Abstract
Microbial fuel cells (MFCs) are a device using microorganisms as biocatalysts for transforming chemical energy into bioelectricity. As soil is an environment with the highest number of microorganisms and diversity, we hypothesized that it should have the potential for energy generation. The soil used for the study was Mollic Gleysol collected from the surface layer (0–20 cm). Four combinations of soil MFC differing from each other in humidity (full water holding capacity [WHC] and flooding) and the carbon source (glucose and straw) were constructed. Voltage (mV) and current intensity (μA) produced by the MFCs were recorded every day or at 2-day intervals. The fastest and the most effective MFCs in voltage generation (372.2 ± 5 mV) were those constructed on the basis of glucose (MFC-G). The efficiency of straw MFCs (MFC-S) was noticeable after 2 weeks (319.3 ± 4 mV). Maximal power density (P max = 32 mW m−2) was achieved by the MFC-G at current density (CD) of 100 mA m−2. Much lower values of P max (10.6–10.8 mW m−2) were noted in the MFC-S at CD of ca. 60–80 mA m−2. Consequently, soil has potential for production of renewable energy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Broadening the energy supply options reduces the increasing demand for fossil fuels [1]. Microbial fuel cells (MFCs) are unique devices that can use microorganisms as catalysts for transforming chemical energy directly into electricity [2–4] and thus have potential to be an innovative system for production of renewable energy [1]. Soil is a place for living of some of the most fascinating and smallest organisms on Earth—soil’s microorganisms [5]. Estimates of the number of bacterial species per gram of soil vary between 2,000 and 8.3 million [6]. Torsvik et al. [7] have indicated that there are 4,000 bacterial “genomic units” only in 1 g of soil. Consequently, the diversity in soils is several orders magnitude higher than above ground, and it seems to be the last frontier for biodiversity on Earth [8]. All organisms in the biosphere depend on microbial activity, i.e., soil microorganisms that colonize the rhizosphere assist plants in the uptake of several vital nutrients; soil microbes can also exert a considerable influence upon the status of plant’s health [5]. This is the reason why there has been a surge of interest in soils as a reservoir of biodiversity in the last several years. Thus, we hypothesized that soil with the enormous diversity of microorganisms contained therein should have the potential for energy generation.
In the MFC, the energy stored in chemical bonds in different organic compounds is indirectly converted into electrical energy (bio-energy) through many enzymatic reactions conducted by microorganisms. Exoelectrogenic bacteria (i.e., Geobacter, Shewanella, Pseudomonas), commonly existing in the soil, are capable of transferring electrons outside their cells through direct contact or using soluble electron shuttles [3]. This capability comes from the fact that some soil bacteria form networks of tiny wires [9], linking individual bacterial cells into a web-like electrical circuit (see Fig. 2). The wires allow the bacteria to get rid of electrons generated during metabolism, transporting them to distant “electron dumps” [10].
A classical MFC consists of two components with an anode and cathode chamber [11]. Microorganisms are situated in the anodic compartment and use the biomass for growth while forming electrons and protons [12], which are either directly transported to an electrode via redox mediators or directly expelled by microorganisms for reducing the substrate [2, 4, 11]. The only by-product released by MFCs is carbon dioxide, which can be fixed by plants during photosynthesis [2]. The performance of MFCs depends on several factors including microbial activity, substrate type, and concentration and electrode material [11]. Considerable attention has been paid to several potential applications of MFCs, e.g., transport and energy generation, implantable power sources, wastewater treatment, and robots [4, 13]. Although there are many studies about MFCs, most cells are constructed on wastes [2, 3, 14], bottom sediments [9], or sewage sludge [1, 15]. Areas under investigation also include the selection of electrode materials [4, 16], maintaining optimal living conditions for microbe colonies [1], improving charge transfer between microbes and material of electrodes either chemically or by mechanical designs [17, 18], and efficient supply of nutrients [2].
Due to reconnaissance character of the current work, no mediators supporting the work of MFC were applied. This study aims at application of two different substrates (glucose and straw) at two different moisture conditions (100 % water holding capacity (WHC) and flooding) to evaluate the Mollic Gleysol potential for electricity generation. To our knowledge, this is the first investigation of the ability and potential of the soil environment for bioelectricity production, without addition of wastes and sludge.
Materials and Methods
Soil Characteristics
The soil used for the experiment was Mollic Gleysol taken in October 2012 from Kosiorów village (51° 13′ N; 21° 51′ E; Fig. 1) located close to the Chodelka River, a tributary of the Vistula River in Poland. The soil was collected from the surface layer (0–20 cm) of an agricultural meadow used for haymaking.
Before MFC construction, soil was characterized by physic-chemical analyses for the moisture percentage (drying samples at 105 °C for 24 h), WHC in a stainless-steel pressure chamber (Soil Moisture Equipment Company, USA), pH and redox potential (Eh) (Hach Lange and Radiometer potentiometric equipment), and total organic carbon content (TOC) by an automatic analyzer Shimadzu TOC-V CSH. In addition, the levels of nutrients were examined in fresh soil samples by Olsen P extraction (as an estimate of plant available P), NaCl-extractable ammonium, and water extraction by a flux analyzer AA3 Bran + Luebbe [19]. The soil investigated was a peaty soil type (upper 20 cm: soil moisture percentage 15.47 %; WHC 41.93 %, TOC 30.7 %; pH 6.019; Eh 446.4 mV; N-NO3 0.0267 mg g−1; N-NH4 0.0081 mg g−1 and P-PO4 0.0053 mg g−1). The soil material was stored at 4 °C prior to the MFC construction.
MFC Construction and Operation
Four combinations of MFCs differing from each other in the water content (100 % WHC, flooding) and the carbon source available for soil microorganisms (glucose and straw) were constructed. The MFCs were prepared in glass cylinders (height 46 cm, diameter 7 cm, and capacity 1,000 cm3). The cylinders were thoroughly filled with the soil (1.14 kg) to a height of 37.5 cm, corresponding to a capacity of 1,000 cm3.
The MFC combinations designed (Fig. 2) were as follows:
-
(1)
Control [1.14 kg of soil + 350 ml of water], coded MFC-C
-
(2)
Glucose [1.14 kg of soil + 350 ml of a 1 % glucose solution instead of water], coded MFC-G
-
(3)
Straw [1.14 kg of soil + 350 ml of water + 75 g of shredded Avena L.], coded MFC-S
-
(4)
Flooding [1.14 kg of soil + 500 ml of water] resulting in 5 cm3 of a stagnant water layer, coded MFC-F
Eight platinum electrodes (5 mm in length, 0.5 mm in diameter), sunk in plastic and previously calibrated in the Michaelis buffer, were inserted into the prepared MFC combinations. Three bottom electrodes (Fig. 2) were located at the depth of approximately 30 cm from the cylinder surface (anode space), while five upper electrodes were placed at the depth of 5 cm from the cylinder surface (cathode space). The laboratory experiment lasted 43 days and was conducted at room temperature (20 °C).
The external circuit resistance was fixed at 1.000 Ω. Water loss via evaporation during the operation was routinely replenished with tap water to maintain a constant condition of 100 % WHC or flooding (5 cm3 of stagnant water on the soil surface).
Analyses
The voltage (mV) produced by MFC was recorded every day or at 2-day intervals, using a precision multimeter CPI-551 (ELMETRON). Moreover, current intensity (μA) was monitored by an ammeter (IA PAS, Lublin, Poland). The current and power densities were calculated based on the footprint area of the anode [20]. Current (I) was calculated according to Ohm’s law: U = I × R, where U is the voltage and R is the external resistance, while power (P) was estimated according to P = I × U. The morphology of the anode surface biofilm (Fig. 2) was studied at the end of the experiment (43 days) using a scanning electron microscope (SEM) (Zeiss Ultra Plus, Germany). Additionally, at the beginning and the end of the study, dehydrogenase activity (DHA) of soil microorganisms with use of TTC as a substrate was determined [5]. Briefly, 6 g of soil with 120 mg CaCO3 was left to react with 1 ml of a 3 % TTC solution at 30 °C for 20 h. Then, solutions were extracted with ethanol and incubated for 1 h in the dark. Absorbance (λ = 485 nm) was measured using a UV-1800 Shimadzu instrument. DHA was expressed as μg TPF g−1 min−1.
Results and Discussion
Electricity Generation from the Soil
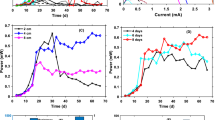
Results for voltage generation from four different combinations of the tested soil MFC are presented in Fig. 3.
MFC-G seemed to be the fastest MFC (already on the 4th day) and the most effective (372.2 ± 5 mV) in voltage generation. This resulted from the fact that glucose is a readily available source of carbon for soil microorganisms, which by having an easy access to this source directly transform the energetic potential included in the organic substances (here at glucose) into electricity, which constitutes the essence of MFC. Generally, it can be stated that the MFC-G was characterized by the highest and most stable potential, as the registered voltage generation values remained at a high level of 230–250 mV throughout the experiment. Only after 42 days, a decrease in the measured potential to the value of 136.6 ± 3 mV was recorded, and simultaneously, it was the lowest potential level achieved in the MFC-G. Probably, the noted decline is the consequence of depletion of the available carbon source and a decrease in the microbial activities.
A natural source of carbon is straw, which decomposes very slowly, typically for all natural materials, such as wood, paper, or stable textiles in cotton. Its structure is primarily formed with cellulose associated with hemicelluloses and lignin. What is important, after hydrolysis, cellulose is a natural source of carbon for microorganisms; therefore, we used it as a substrate in the MFC-S combination. In contrast to the glucose treatment, the efficiency of the MFC-S was noticeable already after 2 weeks of the experiment. Undoubtedly, this fact was linked to the process of a long run of natural carbon stocks contained in cellulose, which became available for soil microorganisms only after hydrolysis of polysaccharides. During the first week of the experiment, the bioelectricity generation from the MFC-S was quite low and remained at the level of ca. 50 ± 2 mV. On days 9 and 11 of incubation, equalization of potentials between the anode and cathode space was noted. An intensive increase in voltage generation after 14 days was recorded with the maximum [319.3 ± 4 mV] on day 29. On subsequent days, voltage variations in the range of 94–180 mV were observed.
The control MFC was characterized by moderate efficiency for bio-energy generation at a rate of 50 ± 1 mV for a greater period of the experiment. We found three cycles of growth in the voltage generation, attributable to days 14–16, 26–29, and 37–38, when the registered values fluctuated in the range of 90–119 mV. Among them, each subsequent increase in voltage generation was higher (c.a. 10–20 mV) than the previous one.
The lowest effectiveness was noted in the MFC-F combination, with a layer of stagnant water on the soil surface. In that case, throughout the experimental period, the generated voltage did not exceed the level of 50 ± 2 mV, with one exception on day 32 when it reached the value of 66.4 ± 3 mV. The voltage of the MFC-F was different from the others, because it decreased quickly due to oxygen depletion in the role of electron acceptor. In addition, the mass transfer limitation in the electron donor that reached the anode might be the cause of this phenomenon [21]. Piechocki et al. [22] emphasized that voltage generation from a single wastewater MFC is on average about 500 mV, which entails a serial connection of several MFCs in order to achieve the required level of voltage and current. Niessen et al. [23] reported that the initial redox potential of a compost soil MFC freshly inoculated with a glucose solution (5 g l − 1) ranged between 350 and 400 mV, analogically to our data from the MFC-G after the fourth day of the experiment. On the contrary, Liang et al. [24] indicated that open-circuit voltages of a wastewater MFC with addition of 50 mM of phosphate buffer were approximately between 670 and 830 mV. In the case of the presented study, all soil MFC combinations revealed a slightly lower efficiency, as the maximum voltage generations of 372.2 and 319.3 mV were achieved by the MFC-G and MFC-S, respectively. Nevertheless, it should be taken into consideration that our experiment was conducted on a small amount of soil (1.14 kg) and that no mediators were used to support the MFC work, as if often practiced by other researchers. On the other hand, Vologni et al. [1] described that after about a 3-month acclimation period, the MFC constructed with primary sludge (sewage sludge) produced a stable voltage of 250 mV, which was by ca. 120 mV lower than in our MFC-G. Özkaya et al. [11] noted even lack of current generation during the first week of their experiment in a MFC constructed on landfill leachate; thereafter, voltage increased steadily to 15 mV within 18 days.
In the course of our experiment, in parallel to voltage generation, the current intensity was also monitored (Fig. 4). At the beginning of the study, the current in all the MFC combinations remained at a similar level ca. 42–65 μA. A substantial increase in the current intensity was observed between the 8th and 10th day, when it reached a value of approximately 80–138 μA. Progressively, from day 13, a rapid growth of the current intensity was noted in the MFC-S, reaching values of up to 182 μA. This was the maximum current, which we managed to register in the course of the whole experiment. In contrast, the lowest current intensity values were found in the MFC-F (24–106 μA).
We also determined power and current densities for each of investigated soil MFC combinations (Fig. 5). We found that the maximum power density (P max = 32 mW m−2) was achieved by the MFC-G at current density (CD) of 100 mA m−2. Much lower values of P max from 10.6 to 10.8 mW m−2 were noted in the case of the MFC-S at CD of ca. 60–80 mA m−2, respectively.
On the other hand, the lowest power density was found in the MFC-F (P max = 1.5 mW m−2 at 100 mA m−2) followed by that of the MFC-C (P max = 4.6 mW m−2 at 80 mA m−2). It should be mentioned that generally it is very difficult to compare directly the power output with performance of other MFCs reported in the literature due to different operating conditions and substrate additions, types of applied electrodes, and different species of microorganisms colonizing the MFC. However, we have found that our data are compatible with those of Song et al. [21], who noted similar P max (31.6 mW m−2 at ca. 100 mA m−2) in the case of a wet-sediment MFC constructed with carbon nanotubes. The same authors also noted a decrease in P max to the level of ca. 10 mW m−2 (analogically to our MFC-S) in the wet-sediment MFC prepared with stainless steel net. Wang et al. [14] noted P max = 30 mV m−2 at 85.49 mA m−2 in a wastewater MFC with acetate addition as a substrate. Additionally, Pant el al. [25] indicated that acetate in particular was well known to generate low anode potentials and high power densities [26]. The value mentioned was close to our data obtained from the MFC with glucose as a substrate, which confirms the ability of soil to generate bio-energy at a comparable level as in the case of wastes. Likewise, Quan et al. [3] demonstrated that in the case of an aerobic-enriched wastewater MFC, the maximum power density amounted to 179 mW/m2 for acetate, 174 mV/m2 for glucose, and 175.4 mW/m2 for ethanol. However, these values still cannot meet the needs of many applications, i.e., lighting lamps or pumping water, which require a power output larger than 100 kW m−2 [27]. Rather than boost conversion, in situ application of this electricity would be a better way as it avoids energy consumption [24]. Consequently, only large-scale treatment is the application closest to industrial realization and is the domain of much current in MFC research [2].
Finally, soil DHA in all the MFC combinations was determined (Fig. 6) in order to obtain information about the biological characteristics of the soil. Dehydrogenases are one of the most important enzymes in the soil environment recommended as an indicator of overall soil microbial activity, as they occur intracellularly in all living microbial cells [5, 28, 29].
Analysis of the data from enzymatic activity revealed that DHA increased on the last day of the experiment in comparison with the initial conditions. The exception was only the MFC-G where the decrease in DHA was noted at the end of the study, assuming that this was connected with higher microbial activities on the first experimental days when glucose, i.e., an easily available carbon source, was accessible to microorganisms without limitation, whereas during the final days of the study, glucose was depleted. Consequently, also microbial activity was lowered, which was also confirmed by the decrease in voltage generation.
Moreover, it was indicated that the highest DHA level (0.00026 μg TPF g−1 min−2) remained in the MFC-S. This relation was confirmed at both the start and the end of the study. What is more, the MFC-S combination with regard to DHA exceeded (p < 0.0001) the other kinds of MFC significantly.
Relatively high activity of dehydrogenases (0.0000819 μg TPF g−1 min−2) was exhibited by the MFC-F with stagnant water on the soil surface, which facilitated formation of anaerobic conditions that promote anaerobic bacterial colonization. Włodarczyk [30] reported that the maximum of DHA occurred precisely in anaerobic microorganisms, which is particularly evident in terms of flooding conditions as in the described MFC-F.
The first step in characterizing the soil bacterial community was SEM observations (Fig. 7), which evidenced that microorganisms colonizing the investigated Mollic Gleysol used for MFC construction form networks of tiny wires, linking individual bacterial cells into a web-like electrical circuit. This is in agreement with the investigations of Ball [10] and Logan [31], who have reported that bacteria can sprout webs of electrical wiring that transform soil into a geological battery.
Conclusions
In this study, we have demonstrated that, after treatment with a carbon source, Mollic Gleysol can be used as a convenient way for electricity generation in microbial fuel cells. Glucose, as a substrate in the soil MFC, was successfully used for high (32 mW m−2) power generation in the first stage of microorganism needs and could be maintained by a soil-straw (MFC-S) combination during the next stage. We have shown that the performance of soil MFC is comparable to wastewaters, sewage sludge, and sediment MFC described in the literature. Consequently, the soil environment has potential for energy generation, and it might be used as a renewable source thereof. Low efficiency should not be a factor discrediting the MFC, as several MFCs in series must only be connected in order to increase performance. The greatest advantage of the soil MFC is the fact that soils are available everywhere, free of charge. Further studies on soil MFCs are required now, including different soil types and different sources of carbon available for soil microorganisms; this would lead to efficient production of bio-energy from soil. Additionally, recognition and identification of soil bacteria species that are the most useful and effective in energy generation is strongly recommended.
References
Vologni, V., Kakarla, R., Angelidaki, I., & Min, B. (2013). Bioprocess and Biosystems Engineering, 36, 635–642.
Mao, L., & Verwoerd, W. S. (2013). International Journal of Energy and Environmental Engineering, 4, 1–18.
Quan, X., Quan, Y., Tao, K., & Jiang, X. (2013). Bioresource Technology, 128, 259–265.
Won, K., Kim, Y. H., An, S., Lee, H. J., Park, S., Choi, Y. K., et al. (2013). Applied Biochemistry and Biotechnology, 171, 1194–1202.
Wolińska, A., Stępniewska, Z., Wołoszyn, A., Pytlak, A., & Dziuba, A. (2011). Acta Agrophysica, 194, 7–11.
Roesch , L. F. W., Fulthorpe, R R., Riva, A., Casella, G., Hadwin, A. K. M., Kent, A. D., et al. (2007). The ISME Journal, 1-8.
Torsvik, V., Goksoyr, J., & Daae, F. (1990). Current Opinion in Microbiology, 5, 240–245.
Black, H. I. J., Perekh, N. R., Chaplow, J. S., Monson, F., Watkins, J., Creamer, R., et al. (2003). Journal of Environmental Management, 67, 255–266.
An, J., Kim, B., Nam, J., Ng, H. Y., & Chang, I. S. (2013). Bioresource Technology, 127, 138–142.
Ball, P. (2007). Nature, 449, 388.
Özkaya, B., Cetnikaya, A. Y., Cakmakci, M., Kardağ, D., & Sahinkaya, E. (2013). Bioprocess and Biosystems Engineering, 36, 399–405.
Rabaey, K., & Verstraete, W. (2005). Trends in Biotechnology, 23, 291–298.
Davis, F., & Higson, S. P. J. (2007). Biosensors and Bioelectronics, 22, 1224–1235.
Wang, H., Jiang, S. C., Wang, Y., & Xiao, B. (2013). Bioresource Technology, 138, 109–116.
Cai, J., Zheng, P., Zhang, J., Xie, Z., Li, W., & Sun, P. (2013). Bioresource Technology, 129, 224–228.
Liu, J. L., Lowy, D. A., Baumann, R. G., & Tender, L. M. (2007). Journal of Applied Microbiology, 102, 177–183.
Oh, S. T., Kim, J. R., Premier, G. C., Lee, T. H., Kim, C., & Sloan, W. T. (2010). Biotechnology Advances, 28, 871–881.
Yang, Y., Sun, G., & Xu, M. (2011). Journal of Chemical Technology and Biotechnology, 86, 625–632.
Banach, A. M., Banach, K., Visser, E. J. W., Stępniewska, Z., Smits, A. J. M., et al. (2009). Biogeochemistry, 92, 247–262.
Reimers, C. E., Tender, L. M., & Lovley, D. R. (2001). Environmental Science and Technology, 35, 192–195.
Song, T., Xiao, P., Wu, X., & Zhou, C. C. (2013). Applied Biochemistry and Biotechnology, 170, 1241–1250.
Piechocki, J., Neugebauerr, M., & Sołowiej, P. (2010). Inżynieria Rolnicza, 3, 165–170.
Niessen, J., Harnisch, F., Rosenbeum, M., Schröder, U., & Scholz, F. (2006). Electrochemistry Communications, 8, 869–873.
Liang, P., Wei, J., & Huang, X. (2013). Frontiers of Environmental Science and Engineering, 7, 913–919.
Pant, D., Bogaert, G. V., Diels, L., & Vanbroekhoven, K. (2010). Bioresource Technology, 101, 1533–1543.
Yang, F., Ren, L., Pu, Y., & Logan, B. E. (2013). Bioresource Technology, 128, 784–787.
Bullen, R. A., Arnot, T. C., Lakeman, J. B., & Wlash, F. C. (2006). Biosensors and Bioelectronics, 21, 2015–2045.
Wolińska, A., & Stępniewska, Z. (2011). Soil Tillage and Microbial Activities, 7, 111–143.
Wolińska, A., & Stępniewska, Z. (2012). Dehydrogenases, 8, 183–210.
Włodarczyk, T. (2000). International Agrophysics, 14, 365–376.
Logan, B. E. (2009). Nature Reviews Microbiology, 7, 375–381.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wolińska, A., Stępniewska, Z., Bielecka, A. et al. Bioelectricity Production from Soil Using Microbial Fuel Cells. Appl Biochem Biotechnol 173, 2287–2296 (2014). https://doi.org/10.1007/s12010-014-1034-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1034-8