Abstract
High tolerance towards ethanol is a desirable property for the Saccharomyces cerevisiae strains used in the alcoholic beverage industry. To improve the ethanol tolerance of an industrial Chinese rice wine yeast, a sequential batch fermentation strategy was used to adaptively evolve a chemically mutagenized Chinese rice wine G85 strain. The high level of ethanol produced under Chinese rice wine-like fermentation conditions was used as the selective pressure. After adaptive evolution of approximately 200 generations, mutant G85X-8 was isolated and shown to have markedly increased ethanol tolerance. The evolved strain also showed higher osmotic and temperature tolerances than the parental strain. Laboratory Chinese rice wine fermentation showed that the evolved G85X-8 strain was able to catabolize sugars more completely than the parental G85 strain. A higher level of yeast cell activity was found in the fermentation mash produced by the evolved strain, but the aroma profiles were similar between the evolved and parental strains. The improved ethanol tolerance in the evolved strain might be ascribed to the altered fatty acids composition of the cell membrane and higher intracellular trehalose concentrations. These results suggest that adaptive evolution is an efficient approach for the non-recombinant modification of industrial yeast strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Saccharomyces cerevisiae is the most exploited and primary microbe used in industrial fermentation processes such as the brewing of alcoholic beverages, ethanol production, and bread manufacture. The enological characteristics of the different yeast strains greatly affect their fermentation performance.
Chinese rice wine, a traditional alcoholic beverage, is produced from glutinous rice by a simultaneous saccharification and fermentation process using “wheat Qu” as the saccharifying agent and S. cerevisiae as the fermentation starter [1–3]. This process protects yeast cells from high concentrations of sugar, and contributes to high ethanol production, which can be over 20 % (v/v) in the final fermentation mash without distillation [3]. However, high concentrations of ethanol are highly toxic to yeast growth and metabolism, which is one of the major reasons for delay or sluggishness in a culture [3]. Therefore, to improve Chinese rice wine production, it is essential to breed yeast strains with high ethanol tolerance.
Recently, many studies have indicated that ethanol tolerance is associated with the interplay of complex networks at the genome level [4]. However, the use of genetically modified organisms is poorly accepted by consumers and is also limited by many legal restrictions, especially in the food industry. Therefore, an adaptive evolution approach (a non-genetic engineering process) has been used as an effective strategy for the selection of non-genetically modified (non-GM) strains with specific phenotypes [5]. This approach has attracted a lot of interest from the food and alcoholic beverage industry [6–9]. Adaptive evolution defines a set of mutations that occur in response to a specific challenge and that are advantageous to some mutants under conditions in which an increase of fitter variants occurs because of natural selection [5, 10–12].
To the best of our knowledge, only four studies have reported the use of this non-genetic engineering approach to improve ethanol tolerance in yeast [13–16]. Brown and Oliver [13] were the first to report the isolation of yeast mutants with increased tolerance to ethanol by a continuous selection technique. Dinh et al. [14] investigated the adaptation process of a laboratory S. cerevisiae for a high level of ethanol tolerance and confirmed the physiological changes in the adapted strain compared with in the non-adapted strain. Stanley et al. [15] generated two yeast mutants with increasing ethanol tolerance by subjecting mutagenized and non-mutagenized populations of the parental strain to adaptive evolution employing extraneous ethanol as the selection pressure. These yeast mutants had higher growth rates than the wild type when grown in sublethal ethanol concentrations. In these studies, the adaptive evolution experiments were carried out based on the long-time cultivation of parental strains in a stressful medium produced by adding extraneous ethanol to the initial medium. In an actual industrial fermentation process however, ethanol stress is not present at the beginning of fermentation. Therefore, industries are more interested in increasing the ethanol tolerance of yeast strains in the middle and late fermentation stages.
In the Chinese rice wine fermentation process, yeast cells die gradually as high concentrations of ethanol begin to accumulate in the medium. This process may have contributed to the unintentional adaptive evolution of contemporary yeast strains. In this study, we describe the creation of ethanol-tolerant mutants of S. cerevisiae using adaptive evolution in a Chinese rice wine-like fermentation environment. The high level of ethanol stress that occurs during the Chinese rice wine fermentation process was used as the selective pressure. Our hypothesis is that, at stressful ethanol concentrations, ethanol-tolerant variants will live longer than less tolerant variants in Chinese rice wine mash and will, therefore, dominate the fermentation mash during long-term cultivation.
Materials and Methods
Yeast Strain and Culture Medium
The Chinese rice wine yeast G85 strain (S. cerevisiae) used in this study was provided by the Guyuelongshan Wine Company (Shaoxing, China). The G85 strain was stored at 4 °C on slants of YPD (1 % yeast extract, 2 % glucose, 2 % peptone, 2 % agar; for the broth, agar was not included).
The adaptive evolution medium was prepared as follows: 4.0 kg cooked glutinous rice was mixed with 7 L water and 0.6 kg wheat Qu. Wheat Qu was used as a source of the saccharification enzymes. The mixture of rice, water, and wheat Qu was incubated at 60 °C for 4 h. During this time, the grains in the mixture were degraded by enzymes in the wheat Qu into sugars, peptides, and amino acids that can be used by the yeast. After 4 h saccharification, the mixture was filtrated with a filter cloth, and then filtrated again with filter paper. The sugar content of the filtrate was adjusted to 22 °Bx with water. This filtrate is the “sugar juice,” which, after sterilization by filtration (0.22 μm), was used in the chemical mutagenesis and adaptive evolution experiments described below. The major chemical composition of the sugar juice was as follows: sugar 207.25 g/L, α-amino nitrogen 0.283 g/L, pH 4.1.
Chemical Mutagenesis and Adaptive Evolution Experiments
To increase the genetic diversity in the starting G85 populations of the adaptive evolution experiments, the chemical mutagen ethyl methane sulfonate (EMS) was used [17]. The G85 strain was grown in YPD medium at 28 °C for 20 h and harvested by centrifugation (5,000 × g), then washed, and suspended in 0.1 M sodium phosphate buffer (pH 7.0). EMS (75 μL) was added to 5.0 mL of the yeast suspension (about 4 × 108 cells/mL) and the mixture was incubated at 25 °C for 60 min. The EMS mutagenesis reaction was stopped by adding 20 mL of 50 g/L sodium thiosulfate. The mutagenized yeast cells were harvested by centrifugation and washed twice with sodium thiosulfate (50 g/L), then resuspended in sterile saline water (9 g/L). The yeast suspension was cultured in sugar juice medium for 24 h at 28 °C and used as the seed culture for the adaptive evolution experiments.
The experiments were based on long-term serial batch transfer fermentation. The seed culture of the mutagenized G85 strain was used to inoculate two 1 L flasks (labeled AE1 and AE2), each containing 500 mL sugar juice medium. These flasks were incubated statically at 30 °C for 48 h, then 65 g (130 g/L) sterilized glucose (dry heat sterilization at 105 °C for 5 h) was fed to each flask to continue the fermentation. After another 48 h of fermentation, the flasks were transferred to 18 °C and maintained at post-fermentation until the proportion of living yeast cells was lower than 1 %. Then, 10 mL of each culture was used to inoculate new flasks with fresh medium. The new flasks were cultivated as described above. This serial transfer was repeated 20 times (approximately 240 generations). During the course of this adaptive evolution process, samples of the evolving populations were taken approximately every 40 generations and maintained in a glycerol stock (30 % glycerol) at −80 °C. These mixed cultures were rescued in YPD medium at 28 °C for 24 h before further analysis.
Isolation of Ethanol-Tolerant Mutants
The fermentation performances of the mixed cultures taken at different times during the evolution process were compared with performance of the parental G85 strain by batch fermentation in glass flasks (1 L) with fermentation bungs on the top. The rescued mixed cultures of the evolved strains and the parental G85 strain were inoculated into flasks with 500 mL sugar juice medium at a cell density of approximately 1 × 106 CFU/mL. The fermentation procedure was carried out with one cycle of the adaptive evolution cultivation process described above. Duplicate fermentations of the parental strain and the mixed cultures were conducted. The concentrations of ethanol and residual sugar, and yeast death rate were measured after 20 days of fermentation.
Based on the batch fermentation results, single-colony clones were isolated by dilution from the mixed cultures with the lower yeast death rates, spread, and incubated on YPD plate. The fermentation performances of these isolates were compared with the performance of the parental G85 strain by batch fermentation. Yeast strains were first inoculated into 10 mL YPD and grown at 28 °C for 24 h. Batch fermentations were conducted by inoculating these strains into 500 mL sugar juice medium at 4 × 106 cells/mL. The fermentations were performed as batch fermentations of one cycle of the adaptive evolution cultivation process described above. Duplicate fermentations of the parental strain and the evolved strains were conducted. The fermentation rate, expressed as CO2 gram per liter loss in the first 72 h of fermentation, was determined as described previously [18, 19]. The concentrations of ethanol and residual sugar, and yeast death rate were measured after 20 days of fermentation.
Stress Tolerance Analyses
Yeast strains were inoculated into 20 mL YPD medium and grown to the stationary phase at 30 °C, then collected by centrifugation (5,000 × g for 5 min at 4 °C), and washed with sterile saline water. For the ethanol tolerance analysis, the yeast cells were resuspended in 20 mL 0.1 M, pH 4.0 lactic acid buffer containing 18 % (v/v) ethanol and 1 % glucose at 2 × 108 cells/mL. The suspension was incubated statically at 30 °C for 7 days and the yeast death rate was analyzed. For the osmotic stress tolerance analysis, the yeast cells were resuspended in sterile saline water and adjusted to OD600 = 1. The suspension was 10-fold serially diluted and spotted (5 μL) on YPD plates containing NaCl (1.0 mol/L) and different concentrations of sorbose (1.0, 1.5, and 2.0 mol/L). After incubation at 30 °C for 4 days, stress tolerance was measured by comparing the colony size or the proportions of viable cells in the cultures of the parental and evolved strains. For the temperature stress tolerance analysis, the yeast cells were resuspended in sterile saline water and adjusted to OD600 = 1. The suspension was incubated in a water bath at 50 °C for 5 or 10 min, and then serially diluted and spotted on YPD plates. Colony growth was inspected after 2–5 days of incubation at 30 °C. Duplicate experiments were conducted for all the stress tolerance treatments.
Fatty Acid and Trehalose Analyses
Yeast cells were cultivated in 20 mL 22 °Bx sugar juice at 30 °C for 24 h without shaking. Then, the cells were harvested by centrifugation (5,000 × g for 5 min at 4 °C) and washed with sterile saline water (9 g/L). Total fatty acid was extracted as described previously [20, 21]. Heptadecanoic acid was used as an internal standard, and the extract was analyzed by gas chromatography. Trehalose content in the yeast cells was determined by the anthrone method [22] and expressed as milligram per gram dry cells weight (DCW).
Laboratory-Scale Chinese Rice Wine Fermentation
Laboratory-scale Chinese rice wine brewing was carried out as described previously [23]. Pre-cultured yeast cells were harvested and mixed with 1,000 g steamed glutinous rice, 160 g wheat Qu, and 700 mL water in a 2-L jar. The inoculum concentration of yeast cells was about 2 × 107 CFU/ml. The fermentation mash was incubated at 30 °C for 4 days (the main fermentation) then at 18 °C for 16 days (the post-fermentation). The experiments were carried out in duplicate. The ethanol and residual sugar levels, and the yeast death rate were monitored during the Chinese rice wine fermentation process. At the end of the fermentation, the mash was centrifuged and the supernatant (Chinese rice wine) was analyzed. The chemical composition of the fresh Chinese rice wine was analyzed as reported previously [23].
Analytical Methods
Ethanol, residual sugar, and glycerol were determined by high-performance liquid chromatography (HPLC). The yeast death rate was determined by staining with methylene blue. The pH was measured using a pH meter. For the yeast DCW analysis, 5 mL of the sugar juice culture was centrifuged (8,000 × g for 10 min), washed twice with distilled water, and dried at 105 °C until a constant weight was achieved. Volatile aroma compounds in the Chinese rice wine were analyzed by headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography–mass spectrometry (GC-MS) as described previously [23]. A sensory evaluation of Chinese rice wine samples was carried out by a group of trained panelists according to the method described previously [24, 25]. Seven aroma terms was selected for the descriptive analysis of the Chinese rice wine. The sensory data were analyzed by one-way analysis of variance (ANOVA).
Statistical Analysis
Statistical analysis of the data was accomplished using one-way ANOVA with Tukey’s honestly significant difference test (p < 0.05) using the SPSS 15.0 software (SPSS Inc., Chicago, Illinois, USA).
Results
Adaptive Evolution and Isolation of Ethanol-Tolerant Yeast Strains
To generate yeast strains with enhanced ethanol tolerance, the industrial Chinese rice wine yeast G85 strain was subjected to adaptive evolution through sequential batch fermentation under Chinese rice wine-like fermentation conditions.
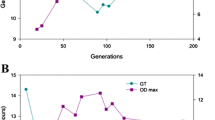
The long-term serial batch fermentation suspensions were sampled approximately every 40 generations. The fermentation performances of the mixed cultures taken at different generation times were compared with the performance of the parental G85 strain by batch fermentation. The results showed that after about 80 generations of adaptive evolution, the yeast death rate began to reduce, indicating that adaptive mutation(s) had begun to accumulate (Fig. 1). After 200 generations of adaptive evolution, the yeast death rates in the evolving populations was lower than 50 %, and more than 90 % in the parental strain population (Fig. 1). Residual sugar levels in the fermentations carried out using the mixed cultures from the 200th and 240th generations were below 8.0 g/L, lower than the result for the parental strain (10.3 g/L). These results indicated that the evolved mutants were better able to complete the fermentation process.
Fermentation performances of the parental G85 strain and adaptively evolving mixed yeast cell cultures (AE1 and AE2) sampled every 40 generations. Fed-batch fermentation was carried out in 1 L flasks containing 500 mL sugar juice medium with 130 g/L glucose fed at 48 h. Yeast cell death rate was determined by staining with methylene blue after 20 days fermentation
Single-cell colonies were isolated from mixed cultures of the 200th and 240th evolved generations. The ethanol tolerance analysis showed that all the evolved mutants had better tolerance to high concentrations of ethanol than the parental strain (Fig. 2). Apart from ethanol tolerance, yeast strains for industrial use should maintain other important fermentation traits. Nine mutant strains were selected to test for other fermentation parameters by batch fermentation (Table 1). No statistical differences between the fermentation rate and the level of ethanol production were found among the selected strains, showing that the evolved strains had maintained the fermentation traits of the parental strain after the long-term adaptive evolution process. However, the residual sugar levels after fermentation showed clear differences among the evolved strains. The lowest concentrations of residual sugar were found in mutant strains G85X-5 (7.35 g/L) and G85X-8 (7.03 g/L), while the highest levels of residual sugar were found in mutant strains G85X-1 (15.36 g/L) and G85X-2 (13.12 g/L). Based on a comprehensive comparison of the fermentation parameters, the G85X-8 and G85X-5 strains were selected for further investigation.
Multi-stress Resistance of Evolved Mutants
Apart from the high levels of ethanol, yeast cells are also exposed to several other stresses including high osmotic and high temperature during the Chinese rice wine fermentation process. After the long-term adaptive evolution process, the tolerance of mutants G85X-8 and G85X-5 for other stresses was investigated. Better growth status was found for the G85X-8 and G85X-5 strains on YPD plates containing different concentrations of sorbose (Fig. 3). For the parental G85 strain, no growth was observed on YPD plates containing 1.0 mol/L NaCl, while clear colonies were seen for the G85X-8 and G85X-5 strains. Of these three yeast strains, G85X-8 displayed the best osmotic stress tolerance. The heat shock test was carried out for the G85X-8 and G85 strains (Fig. 4). The G85 strain showed poor growth after 5 min and no growth after 10 min of heat shock treatment at 55 °C. However, the G85X-8 strain grew well even after 10 min of heat shock treatment at 55 °C. These results indicated that the evolved mutants not only showed increased ethanol tolerance but also osmotic and temperature tolerance compared with the parental strain.
Determination of osmotic stress tolerance of strains G85, G85X-5, and G85X-8. Yeast cells were grown in YPD medium at 30 °C to the stationary phase. Tenfold serial dilutions of each sample were spotted onto a YPD plate containing different concentrations of sorbose or NaCl. Stress tolerance was measured by comparing the colony size or viable cells among the different strains in the stressed cultures
Determination of heat shock tolerance of strains G85 and G85X-8. Yeast cells were grown in YPD medium at 30 °C to the stationary phase, then were incubated at 50 °C in a water bath for 5 or 10 min. Tenfold serial dilutions of each sample were spotted onto a YPD plate. Stress tolerance was measured by comparing the colony size or viable cells among the different strains in the stressed cultures
Characterization of the Ethanol-Tolerant Yeast Strains
The cell plasma membrane is the major point at which ethanol can affect the activity of the yeast cell. Under ethanol stress conditions, yeast cells can change the composition of the membrane to adjust membrane fluidization and stabilize the plasma membrane [26–28]. Therefore, the fatty acid composition of the plasma membrane in the evolved mutant and parental strains was analyzed (Table 2). No statistical differences in the total fatty acid content of the plasma membranes were found between the parental and evolved strains; however, there were clear differences in the fatty acid composition of the plasma membranes between the strains. The evolved strains had markedly higher proportions of oleic acid and linoleic acid and lower proportions of myristic acid than the parental strain. The ratio of C18/C16 fatty acids was also higher in the plasma membranes of the evolved strains.
The correlation between trehalose content and stress resistance has been reported for a variety of stresses, especially ethanol stress [29, 30]. For the strains grown in sugar juice medium, the amounts of trehalose in the mutant G85X-8 and G85X-5 strains were 85.62 and 68.53 %, respectively, higher than of the amount in the parental strain (Fig. 5). These results indicate that the evolved mutant yeast strains might accumulate trehalose in their cells as a protective response to ethanol stress.
Laboratory-Scale Chinese Rice Wine Fermentation with Evolved Yeast Strain
Laboratory-scale Chinese rice wine fermentation was conducted using the G85 and G85X-8 strains to verify the fermentation performance of the evolved strain. Residual sugar concentrations, ethanol production, and yeast death rate were monitored during the fermentation process (Fig. 6). Similar variations in residual sugar and ethanol were found for both strains, indicating that the evolved G85X-8 strain maintained the important fermenting properties of the parental strain during the Chinese rice wine fermentation process. Slightly lower residual sugar concentrations and higher ethanol levels were found for the G85X-8 strain after 100 h fermentation, indicating the evolved strain may be better able to complete fermentation than the parental strain. During the fermentation process, the yeast death rate of the parental strain increased quickly after 3 days fermentation and was higher than 80 % after 14 days fermentation, while the cell viability of the evolved G85X-8 strain was stable after 14 days when the yeast dead rate was below 20 %.
General oenological parameters of fresh Chinese rice wine were analyzed after fermentation (Table 3). No statistical differences for most of the general oenological parameters of the Chinese rice wine samples fermented by either the G85X-8 or G85 strains were found. The exceptions were residual sugar and α-amino nitrogen, which were significantly lower in the Chinese rice wine sample fermented with the evolved G85X-8 strain (4.13 and 0.49 g/L, respectively) than in the sample fermented with the parental strain (6.71 and 0.58 g/L, respectively). The levels of the major aroma compounds, which correlated with the yeast metabolism, were analyzed in the Chinese rice wine samples produced by the evolved and parental yeast strains (Table 4). In general, the differences between the samples were small. The higher alcohols (1-propanol, 2-methylpropanol, and 3-methylbutanol) were about 14 % lower in Chinese rice wine samples made by the G85X-8 strain. Acetic acid and ethyl acetate were roughly 22 and 14 % higher, respectively, in Chinese rice wine made with the G85 strain compared with Chinese rice wine made with the G85X-8 strain, while the concentration of 3-methylbutyl acetate was about 19 % higher in Chinese rice wine made by the G85X-8 strain compared with Chinese rice wine made by the G85 strain. Although there were differences between the concentrations of some of the major aroma compounds, no significant sensory differences were observed between the G85X-8 and G58 strains (data not shown).
Discussion
The high level of ethanol produced during alcohol fermentation was used as a selective pressure to trigger adaptive evolution in the parental yeast G85 strain. Most of the yeast mutants that were obtained showed enhanced ethanol tolerance compared with the parental strain after 200 generations of adaptive evolution. A mutant G85X-8 strain was selected for its markedly enhanced ethanol tolerance and fermentation characteristics. A laboratory-scale Chinese rice wine fermentation process confirmed the improved ethanol tolerance of the G85X-8 strain compared with the parental strain, and no significant changes were observed in the profile of the yeast-derived aroma compounds in the wine produced with the mutant strain.
The generation of ethanol-tolerant yeast mutants using adaptive evolution strategy has been described previously [13–16]. In all of these reports, extrinsic ethanol was used as the selective pressure in the initial adaptive medium. In our study, the high level of ethanol that was produced during fermentation was used as the selective pressure, which is closer to the real environment during the Chinese rice wine fermentation process. By monitoring the adaptive evolution process, we found that after about 80 generations, the advantageous mutants began to accumulate. The rate of evolution achieved in this study was faster than the rates reported previously, where more than 150 generations were required for adaptation to occur [6, 9]. The EMS mutagenesis treatment of the parental strain before beginning the adaptive evolution process may be the reason for the higher adaptive efficiency obtained in our study. The rate of evolution is determined by mutation rate, population size, and the strength of selection. Adaptive evolution can be accelerated by increasing the genetic variation in the starting culture [5]. Our results are in good agreement with the results of Stanley [15] who reported that the adaptive evolution of mutagenized yeast cultures required considerably less time to generate ethanol-tolerant mutants than the non-mutagenized cultures.
In our study, after 200 generations of adaptive evolution, all the evolved strains showed significantly increased ethanol tolerance, indicating that these strains were likely to be better adapted to the stressful Chinese rice wine fermentation environment. Interestingly, the ethanol-tolerant evolved yeast strains also showed increased tolerance to high osmotic and temperature stresses. These characteristics will be highly prized by Chinese rice wine-makers because high osmotic pressure and temperature are common stresses faced by yeast cells during Chinese rice wine fermentation. A similar result was reported by Aguilera et al. [7], who isolated a freeze-tolerant industrial baker’s yeast by adaptive evolution in a dough-like environment that also showed a progressive increase in salt tolerance.
We found that some physiological characteristics changed between the parental and evolved strains; for example, the higher ethanol-tolerant strains had higher ratios of C18 to C16 fatty acids and higher oleic acid (C18:1) content in their plasma membranes than the parental strain. The results from this study correlated well with the results of Tao et al. [31], but not with the results reported by Dinh et al. [14] who found that the palmitic acid content rather than oleic acid content changed after adaptation. Higher proportions of unsaturated fatty acids, especially oleic acid, in the plasma membrane might be the principal mechanism used by yeast to adapt to the presence of ethanol [32]. In the present study, for fatty acids analysis, the yeast cells were harvested at the early stationary stage when there was no obvious ethanol stress, suggesting that the observed differences in fatty acids composition between the evolved and parental strains were present even before the cells became ethanol-stressed. These results suggest that the fatty acids composition of the plasma cell membrane of the evolved strains changed after the long-time adaptive evolution process.
The present work introduces an efficient tool for food industrial strain breeding. To the best of our knowledge, this report is the first to generate ethanol-tolerant yeast strains using an adaptive evolution strategy under an industrial fermentation-like environment. The evolved strains obtained in this study can be used directly in the Chinese rice wine industrial. Our results show that the stresses generated during fermentation are useful selective pressures for adaptive evolution. This proposed strategy could also be applied to other trait improvements or to other microorganisms. However, without using genome analysis techniques, the mutations responsible for the improved ethanol tolerance of the evolved variants could not be identified and further work is required to determine the underlying mechanisms involved.
References
Xie, G. F., Li, W. J., Lu, J., Cao, Y., Fang, H., Zou, H. J., et al. (2007). Journal of the Institute of Brewing, 113, 272–279.
Xu, Y., Wang, D., Fan, W. L., Mu, X. Q., & Chen, J. A. (2010). In G. T. Tsao, P. Ouyang, & J. Chen (Eds.), Traditional Chinese biotechnology (pp. 189–233). Berlin: Springer-Verlag Berlin.
Zhou, J. Q. (1996). Chinese rice wine brewing process. Beijing: China light industry Press.
Ma, M. G., & Liu, Z. L. (2010). Applied Microbiology and Biotechnology, 87, 829–845.
Chambers, P. J., Bellon, J. R., Schmidt, S. A., Varela, C., & Pretorius, I. S. (2009). Non-genetic engineering approaches for isolating and generating novel yeasts for industrial applications. In T. Satyanarayana & G. Kunze (Eds.), Yeast biotechnology: Diversity and applications (pp. 433–457). Dordrecht: Springer.
McBryde, C., Gardner, J. M., Lopes, M. D., & Jiranek, V. (2006). American Journal of Enology and Viticulture, 57, 423–430.
Aguilera, J., Andreu, P., Randez-Gil, F., & Antonio Prieto, J. (2010). Microbial Biotechnology, 3, 210–221.
Cadière, A., Ortiz-Julien, A., Camarasa, C., & Dequin, S. (2011). Metabolic Engineering, 13, 263–271.
Kutyna, D. R., Varela, C., Stanley, G. A., Borneman, A. R., Henschke, P. A., & Chambers, P. J. (2012). Applied Microbiology and Biotechnology, 93, 1175–1184.
Çakar, Z. P., Turanlı-Yıldız, B., Alkım, C., & Yılmaz, Ü. (2012). FEMS Yeast Research, 12, 171–182.
Chen, C. Y., Tang, X. Y., Xiao, Z. Y., Zhou, Y. H., Jiang, Y., & Fu, S. W. (2013). Applied Biochemistry and Biotechnology, 169, 2362–2373.
Laluce, C., Schenberg, A. C. G., Gallardo, J. C. M., Coradello, L. F. C., & Pombeiro-Sponchiado, S. R. (2012). Applied Biochemistry and Biotechnology, 166, 1908–1926.
Brown, S. W., & Oliver, S. G. (1982). Applied Microbiology and Biotechnology, 16, 119–122.
Dinh, T., Nagahisa, K., Hirasawa, T., Furusawa, C., & Shimizu, H. (2008). PLoS ONE, 3, e2623.
Stanley, D., Fraser, S., Chambers, P. J., Rogers, P., & Stanley, G. A. (2010). Journal of Industrial Microbiology and Biotechnology, 37, 139–149.
Fiedurek, J., Skowronek, M., & Gromada, A. (2011). Polish Journal of Microbiology, 60, 51–58.
Amberg, D. C., Burke, D. J., & Strathern, J. N. (2006). Cold Spring Harbor Protocols, 2006: pdb.prot4180.
Nikolaou, E., Soufleros, E. H., Bouloumpasi, E., & Tzanetakis, N. (2006). Food Microbiology, 23, 205–211.
Pérez-Coello, M. S., Briones Pérez, A. I., Ubeda Iranzo, J. F., & Martin Alvarez, P. J. (1999). Food Microbiology, 16, 563–573.
Razes, N., Garcia-Jares, C., Larue, F., & Lonvaud-Funel, A. (1992). Journal of the Science of Food and Agriculture, 59, 351–357.
Beltran, G., Novo, M., Guillamon, J. M., Mas, A., & Rozes, N. (2008). International Journal of Food Microbiology, 121, 169–177.
Mahmud, S. A., Nagahisa, K., Hirasawa, T., Yoshikawa, K., Ashitani, K., & Shimizu, H. (2009). Yeast, 26, 17–30.
Chen, S., & Xu, Y. (2010). Journal of the Institute of Brewing, 116, 190–196.
Chen, S., Xu, Y., & Qian, M. C. (2013). Journal of Agricultural and Food Chemistry, 61, 11295–11302.
Chen, S., Wang, D., & Xu, Y. (2013). Journal of Agricultural and Food Chemistry, 61, 9712–9718.
Aguilera, F., Peinado, R. A., Millán, C., Ortega, J. M., & Mauricio, J. C. (2006). International Journal of Food Microbiology, 110, 34–42.
Alexandre, H., Rousseaux, I., & Charpentier, C. (1994). Biotechnology and Applied Biochemistry, 20, 173–183.
Mannazzu, I., Angelozzi, D., Belviso, S., Budroni, M., Farris, G. A., Goffrini, P., et al. (2008). International Journal of Food Microbiology, 121, 84–91.
Mansure, J. J. C., Panek, A. D., Crowe, L. M., & Crowe, J. H. (1994). Biochimica et Biophysica Acta (BBA) - Biomembranes, 1191, 309–316.
Piper, P. W. (1995). FEMS Microbiology Letters, 134, 121–127.
Tao, X., Zheng, D., Liu, T., Wang, P., Zhao, W., Zhu, M., et al. (2012). PLoS ONE, 7, e31235.
You, K. M., Rosenfield, C.-L., & Knipple, D. C. (2003). Applied and Environmental Microbiology, 69, 1499–1503.
Acknowledgments
This work was supported by grants from the National High Technology Research and Development Program of China (863 Program; no. 2013AA102108), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the 111 Project (No. 111-2-06), and the Jiangsu province "Collaborative Innovation Center for Advanced Industrial Fermentation" industry development program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., Xu, Y. Adaptive Evolution of Saccharomyces cerevisiae with Enhanced Ethanol Tolerance for Chinese Rice Wine Fermentation. Appl Biochem Biotechnol 173, 1940–1954 (2014). https://doi.org/10.1007/s12010-014-0978-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0978-z