bukhari AI, Aftab MN, Haq IU (2012) Wheat bran as a brown gold: Nutritious value and its biotechnological applications. Afr J Microbiol Res 6:724–733. https://doi.org/10.5897/AJMRX11.035
Jilani SB, Olson DG (2023) Mechanism of furfural toxicity and metabolic strategies to engineer tolerance in microbial strains. Microb Cell Fact 22:221. https://doi.org/10.1186/s12934-023-02223-x
Katileviciute A, Plakys G, Budreviciute A, Onder K, Damiati S, Kodzius R (2019) A sight to wheat bran: high value-added products. Biomolecules 9:887. https://doi.org/10.3390/biom9120887
Kumar D, Singh V (2019) Bioethanol Production From Corn. In: Serna-Saldiva SO (ed) Corn Chemistry and Technology, 3rd edn. Elsevier, Netherlands, Oxford, pp 405–433
Kunamneni A, Pillai S, Singh S (2005) Response surface methodological approach to optimize the nutritional parameters for enhanced production of α-amylase in solid state fermentation by Thermomyces lanuginosus. Afr J Biotechnol 4:708–716. https://doi.org/10.5897/ajb2005.000-3138
Lago MC, Santos FC, Bueno PSA, Oliveira MAS, Barbosa-Tessmann IP (2021) The glucoamylase from Aspergillus wentii: purification and characterization. J Basic Microbiol 61:443–458. https://doi.org/10.1002/jobm.202000595
Läufer A (2017). Starch Biorefinery Enzymes. In: Wagemann, K., Tippkötter, N. (eds) Biorefineries. Adv Biochem Eng Biotechnol 166:137–152
Li H, Chi Z, Wang X, Duan X, Ma L, Gao L (2007) Purification and characterization of extracellular amylase from the marine yeast Aureobasidium pullulans N13d and its raw potato starch digestion. Enzyme Microb Technol 40:1006–1012. https://doi.org/10.1016/j.enzmictec.2006.07.036
Li Z, Cai L, Gu Z, Shi YC (2014) Effects of granule swelling on starch saccharification by granular starch hydrolyzing enzyme. J Agric Food Chem 62:8114–8119. https://doi.org/10.1021/jf500814g
Lim SL, Hazwani-Oslna NS, Oslna SN (2020) Purification and characterisation of thermostable α-amylases from microbial sources. BioResources 15(1):2005–2029. https://doi.org/10.15376/biores.15.1.Lim
MacNeill GJ, Mehrpouyan S, Minow MAA, Patterson JA (2017) Tetlow IJ (2017) Starch as a source, starch as a sink: the bifunctional role of starch in carbon allocation. J Exp Bot 68:4433–4453. https://doi.org/10.1093/jxb/erx291
Masui DC, Zimbardi ALRL, Souza FH, Guimarães LHS, Furriel RPM, Jorge JA (2012) Production of a xylose-stimulated β-glucosidase and a cellulase-free thermostable xylanase by the thermophilic fungus Humicola brevis var. thermoidea under solid state fermentation. World J Microbiol Biotechnol 28:2689–2701. https://doi.org/10.1007/s11274-012-1079-1
Melnichuk N, Beaia MJ, Anselmi P, Meini MR, Romanini D (2020) Valorization of two agroindustrial wastes to produce alpha-amylase enzyme from Aspergillus oryzae by solid-state fermentation. Waste Manage 106:155–216. https://doi.org/10.1016/j.wasman.2020.03.025
Mesbah NM (2022) Industrial biotechnology based on enzymes from extreme environments. Front Bioeng Biotechnol 10:870083. https://doi.org/10.3389/fbioe.2022.870083
Meyer V, Basenko EY, Benz JP, Braus GH, Caddick MX, Csukai M, Vries RP, Endy D, Frisvad JC, Cimerman NG, Haarmann T, Hadar Y, Hansen K, Johnson RI, Keller NP, Kraševec N, Mortensen UH, Perez R, Ram AFJ, Record E, Ross P, Shapaval V, Steiniger C, Brink H, Munster J, Yarden O, Wösten HAB (2020) Growing a circular economy with fungal biotechnology: a white paper. Fungal Biol Biotechnol 7:5. https://doi.org/10.1186/s40694-020-00095-z
Michelin M, Silva TM, Benassi VM, Peixoto-Nogueira SC, Moraes LAB, Leão JM, Jorge JA, Terenzi HF, Polizeli MLTM (2010) Purification and characterization of a thermostable α-amylase produced by the fungus Paecilomyces variotii. Carbohydr Res 345:2348–2353. https://doi.org/10.1016/j.carres.2010.08.013
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Møller MS, Henriksen A, Svensson B (2016) Structure and function of α-glucan debranching enzymes. Cell Mol Life Sci 73:2619–2641. https://doi.org/10.1007/s00018-016-2241-y
Mondal S, Mondal K, Halder SK, Thakur N, Mondal KC (2022) Microbial amylase: old but still at the forefront of all major industrial enzymes. Biocatal Agric Biotechnol 45:102509. https://doi.org/10.1016/j.bcab.2022.102509
Muras A, Romero M, Mayer C, Otero A (2021) Biotechnological Applications of Bacillus Licheniformis. Crit Rev Biotechnol 41:609–627. https://doi.org/10.1080/07388551.2021.1873239
Ngo ST, Tran-Le PD, Ho GT, Le LQ, Bui LM, Vu BK, Phung HT, Nguyen HD, Vo TS, Vu VV (2019) Interaction of carbohydrate binding module 20 with starch substrates. RSC Adv 9:24833–24842. https://doi.org/10.1039/C9RA01981B
Nguyen QD, Rezessy-Szabó JM, Claeyssens M, Stals I, Hoschke Á (2002) Purification and characterisation of amylolytic enzymes from thermophilic fungus Thermomyces lanuginosus strain ATCC 34626. Enzyme Microb Technol 31:345–352. https://doi.org/10.1016/S0141-0229(02)00128-X
Norouzian D, Akbarzadeh A, Scharer JM, Moo Young M (2006) Fungal glucoamylases. Biotechnol Adv 24:80–85. https://doi.org/10.1016/j.biotechadv.2005.06.003
Oh SM, Lee BY, Seo DH, Choi HW, Kim BY, Baik MY (2020) Starch nanoparticles prepared by enzymatic hydrolysis and self-assembly of short-chain glucans. Food Sci Biotechnol 29:585–598. https://doi.org/10.1007/s10068-020-00768-w
Oliveira APA, Silvestre MA, Garcia NFL, Alves-Prado HF, Rodrigues A, Paz MF, Fonseca GG, Leite OLIVEIRARSRL (2016) Production and catalytic properties of amylases from Lichtheimia ramosa and Thermoascus aurantiacus by solid-state fermentation. Scientific World J. https://doi.org/10.1155/2016/7323875
Olorunnisola KS, Jamal P, Alam MZ (2018) Growth, substrate consumption, and product formation kinetics of Phanerochaete chrysosporium and Schizophyllum commune mixed culture under solid-state fermentation of fruit peels. Biotech. https://doi.org/10.1007/s13205-018-1452-3
Onipe OO, Jideani AIO, Beswa D (2015) Composition and functionality of wheat bran and its application in some cereal food products. Int J Food Sci Tecnol 50:2509–2518. https://doi.org/10.1111/ijfs.12935
Pandey A, Nigam P, Soccol C, Soccol VT, Singh D, Mohan R (2000) Advances in microbial amylases. Biotechnol Appl Biochem 31:135–152. https://doi.org/10.1042/ba19990073
Parashar D, Satyanarayana T (2018) An insight into ameliorating production, catalytic efficiency, thermostability and starch saccharification of acid-stable α-amylases from acidophiles. Front Bioeng Biotechnol 6:125. https://doi.org/10.3389/fbioe.2018.00125
Pathania S, Sharma N, Handa S (2018) Utilization of horticultural waste (Apple Pomace) for multiple carbohydrase production from Rhizopus delemar F(2) under solid state fermentation. J Genet Eng Biotechnol 16:181–189. https://doi.org/10.1016/j.jgeb.2017.10.013
Paul JS, Gupta N, Beliya E, Tiwari S, Jadhay SK (2021) Aspects and recent trends in microbial α-amylase: a review. Appl Biochem Biotechnol 193:2649–2698. https://doi.org/10.1007/s12010-021-03546-4
Pervez S, Aman A, Iqbal S, Siddiqui N, Qader SAU (2014) Saccharification and liquefaction of cassava starch: an alternative source for the production of bioethanol using amylolytic enzymes by double fermentation process. BMC Biotechnol 14:49. https://doi.org/10.1186/1472-6750-14-49
Petrova SD, Ilieva SZ, Bakalova NG, Atev AP, Bhat MK, Kolev DN (2000) Production and characterization of extracellular α-amylases from the thermophilic fungus Thermomyces lanuginosus (wild and mutant strains). Biotechnol Lett 22:1619–1624. https://doi.org/10.1023/A:1005685226480
Pothiraj C, Eyini M (2007) Enzyme activities and substrate degradation by fungal isolates on cassava waste during solid state fermentation. Mycobiology 35(4):196–204. https://doi.org/10.4489/MYCO.2007.35.4.196
Raza ZA, Rehman A (2016) Optimization of amylase activity in the presence of various metal ions and surfactants in aqueous system. J Biochem Tech 7:1058–1062
Read SM, Northcote DH (1981) Minimization of variation in the response to different proteins of the coomassie blue G dye-binding assay for protein. Anal Biochem 116:53–64. https://doi.org/10.1016/0003-2697(81)90321-3
Riaz M, Perveen R, Javed MR, Nadeen H, Rashid MH (2007) Kinetic and thermodynamic properties of novel glucoamylase from Humicola sp. Enzyme Microb Technol 41:558–564. https://doi.org/10.1016/j.enzmictec.2007.05.010
Roth C, Moroz OV, Turkenburg JP, Blagova E, Waterman J, Ariza A, Ming L, Tiangi S, Andersen C, Davies GJ, Wilson KS (2019) Structural and functional characterization of three novel fungal amylases with enhanced stability and pH tolerance. Int J Mol Sci 20:4902. https://doi.org/10.3390/ijms20194902
Ruller R, Alponti J, Deliberto LA, Zanphorlin LM, Machado CB, Ward RJ (2014) Concommitant adaptation of a GH11 xylanase by directed evolution to create an alkali-tolerant/thermophilic enzyme. Protein Eng Des Sel 27:255–262. https://doi.org/10.1093/protein/gzu027
Sauer J, Sigurskjold BW, Christensen U, Frandsen TP, Mirgorodskaya E, Harrison M, Roepstorff P, Svensson B (2000) Glucoamylase: structure/function relationships, and protein engineering. Biochim Biophys Acta 1543:275–293. https://doi.org/10.1016/S0167-4838(00)00232-6
Saxena R, Singh R (2011) Amylase production by solid-state fermentation of agro-idustrial wastes using Bacillus sp. Braz J Microbiol 42:1334–1342. https://doi.org/10.1590/S1517-83822011000400014
Serna-Saldivar SO, Carrillo EP (2019) Food uses of whole corn and dry-milled fractions corn 3ed. Chem Technol. https://doi.org/10.1016/B978-0-12-811971-6.00016-4
Sethi BK, Jana A, Nanda PK, DasMohapatra PK, Sahoo SL, Patra JK (2016) Production of α-amylase by Aspergillus terreus NCFT 4269. 10 using pearl millet and its structural characterization. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00639
Seung DJNP (2020) Amylose in starch: Towards an understanding of biosynthesis, structure and function. New Phytol 228:1490–1504. https://doi.org/10.1111/nph.16858
Shruthi BR, Achur RNH, Boramuthi TN (2020) Optimized solid-state fermentation medium enhances the multienzymes production from Penicillium citrinum and Aspergillus clavatus. Curr Microbiol 77:2192–2206. https://doi.org/10.1007/s00284-020-02036-w
Silva TM, Maller A, Damasio RL, Michelin M, Ward RJ, Hirata IY, Jorga JA, Terenzi HF, Polizeli MLTM (2009) Properties of a purified thermostable glucoamylase from Aspergillus niveus. J Ind Microbiol Biotechnol 36:1439–1446. https://doi.org/10.1007/s10295-009-0630-z
Sindhu R, Binod P, Madhavan A, Beevi US, Mathew AK, Abraham A, Pandey A (2017) Kumar V (2017) Molecular improvements in microbial α-amylases for enhanced stability and catalytic efficiency. Bioresour Technol 245:1740–1748. https://doi.org/10.1016/j.biortech.2017.04.098
Singh S, Singh S, Bali V, Sharma L, Mangla J (2014) Production of fungal amylases using cheap, readily available agriresidues, for potential application in textile industry. BioMed Res Int 2014:215748. https://doi.org/10.1155/2014/215748
Spinelli LBB, Polizeli MLTM, Terenzi HF, Jorge JA (1996) Biochemical characterization of glucoamylase from the hyperproducer exo-1 mutant strain of Neurospora crassa. FEMS Microbiol Lett 138:173–177. https://doi.org/10.1016/0378-1097(96)00099-7
Tallapragada P, Dikshit R, Jadhav A, Sarah U (2017) Partial purification and characterization of amylase enzyme under solid state fermentation from Monascus sanguineus. J Genet Eng Biotechnol 15:95–101. https://doi.org/10.1016/j.jgeb.2017.02.003
Tomasik P, Horton D (2012) Enzymatic conversions of starch. Adv Carbohydr Chem Biochem 68:59–436. https://doi.org/10.1016/B978-0-12-396523-3.00001-4
Tosi LRO, Terenzi HF, Jorge JA (1993) Purification and characterization of an extracellular glucoamylase from the thermophilic fungus Humicola grisea var. thermoidea. J Microbiol 39:846–852. https://doi.org/10.1139/m93-126
Traaen AE (1914) Untersuchuggen uber bodenpilze aus norwegen. Nytt Mag Naturwiss Christiania 52:1–121
Troiano D, Orsat V, Dumont MJR (2020) Status of filamentous fungi in integrated biorefineries. Renew Sustain Energy Rev 117:109472. https://doi.org/10.1016/j.rser.2019.109472
Wang S, Copeland L (2015) Effect of acid hydrolysis on starch structure and functionality: a review. Crit Rev Food Sci Nutr 55:1081–1097. https://doi.org/10.1080/10408398.2012.684551
Wang J, Li Y, Lu F (2018) Molecular cloning and biochemical characterization of an α-amylase family from Aspergillus niger Electron. J Biotechnol 32:55–62. https://doi.org/10.1016/j.ejbt.2018.01.004
Wang YC, Hu HF, Ma JW, Yan QY, Liu HJ, Liu HJ (2020) A novel high maltose-forming α-amylase from Rhizomucor miehei and its application in the food industry. Food Chem 305:125447. https://doi.org/10.1016/j.foodchem.2019.125447
Xian L, Wang F, Luo X, Feng YL, Feng JX (2015) Purification and characterization of a highly efficient calcium-independent α-amylase from Talaromyces pinophilus 1–95. PLoS ONE 10:e0121531. https://doi.org/10.1371/journal.pone.0121531
Xu QS, Yan Y, Feng JX (2016) Efficient hydrolysis of raw starch and ethanol fermentation: a novel raw starch-digesting glucoamylase from Penicillium oxalicum. Biotechnol Biofuels 9:216. https://doi.org/10.1186/s13068-016-0636-5
Yoshizaki Y, Susuki T, Takamine K, Tamaki H, Ito K, Sameshima Y (2010) Characterization of glucoamylase and α-amylase from Monascus anka: enhanced production of α-amylase in red koji. J Biosci Bioeng 110:670–674. https://doi.org/10.1016/j.jbiosc.2010.07.005
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marciano, C.L., de Almeida, A.P., Bezerra, F.C. et al. Enhanced saccharification levels of corn starch using as a strategy a novel amylolytic complex (AmyHb) from the thermophilic fungus Humicola brevis var. thermoidea in association with commercial enzyme. 3 Biotech 14, 198 (2024). https://doi.org/10.1007/s13205-024-04038-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-024-04038-y