Abstract
This study aims at evaluating the performance of a two-chambered continuously fed microbial fuel cell with new Ti–TiO2 electrodes for bioelectricity generation from young landfill leachate at varying strength of wastewater (1–50 COD g/L) and hydraulic retention time (HRT, 0.25–2 days). The COD removal efficiency in the MFC increased with time and reached 45 % at full-strength leachate (50 g/L COD) feeding. The current generation increased with increasing leachate strength and decreasing HRT up to organic loading rate of 100 g COD/L/day. The maximum current density throughout the study was 11 A/m2 at HRT of 0.5 day and organic loading rate of 67 g COD/L/day. Coulombic efficiency (CE) decreased from 57 % at feed COD concentration of 1 g/L to less than 1 % when feed COD concentration was 50 g/L. Increase in OLR resulted in increase in power output but decrease in CE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Landfill leachate, generated from the disposal of municipal solid waste, is highly contaminated with a wide range of organic and inorganic nutrients, various chemical compounds and heavy metals [1]. Its characteristics depend on many factors including age, precipitation, weather, waste type and composition [2, 3]. Among those factors, age is the most crucial parameter determining the changes of organic content and biodegradability (BOD5/COD). Young leachate (1–2 years) has a high COD concentration, over 10 g/L, with a BOD5/COD ratio of around 0.4–0.6 [4, 5]. As aerobic biological treatment consumes high energy, economically and environmentally sustainable processes for the treatment of leachate are in growing demand [6, 7].
Microbial fuel cell (MFC) employs microbes to directly generate electricity from biochemical energy in addition to a novel approach for wastewater treatment [8–11]. A classical MFC consists of two components with an anode and cathode chamber. In the anodic chamber, electrons and protons are generated due to the anaerobic oxidation of organics [12]. Electrons diffuse to the cathode through an external circuit and the protons are transferred to the cathode chamber via cation exchange membrane. Electrons and protons are combined in the cathodic chamber by an electron acceptor such as O2 or potassium ferricyanide [13]. The performance of an MFC depends on several factors; including microbial activity, substrate type and concentration, anode and cathode materials [13, 14]. Substrate type and concentration affect both the performance and microbial diversity of MFC [12, 15, 16]. Kiely et al. [17] studied long-term cathode performance and the microbial communities in MFC fed with different fermentation end products. The MFCs were fed in a fed-batch mode for more than one year with individual end products of lignocellulose fermentation (acetic acid, formic acid, lactic acid, succinic acid, or ethanol). Depending on the substrate, the power densities ranged from 835 to 62 mW/m3. In another study, Sharma and Li [18] studied the power generation from three different substrates (acetate, ethanol, and glucose) over a concentration range of 0.5–35 mM in a single-chamber MFC. The maximum power density of 401 mW/m2 was generated from glucose. In contrast to the findings of Sharma and Li [18], Lee et al. [7] reported that the acetate-fed MFC had much higher power output (360 mW/m2), compared with the glucose-fed MFC (9.8 mW/m2). Tugtas et al. [19] evaluated power production performance of a continuous flow membrane-less cathode MFC fed with synthetic wastewater-containing acetate (180 mg/L). The air-facing side of the cathode was covered with a spunbonded olefin sheet to control oxygen diffusion and water loss. The configuration produced maximum power density of 750 mW/m2. However, loss of platinum catalyst and biomass growth on cathode resulted in gradual decrease of power density to 280 mW/m2.
High-strength wastewater can be used for electricity generation in MFC. By this way, both wastewater treatment and energy generation can be achieved simultaneously. Although there are several studies with synthetic wastewater, limited studies have been conducted with high-strength real wastewater, especially in continuous mode of operation. Cercado-Quezada et al. [20] and Wang et al. [21] obtained promising results using food and brewery industry wastewaters. Velasquez-Orta et al. [22] compared four different industrial wastewaters (bakery, brewery, paper and dairy) and obtained highest energy production with paper industry wastewater independently from biodegradability and COD concentration. Additionally, Pant et al. [23] reviewed MFC performance of various synthetic and high-strength real wastewaters. So far, researchers have used carbon-based electrodes for the treatment of leachate in MFC [24–26].
Electrode material is also another important parameter on the performance of MFC. Carbon-based and platinum electrodes have been widely used in MFC studies [8, 27]. However, researchers have recently obtained higher current production using new metal-based electrodes [28]. Our previous research indicated that Ti–TiO2 electrode (476.6 mA/m2) achieved 15 times higher current density than carbon-based electrode (31 mA/m2) with synthetic glucose solution in a dual-chambered MFC [29]. In the present study, the same MFC reactor configuration was used for electricity generation from young landfill leachate. The MFC reactor performance was evaluated under continuous mode with varying operational conditions.
Materials and methods
Leachate collection and characterization
Leachate samples were collected from Odayeri municipal landfill in Istanbul and transferred to the laboratory and kept at 4 °C throughout the MFC study. The characterization of the leachate is given in Table 1. The high COD and BOD5/COD ratio indicated that the leachate is young and highly biodegradable.
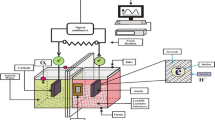
Microbial fuel cell design and setup
Dual-chambered plexiglass MFC was used in the study (see Ref. [29] for details). Each chamber has a volume of 275 mL while two compartments were separated by a cation exchange membrane (CMI 700, for a detail see Ref. [29]). In order to increase the porosity, the membrane was conditioned by boiling in 30 % H2O2 for 15 min and then rinsed with deionized water. Remaining H2O2 on the membrane surface was cleaned using 0.5 M H2SO4 and then rinsed with deionized water. Anode and cathode electrodes have the same dimensions of 5 cm height, 2 cm of length and 1.5 mm of thickness. The electrodes were mixed metal oxide titanium (Ti–TiO2 from Akat Engineering Company in Turkey). Same electrodes were used in both anode and cathode compartments. Electro catalytic coating was carried out with the thermal decomposition of mixed metal salts sprayed on titanium. The electrode properties were taken from the product catalog (Akat Engineering Company, Turkey) and it has crystal structure with density of 6–12 g/cm3, resistivity of 0.00001 Ω × cm, a large surface area with the Brunauer–Emmett–Teller (BET) surface of 20–50 m2/g as physical and chemical properties with a surface area of 10 cm2.
A 10 Ω resistance was used to transfer the electrons from anode to cathode electrode. Prior to the operation, both electrodes were washed with ethyl alcohol and rinsed with distilled water to remove impurities. The cathode compartment was filled with distilled water and aerated. Both chambers were mixed by a magnetic stirrer at 350 rpm. The anode chamber was sparged with nitrogen to remove oxygen to ensure anaerobic condition during the startup of the MFC. The MFC was not inoculated from any other source and the anode-respiring microorganisms in the original leachate samples were enriched during the operation.
Analysis
All analyses were made according to standard methods [30]. COD and BOD5 tests were performed as recommended in the open reflux method 5,220 and 5,210 B, respectively. Total organic carbon (TOC) was measured using a TOC analyzer (Hach Lange IL 550 model). Conductivity and pH were measured by multimeter (WTW). Anions and cations were determined by Ion Chromatography instrument (Dionex AS ICS3000). Sulfate was measured according to turbidimetric method. Each test was performed in triplicate and the mean values were given with standard deviations.
Potential in anode and cathode chambers was on-line measured by Ag/AgCl reference electrodes (+0.197 V according to SHE). The voltage (V) across an external resistance (10 Ω) in the MFC circuit was on-line monitored at 5-min intervals using a four-channel precision multimeter (Fluke 8846A) connected to a personal computer.
Current (I) and power (P = IV) were calculated according to Ohm’s Law and normalized by the wetted surface area of anode (7 cm2) or the volume of liquid media in the anode chamber. Coulombic efficiency (CE) was calculated according to Sleutels et al. [31].
Result
Start-up period
The MFC operation was started at an influent COD concentration of 1 g/L and HRT of 2 days, corresponding to OLR of 0.5 g COD/L/day. There was no current generation during first week; thereafter, voltage increased steadily to 15 mV within 18 days (Fig. 1). During this period, COD removal efficiency was around 5 % within the first week and then steadily increased to around 20 % within 15 days. As the anaerobic conditions developed, the anode potential decreased and become more negative as it decreased from its initial value of around −100 to −230 mV within 15 days.
Impact of feed COD on MFC performance
The MFC was operated in a continuous mode of operation at room temperature (25 ± 2 °C) for around 65 days at different OLRs by changing hydraulic retention time (HRT) or feed COD concentration. The leachate was diluted with tap water to adjust its COD concentration to the desired values. Between days 0 and 16, HRT and feed COD were 2 days and 1,000 mg/L, respectively. The OLR was kept low in the first period to enrich anode-respiring bacteria on the electrode. Then, HRT was decreased to 1 day and feed COD concentration was increased gradually up to 50,000 mg/L. Hence, the OLR was increased from 0.5 g COD/L/day on day 16 to 50 g COD/L/day on day 34. Then, the impact of increasing OLR on the MFC performance was evaluated by keeping the feed COD constant at 50,000 mg/L and decreasing HRT from 1.0 to 0.25 day. On day 55, to recover the MFC performance, the HRT was increased from 0.25 day back to 0.5 day (Fig. 2).
The impact of increasing feed COD concentration from 1,000 to 50,000 mg/L (HRT 1 day) on voltage generation was investigated between days 15 and 40. COD removal efficiency increased steadily and reached 35–40 %. As the feed COD concentration increased, the cell voltage increased from around 15 mV on day 15 to 60 mV on day 40. Similarly, the current density and power output reached 8.7 A/m2 and 515 mW/m2 (1,350 mW/m3), respectively, on day 40 (Fig. 2). Throughout the study, sulfate reduction efficiency averaged 15 ± 5 %.
Impact of HRT on MFC
After day 40, the impact of decreasing HRT on the MFC performance was evaluated. Decreasing the HRT from 1 day to 0.75 between days 42 and 48, corresponding to OLR of 67 g COD/L/day, did not adversely affect the COD removal efficiency as it remained between 35 and 40 %. The cell voltage and power output increased to around 70 mV and 720 mW/m2 (or 1,920 mW/m3) respectively. Similarly, current density increased to around 10 A/m2. Between days 48 and 52, HRT was decreased to 0.5 day, corresponding to OLR of 100 g COD/L/day. The COD removal efficiency averaged around 43 % and the cell voltage increased slightly around 77 mV. The current density and the power output also slightly increased to around 11 A/m2 and 900 mW/m2 (or 2,250 mW/m3). Hence, the reactor performed well even at OLR of 100 g COD/L/day.
Further decreasing HRT to 0.25 day (OLR 200 g COD/L/day) adversely affected system performance as the COD removal efficiency sharply decreased to below 5 %. Similarly, cell voltage and current decreased sharply close to zero (Fig. 2). In order to recover the process performance, HRT was increased back to 0.5 day on day 55. Then, the COD removal efficiency increased again to around 40 %. Similarly, current and power densities increased back to around 10 A/m2 and 700 mW/m2 (1,815 mW/m3), respectively.
Impact of OLR on coulombic efficiency (CE) and power generation
The impact of OLR on the MFC performance was investigated by changing the feed COD and HRT. The impact of OLR on the current and power densities is shown in Fig. 3. The maximum current and power densities throughout the study were 11 A/m2 and 900 mW/m2 (or 2,250 mW/m3), respectively (Fig. 3). The current and power densities increased linearly up to OLR of 67 g COD/L/day and then remained constant when the OLR was increased to 100 g COD/L/day. Further increase of the OLR to 200 g COD/L/day caused a sharp decrease in power production.
The CE is very important in electricity generation as it is the ratio of the electrons used for the current generation. As the OLR was increased from 1 to 5 g COD/L/day, the CE decreased sharply from around 57 to 7 % and further increase of OLR caused a sharp decrease of CE to below 1 % (Fig. 4). Although the increase of OLR increased the voltage output, it decreased CE, which may be due to use of organics for non-electricity generating processes such as methane production.
Discussion
Electricity generation from landfill leachate
High-strength wastewaters are good source for MFCs as both wastewater treatment and electricity generation can be achieved simultaneously. Lu et al. [32] showed that starch processing wastewater with 4,852 mg/L COD could be used for electricity generation in an MFC with a maximum voltage of 239.4 mV/m2 (a current density of 893.3 mA/m2) corresponding to COD removal efficiency and maximum coulombic efficiency of 98 and 8 %, respectively. Wen et al. [33] studied electricity generation in an MFC fed with brewery wastewater in a continuous mode of operation. The maximum voltage and COD removal efficiency were 264 mW/m2 and 40–43 %, respectively. The maximum coulombic efficiency was close to 20 %. In an MFC with graphite electrode, the maximum power density and COD removal efficiency were 344 mW/m3 and 37 %, respectively, when landfill leachate was used [26]. The coulombic efficiency was very low (below 2 %) indicating the loss of substrate in non-electricity-generating processes. In spite of the low coulombic efficiency, the power density was higher than that observed in studies conducted with municipal wastewater due to high organic content of landfill leachate [26]. In our study, much higher power density values (900 mW/m2 or 2,250 mW/m3) were reached, which should be due to higher influent COD concentration (50 g/L), high biodegradability of young landfill leachate and continuous mode of MFC operation. In the study of Kiely et al. [17], similar power density (835 mW/m2) was obtained in an MFC fed with 1 g/L acetate. Although landfill leachate used in our study contained much higher COD than 1 g/L acetate, the possible reasons for obtaining similar power outputs are the lower biodegradability of leachate due to the presence of inhibitory compounds (heavy metals, Table 1) and the presence of other electron acceptors [nitrate, sulfate (Table 1)] in landfill leachate. Also, it is known that non-fermentable compounds, especially acetate, are more efficient for power generation in MFCs [7]. The obtained maximum COD removal rate in the present study was quite high, around 40 g COD/L/day, which points out that MFC can be used for both treatment of high-strength wastewaters and electricity generation.
Power generation increased with increasing the feed COD concentration and decreasing HRT up to OLR of 100 g COD/L/day (Fig. 2). Greenman et al. [6] studied electricity generation from landfill leachate using a continuous flow MFCs. In their study, the COD and BOD5 concentrations of the leachate were in the range of 12,900–35,300 and 5,800–18,000 mg/L, respectively. The high BOD5/COD ratio of 0.34–0.57 showed relatively high biodegradability. They reported that the decrease of HRT and increase of leachate strength increased the power output. The highest power density was 1.38 mW/m2 obtained with full-strength leachate and the BOD5 removal efficiency was lower than 35 %. The obtained maximum power density in our study (900 mW/m2) is much higher although the organic removal efficiencies are comparable. Obtaining much lower power output in the study of Greenman et al. [6] may be due to loss of a significant fraction of COD in non-electricity generating processes. Oxygen diffusion from air-cathode system, denitrification, biomass production and methane production may be alternative ways of organic consumptions.
Impact of loading rate on coulombic efficiency
The use of MFC for electricity generation is challenging due to generating combustion-less and pollution-free bioelectricity directly from organic matters [34]. The use high-strength wastewaters in MFC seems to be a good approach as the power generation per volume of the wastewater can be increased and at the same time wastewater treatment can be achieved. Hamelers et al. [35] reported that the coulombic efficiency should be at least 80 % for an MFC to be competitive with anaerobic digestion. However, studies showed that increasing the organic concentration in wastewater may cause decrease of CE [31], which means most of the electrons produced from organic oxidation are diverted to non-electricity-generating processes. Sleutels et al. [31] reported that both increasing the influent acetate concentration and decreasing anode potential might decrease the CE. Increase in substrate concentration from 1 to 35 mM increased current density to 21.1 A/m2, while decreased CE to 52 %. In our study, although increasing OLR, either decreasing HRT or increasing strength of leachate, generated higher power output (Fig. 2); it decreased CE sharply (Fig. 4). The possible reasons for decrease in CE at higher OLRs are increasing sulfate reduction or methane production at high OLRs. The decrease of anode potential at higher OLRs may produce better environmental conditions for non-electricity-generating processes (sulfate reduction or methane production). Sleutels et al. [31] reported that decreasing anode potential from −250 to −450 mV decreased both current density and CE appreciably. Similarly, Min et al. [8] reported that increasing initial COD concentration of swine wastewater in MFC resulted in decrease of coulombic efficiency. Swine wastewater with 8,320 mg/L COD produced maximum power density of 45 and 261 mW/m2 in a two-chambered and single-chambered MFCs, respectively, although the coulombic efficiency was quite low (8 %). In another study, Sharma and Li [18] used different carbon sources (acetate, ethanol, and glucose) in single-chambered MFCs. They reported that the CE values changed dramatically with the substrate types and acetate exhibited highest CE value among the studied substrates. The CE decreased from 38 % at the initial acetate concentration of 0.5 mM to 6 % at 8 mM and to 3 % at 35 mM. The inverse relationship between OLR and CE may impede the production of high power density with high-strength wastewater and more studies are needed to increase the power output and CE from high-strength wastewaters.
The COD removal efficiency reached 45 % at full-strength leachate. In real MFC applications for power generation from high-strength wastewater, the remaining COD should be treated by anaerobic and/or aerobic processes.
Conclusion
Two-chambered continuously fed MFC with new Ti–TiO2 electrodes was able to generate electricity and simultaneously treat landfill leachate with a maximum COD removal efficiency of 45 % and current production of 11 A/m2. Although increasing loading rate up to 100 g COD/L/day increased current generation, it decreased coulombic efficiency appreciably. It was found that Ti is suitable as an anode and cathode material in MFC application due to its stability and the formation of ohmic contact between TiO2 and Ti.
References
Renou S, Givaudan JG, Poulain S, Dirassouyan F, Moulin P (2008) Landfill leachate treatment: review and opportunity. J Hazard Mater 150:468–493
Christensen TH, Kjeldsen P, Bjerg PL, Jensen DL, Christensen JB, Baun A, Albrechtsen HJ, Heron G (2001) Biogeochemistry of landfill leachate plumes. Appl Geochem 16(7–8):659–718
Mangimbulude JC, Van Breukelen BM, Krave AS, Van Straalen NM, Roling WFM (2009) Seasonal dynamics in leachate hydrochemistry and natural attenuation in surface run-off water from a tropical landfill. Waste Manag 29:829–838
Ozkaya B, Demir A, Bilgili MS (2006) Soluble substrate concentrations in leachate from field scale MSW test cells. J Hazard Mater 134(1–3):19–26
Gotvajn AZ, Tisler T, Zagorc-Koncan J (2009) Comparison of different treatment strategies for industrial landfill leachate. J Hazard Mater 162:1446–1456
Greenman A, Gálvez L, Giusti I, Ieropoulos (2009) Electricity from landfill leachate using microbial fuel cells: comparison with a biological aerated filter. Enzyme Microb Technol 44:112–119
Lee HS, Parameswaran P, Kato-Marcus A, Torres CI, Rittmann BE (2008) Evaluation of energy-conversion efficiencies in microbial fuel cells (MFCs) utilizing fermentable and non-fermentable substrate. Water Res 42:1501–1510
Min B, Kim JR, Oh SE, Regan JM, Logan BE (2005) Electricity generation from swine wastewater using microbial fuel cells. Water Res 39:4961–4968
Logan BE, Min B (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7:375–381
Logan BE (2007) Microbial fuel cells, Wiley-Interscience, Wiley, New York, ISBN 978-0-470-23948-3
Torres CI, Marcus AK, Lee H-S, Parameswaran P, Krajmalnik-Brown R, Rittmann BE (2010) A kinetic perspective on extracellular electron transfer by anode-respiring bacteria. FEMS Microbiol Rev 34:3–17
Chae KJ, Choi MJ, Lee JW, Kim KY, Kim IS (2009) Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour Technol 100(14):3518–3525
Logan BE, Hamelers B, Rozendal R, Schroder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2007) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Zhuang L, Zheng Y, Zhou S, Yuan Y, Yuan H, Chen Y (2012) Scalable microbial fuel cell (MFC) stack for continuous real wastewater treatment. Bioresour Technol 106:82–88
Kim JR, Jung SH, Regan JM, Logan BE (2007) Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour Technol 98:2568–2577
Pant D, Bogaert GV, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol 101(6):1533–1543
Kiely PD, Rader G, Regan JM, Logan BE (2011) Long-term cathode performance and the microbial communities that develop in microbial fuel cells fed different fermentation endproducts. Bioresour Technol 102(1):361–366
Sharma Y, Li B (2010) The variation of power generation with organic substrates in single-chamber microbial fuel cells (SCMFCs). Bioresour Technol 201:1844–1850
Tugtas AE, Cavdar P, Calli B (2011) Continuous flow membrane-less air cathode microbial fuel cell with spunbonded olefin diffusion layer. Bioresour Technol 102:10425–10430
Cercado-Quezada B, Delia ML, Bergel A (2010) Testing various food-industry wastes for electricity production in microbial fuel cell. Bioresour Technol 101(8):2748–2754
Wang X, Feng YJ, Lee H (2008) Electricity production from beer brewery wastewater using single chamber microbial fuel cell. Water Sci Technol 57(1):1117–1121
Velasquez-Orta SB, Head IM, Curtis TP, Scott K (2011) Factors affecting current production in microbial fuel cells using different industrial wastewaters. Bioresour Technol 102(8):5105–5112
Pant D, Bogaert GV, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol 101(6):1533–1543
Galves A, Greenman J (2009) Ieropoulos landfill leachate treatment with microbial fuel cells; scale-upthrough plurality. Bioresour Technol 100(21):5081–5091
You SJ, Zhao QL, Jiang Q, Zhang JN, Zhao SQ (2006) Sustainable approach for leachate treatment: electricity generation in microbial fuel cell. J Environ Sci Health A Tox Hazard Subst Environ Eng 41(12):2721–2734
Puig S, Serra M, Coma M, Cabréa M, Balaguer MD, Colprim J (2011) Microbial fuel cell application in landfill leachate treatment. J Hazard Mater 185(2–3):763–767
Logan BE (2004) Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ Sci Technol 38:5809–5814
Morris JM, Jin S, Wang JQ, Zhu CZ, Urynowicz MA (2007) Lead dioxide as an alternative catalyst to platinum in microbial fuel cells. Electrochem Commun 9:1730–1734
Ozkaya B, Akoğlu B, Karadag D, Aci G, Taksan E, Hasar H, Bioelectricity production using a new electrode material in microbial fuel cell, Bioprocess and Biosystems Engineering (2012), doi:10.1007/s00449-012-0709-1
APHA (2005) Standard methods for the examination of water and wastewater, American public health association, American water works association, water environmental federation, 21st edn. Washington, USA
Sleutels THJA, Hamelers HVM, Buisman CJN (2011) Effect of operational parameters on coulombic efficiency in bioelectrochemical systems. Bioresour Technol 102:11172–11176
Lu N, Zhou SG, Zhuang L, Zhang J, Ni J (2009) Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochem Eng J 43:246–251
Wen Q, Wu Y, Cao D, Zhao L, Sun Q (2009) Electricity generation and modeling of microbial fuel cell from continuous beer brewery wastewater. Bioresour Technol 100:4171–4175
Rittmann BE (2008) Opportunities for renewable bioenergy using microorganisms. Biotechnol Bioeng 100:203–212
Hamelers HVM, ter Heijne A, Stein N, Rozendal RA, Buisman CJN (2011) Butler-Volmer-Monod model for describing bio-anode polarization curves. Bioresour Technol 102:381–387
Acknowledgments
The authors gracefully acknowledge the financial support of TÜBİTAK, Project no. 109Y269.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özkaya, B., Cetinkaya, A.Y., Cakmakci, M. et al. Electricity generation from young landfill leachate in a microbial fuel cell with a new electrode material. Bioprocess Biosyst Eng 36, 399–405 (2013). https://doi.org/10.1007/s00449-012-0796-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0796-z