Abstract
The development of new wine yeast strains with improved characteristics is critical in the highly competitive wine market, which faces the demand of ever-changing consumer preferences. Although new strains can be constructed using recombinant DNA technologies, consumer concerns about genetically modified (GM) organisms strongly limit their use in food and beverage production. We have applied a non-GM approach, adaptive evolution with sulfite at alkaline pH as a selective agent, to create a stable yeast strain with enhanced glycerol production; a desirable characteristic for wine palate. A mutant isolated using this approach produced 41% more glycerol than the parental strain it was derived from, and had enhanced sulfite tolerance. Backcrossing to produce heterozygous diploids revealed that the high-glycerol phenotype is recessive, while tolerance to sulfite was partially dominant, and these traits, at least in part, segregated from each other. This work demonstrates the potential of adaptive evolution for development of novel non-GM yeast strains, and highlights the complexity of adaptive responses to sulfite selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Constantly facing new demands in an overcrowded market place, the wine industry is involved in the ongoing development of yeast strains with desirable and stable phenotypes. To date the development of Saccharomyces cerevisiae strains for wine fermentations has largely focused on aspects of fermentation reliability including: the early initiation of fermentation, improving stress tolerance, and increasing fermentation efficiency (Pretorius 2000; Rainieri and Pretorius 2000). However, the wine industry is also very interested in the development of wine yeasts that generate wines with improved organoleptic properties (Pretorius 2000). Among other phenotypes, yeast strains with enhanced glycerol production are in demand, as this metabolite is recognized as having a favourable influence on wine by enhancing sweetness, smoothness and overall body (Eustace and Thornton 1987; Gawel et al. 2007; Noble and Bursick 1984).
There are several approaches available to achieve this end, mostly involving genetic engineering techniques, which can be implemented to divert yeast metabolism towards increased glycerol production, which results in decreased ethanol formation. One example is the overexpression of GPD1 and/or GPD2 genes, which encode isozymes of glycerol 3-phosphate dehydrogenases (Gpd1/2p) (Cambon et al. 2006; de Barros Lopes et al. 2000; Eglinton et al. 2002; Michnick et al. 1997; Nevoigt and Stahl 1996; Remize et al. 1999, 2001). Another genetically modified (GM) modification that leads to enhanced glycerol production is to decrease pyruvate decarboxylase (Pdc) levels (Nevoigt and Stahl 1996), or to impair alcohol dehydrogenase (ADH/Adh) expression/activity (Drewke et al. 1990; Johansson and Sjöström 1984).
While redirecting yeast metabolism towards increased glycerol production using GM approaches is relatively easy to achieve, the use of GM strains for winemaking in many parts of the world is not permitted, and consumer acceptance of GMOs remains a contentious issue (Grossmann et al. 2011). Thus, non-GMO approaches, such as adaptive evolution, must be relied upon (Chambers et al. 2009).
Adaptive evolution involves applying culture conditions that provide a selection pressure favouring the growth of mutants in the evolving population that confer the trait of interest. Thus, culturing populations in a specific selective environment (e.g., medium containing a high ethanol concentration as a selective pressure), will direct the accumulation of adaptation(s) towards desired phenotype(s) (e.g., enhanced ethanol tolerance) (Cakar et al. 2005; Chambers et al. 2009; Zeyl 2005). There are a number of examples illustrating the power of this approach, such as the creation of yeast strains that can anaerobically utilize xylose (Kuyper et al. 2005; Sonderegger and Sauer 2003), yeast with enhanced maltose utilisation and osmotolerance (Higgins et al. 2001), yeast with enhanced ethanol tolerance (Brown and Oliver 1982; Stanley et al. 2010) and very recently, wine yeast with improved fermentation rate, enhanced production of volatile aroma compounds and decreased formation of acetate (Cadiere et al. 2011).
Several selection pressures have the potential to stress yeast cells and induce glycerol overproduction. The most commonly used are osmotic stress inducers (i.e., potassium chloride or sodium chloride), alkalis such as sodium carbonate, and sulfites, such as potassium sulfite or sodium sulfite. Previous reports in the literature show that incubation of S. cerevisiae in medium containing sodium chloride led to a 2.4-fold increase in glycerol production (Petrovska et al. 1999), while addition of sodium carbonate led to 4.6-fold increase (Freeman and Donald 1957). Addition of sodium sulphite, however, has the greatest potential to drive an increase in glycerol production with reported increments of 12.7-fold (Petrovska et al. 1999).
Sulfite addition to fermenting yeast cultures was used for mass glycerol production in Germany during the First and Second World Wars (Freeman and Donald 1957; Taherzadeh et al. 2002). The mechanism underpinning sulfite-induced glycerol overproduction involves a reaction between sulfite and acetaldehyde, to produce a stable, non-toxic compound, 1-hydroxyethanesulfonate (Taylor et al. 1986). This reaction is driven by SO 2−3 , which is the predominant ionic form of sulfite at alkaline pH. In yeast, binding of sulfite to acetaldehyde significantly decreases the concentration of the latter, consequently reducing its availability for ethanol production and oxidation of NADH. This results in a decrease in intracellular availability of NAD+, which, in turn, impairs glycolysis. The cell partially restores glycolytic flux by redirecting a portion of its carbon (as dihydroxyacetone phosphate) through the glycerol synthesis pathway, which uses NADH as a cofactor, thus regenerating NAD+. Under such conditions, glycerol synthesis provides a selection advantage by restoring the intracellular NAD+/NADH balance.
In this study, we developed a proof of concept adaptive evolution approach to generate new, genetically stable yeast strains with enhanced glycerol production phenotypes. To achieve this we used the laboratory strain of S. cerevisiae BY4742, and sulfite at alkaline pH as a selection pressure.
Materials and methods
Adaptive evolution experiments
S. cerevisiae BY4742 haploid (MATα, his3∆1, leu2∆0, lys2∆0, ura3∆0) was used throughout this study. All yeast strains described in this paper are deposited in the Australian Wine Research Institute Culture Collection, with the following reference numbers: BY4742 (AWRI2535), B2-c3 (AWRI2536), BY4742(2n) (AWRI2537), B2-c3(2n) (AWRI2538), BY4742-a x B2-c3-α (2n) (AWRI2539) and BY4742- α x B2-c3-a (2n) (AWR2540). For adaptive evolution experiments triplicate evolving populations of BY4742 were established from three separate colonies, randomly chosen from the same culture plate. These were inoculated into YPD (yeast extract 10 g/l; peptone 20 g/l; glucose 20 g/l) and grown overnight in a rotary shaker (140 rev/min) at 30 °C. These triplicate starter populations, B1, B2 and B3, were used to inoculate founding populations, FB1, FB2 and FB3, in selective medium containing sodium sulfite. Selective medium YPD10S consisted of yeast extract 10 g/l, peptone 20 g/l, glucose 100 g/l and sodium sulfite 30 g/l, pH 8. Founding populations were inoculated at OD600 0.1 in 100 ml of YPD10S and grown at 30 °C with shaking. After approximately five generations (OD600 of 3.2) founding populations were transferred into the same volume of fresh YPD10S to an OD600 0.1 and grown as described previously, giving rise to subsequent evolving populations EB1, EB2 and EB3. This serial transfer was repeated until evolving populations reached approximately 300 generations. During the course of adaptive evolution samples of evolving populations were taken every 50 generations, frozen at −80 °C and stored for future characterization.
Characterization of isolates from evolving populations and dissected tetrads
Isolates from evolving populations were characterized in fermentation performed in 96-well microplates and shake flasks. Microplate cultures were inoculated at an initial OD600 of 0.2, plates were sealed with Breathe-Easy® gas permeable membranes (Diversified Biotech, U.S. Patent No. 5,858,770) and incubated at 30 °C in a Therumo Multiscan Ascent plate reader. Shake flasks were inoculated at an initial OD600 of 0.1 and incubated under: aerobic conditions in flasks covered with aluminium foil that allowed free gas exchange with the environment; semi-aerobic conditions in flasks fitted with air-locks, which ensure anaerobic conditions after oxygen in the headspace is consumed; and in an anaerobic hood (Coy Laboratory Products Inc.), for which media was de-oxygenated for 24 h with agitation prior to inoculation. Inocula for anaerobic cultures were grown overnight under semi-aerobic conditions, de-oxygenated in the anaerobic pre-chamber of the anaerobic hood, and subsequently inoculated, in the anaerobic hood, into flasks containing anaerobic medium. The medium used contained yeast extract 10 g/l, peptone 20 g/l and glucose 100 g/l, at pH 4.5 (YPD10) for assessment of the ‘high glycerol’ phenotype of both isolates and dissected tetrads, or pH 8 for assessment of sulfite tolerance (YPD10S) of isolates; sulfite was added at 40 g/l to this latter medium as this concentration gave a clear separation between tolerant and sensitive phenotypes. Cultures were incubated at 30 °C with shaking (140 rev/min). Samples were taken at regular intervals, then centrifuged, and supernatants were kept for fermentation products analysis. Final glycerol and ethanol concentrations were obtained immediately after glucose was depleted from the medium.
Analytical methods
Yeast growth was followed spectrophotometrically by absorbance at 600 nm. Dry cell weight (DCW) was determined by drying and weighing 20 ml of yeast culture. A calibration curve between OD600 and DCW was constructed for each strain tested. Glucose, glycerol, ethanol and acetic acid levels were determined using high-performance liquid chromatography (HPLC) on a Bio-Rad HPX-87H column as described previously (Nissen et al. 1997).
Molecular techniques
To ensure that changes in phenotype in adaptively evolving populations were not due to contamination, samples were tested by DNA fingerprinting, targeting Ty1 transposon (Ness et al. 1993), and the mating type locus (Huxley et al. 1990).
GPD1 overexpression was carried out by promoter replacement using the method described by Storici and Resnick (2006) with some modifications. Briefly, a cassette containing a ClonNAT resistance gene, natMX, and GIN11 (which, when expressed, is lethal to S. cerevisiae), behind the GAL1 promoter. The cassette was PCR amplified from plasmid pAG25-GIN11, transformed into yeast where it was targeted to insert upstream of GPD1. In a counter-selection step a PCR product containing the strong FBA1 promoter was inserted stably into the yeast chromosome replacing the native GPD1 promoter and removing the counter-selectable cassette introduced in the first transformation step. All yeast transformations were conducted using the lithium acetate/polyethylene glycol method (Ausubel et al. 2007).
Mating, sporulation and dissection of tetrads
To enable mating type interconversions for backcrossing and selfing, a functional HO gene was introduced into B2-c3 and its parent on a plasmid essentially as described by Burke et al. (2000). Mating of haploids was conducted on YPD agar plates as described by Ausubel et al. (2007). Colonies derived from these cells were tested for ploidy using mating-type PCR (Huxley et al. 1990). Sporulation was conducted on solid medium containing 2% (w/v) potassium acetate and agar as described elsewhere (Iverson 1967). After 5 to 7 days, sporulation was confirmed by microscopic examination. Spores were then dissected using a micromanipulator essentially as described previously (Burke et al. 2000).
Results
Adaptive evolution and isolation of high-glycerol producing mutants
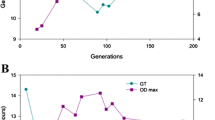
In order to generate non-GM yeast strains with enhanced glycerol production phenotypes we applied an adaptive evolution approach using sulfite at alkaline pH as the selection pressure. Triplicate evolving populations derived from S. cerevisiae BY4742 were grown under aerobic, sulphite-selective conditions for 300 generations. After about 150 generations, samples of all populations were tested for glycerol production under non-selective semi-aerobic conditions, and all were found to generate increased glycerol yields compared to the parental strain, with a further increase for EB2 after 200 generations (Fig. 1), indicating different rates of evolution of the ‘high glycerol’ phenotype in the different populations. By 200 generations all evolving populations were similar with respect to glycerol production.
After every 50 generations, up to 300 generations, a sample of each population was plated and five random colonies from each were picked and tested for glycerol production in non-selective, semi-aerobic conditions. Cultures derived from these colonies produced a range of glycerol concentrations, some generating more, others similar or less than the parental strain BY4742. Although the highest glycerol concentration was observed for population EB1 at 150 generations, the individual clone that produced the highest glycerol concentration was found in population EB2 after 300 generations. Colony B2-c3 produced 41% more glycerol concentration than the parental strain, 3.7 and 2.6 g/l, respectively.
Ty1 transposon PCR confirmed that all evolved populations as well as individual variants had the same Ty1 transposon pattern as the parental strain BY4742 (data not shown) and therefore were of the same parentage. After growing in YPD10 non-selective medium for 50 generations, mutant B2-c3 produced the same increased glycerol yields as the original isolate, indicating that the high-glycerol phenotype was a stable trait in this mutant.
Characterization of the high-glycerol producing mutant B2-c3 in non-selective conditions
Adaptive evolution experiments were carried out in aerobic conditions. To assess whether enhanced glycerol formation was conserved by the mutant in an environment with depleted oxygen, as in wine fermentations, we compared glycerol production under aerobic and anaerobic conditions (Table 1). Under aerobic conditions, B2-c3 produced 3.5 g/l glycerol, which was approximately 46% more than the parent BY4742, which produced 2.4 g/l. Under anaerobic conditions, B2-c3 produced 4.2 g/l of glycerol, over 30% more than its anaerobically grown parent, which produced 3.2 g/l of glycerol. Increased glycerol production by B2-c3 was accompanied by a slight decrease in ethanol formation; B2-c3 produced approximately 1.9% less ethanol in both aerobic and anaerobic conditions. Acetic acid production by B2-c3 decreased by 9.2% in aerobic conditions, and increased by 11.5% in anaerobic conditions. Overall glucose utilization rate and biomass yield were significantly lower for B2-c3 compared to the parental BY4742 in both aerobic and anaerobic conditions. Calculated glycerol yields on glucose (Y GLY/GLU, expressed as g glycerol produced per g glucose consumed) and on biomass (YGLY/DCW, expressed as g glycerol produced per g DCW formed) were significantly higher for B2-c3 in both conditions (Table 1). Assuming CO2 is produced in equimolar amounts to ethanol for BY4742 and B2-c3 grown under anaerobic conditions, the carbon recoveries for BY4742 and B2-c3 in these experiments were 98.3% and 99.3%, respectively.
The high-glycerol phenotype is a recessive, multigenic trait, which segregates independently from adaptively evolved sulphite tolerance
To characterize the genetics of the high-glycerol phenotype, B2-c3 was backcrossed to the parental strain BY4742. Two heterozygous diploids derived from reciprocal crosses (‘a’ × ‘α’, and ‘α’ × ‘a’) and two homozygous diploids, one produced by self-mating BY4742 and the other from self-mating B2-c3, were tested for glycerol production in non-selective medium under semi-anaerobic conditions (Table 2). Both heterozygous diploids produced similar amounts of glycerol to BY4742, which is consistent with the high-glycerol phenotype being recessive; the homozygous diploids of the parents used in the backcrosses gave only slightly lower glycerol concentration than the haploids they were derived from, indicating that ploidy is not a major determining factor for glycerol production in this genetic background.
Two of the heterozygotes, BY4742-αxB2-c3-a (2n) and BY4742-axB2-c3-α (2n), were sporulated and haploid progeny tested for glycerol yields. Segregation of glycerol yields for ten tetrads, five derived from each heterozygote, did not follow any obvious pattern (Fig. 2). Some tetrads generated single, high-glycerol yielding spores whereas others were of intermediate phenotype; phenotypic ratios were highly variable. This indicates that the ‘high-glycerol’ phenotype is probably multigenic.
Samples of the three evolving populations showed similar growth kinetics to each other and significantly higher growth rates than the starting populations and BY4742 when grown in fresh medium containing 30 g/l sulfite at pH 4.5 (data not shown). To characterize sulfite tolerance in B2-c3, a heterozygote, BY4742 × B2-c3, was grown under aerobic conditions, in selective medium containing sulfite at pH 8.0 (Fig. 3). The heterozygote displayed a level of sulfite tolerance that was between that of homozygous diploid BY4742(2n) and homozygous diploid B2-c3(2n), indicating incomplete dominance for this trait.
To determine whether or not increased glycerol production in variant B2-c3 was associated with increased sulfite tolerance, parent BY4742, B2-c3, and four haploid segregants, H1–H4, derived from a heterozygous diploid BY4742-αxB2-c3-a (2n), were tested for growth and glycerol yields in non-selective (Fig. 4a), as well as selective, aerobic conditions (Fig. 4b).
Yeast growth and glycerol yields in non-selective (YPD10) and selective (YPD10S) conditions. Yeast growth for parental BY4742 (black circles), variant B2-c3 (black squares), and segregants H1 (white circles), H2 (white squares), H3 (white diamonds), H4 (white triangles) grown in non-selective conditions (a) and selective conditions (b). Bar plots show glycerol yields for the indicated time points, BY4742 (black), mutant B2-c3 (white), and segregates H1–H4 (gray scale)
Clearly, the ‘high glycerol’ phenotype is not wholly associated with sulfite tolerance and vice versa: in non-selective conditions, spore H3 produces high glycerol yields but is not particularly sulfite tolerant, whereas H2 and H4 produce similar levels of glycerol to BY4742 but have a high level of sulfite tolerance (Fig. 4a). In selective conditions (medium containing sulfite at pH 8.0), glycerol yields generally reflected growth rates (Fig. 4b), and by the end of fermentation the four spores had produced similar levels of glycerol to each other (ranging from 22. to 22.6 g/l), but this was more than BY4742 (18.9 g/l) and less than B2-c3 (24.1 g/l) (Fig. 4b).
Sulfite tolerance is not associated with glycerol overproduction in an engineered strain overexpressing GPD1
To test whether sulfite tolerance could be engineered into yeast simply by increasing glycerol production, S. cerevisiae BY4742 was GM to overexpress the glycerol-3-phosphate dehydrogenase gene, GPD1. Compared to its parent, BY4742 FBA-GPD1 produced 3.5 times more glycerol (8.2 g/l) in non-selective, aerobic conditions. However it showed no significant increase in sulfite tolerance (Fig. 5). These results suggest that simply boosting glycerol production does not confer increased sulfite tolerance.
Discussion
A stable mutant yeast strain, B2-c3, with an enhanced glycerol production phenotype was generated using a non-GM, adaptive evolution strategy, which employed sodium sulfite at pH 8.0 as the selection pressure. When grown aerobically in YPD medium, B2-c3 produced up to 46% more glycerol than its parent, BY4742. Under anaerobic conditions the difference was more modest, but was still about 30% more than the parental strain. An additional potential beneficial feature of B2-c3 is that it produced slightly less ethanol than BY4742; although the drop in ethanol production was minor, these results suggest that the adaptive evolution strategy used here might, with some modifications, be useful for the generation of non-GM, low-ethanol producing yeast strains, for which there is increasing demand in the wine industry (Kutyna et al. 2010).
When applying adaptive evolution strategies to generate new microbial strains with desirable traits, it is unlikely that the selection pressure used, particularly when applied over many generations, will impact solely on the phenotype of interest. In the case of using sulfite as the agent of selection one would predict, for example, that mechanisms of sulfite resistance, other than that associated with increased glycerol production, would come into play. It is known, for example, that sulfite resistance in S. cerevisiae is affected by mutations of the sulfite transporter gene SSU1 (Avram and Bakalinsky 1997; Goto-Yamamoto et al. 1998; Park and Bakalinsky 2000) and a transcription factor that regulates its expression FZF1-4 (Park and Bakalinsky 2000). Indeed, genetics experiments following segregation of sulfite tolerance and the ‘high-glycerol’ phenotypes, showed that these traits do not co-segregate in B2-c3; in fact, we isolated a spore, H3, that retained the high glycerol phenotype with very little sulfite tolerance, and spore H2, which had almost lost the ‘high-glycerol’ phenotype but retained sulfite tolerance.
On the face of it, the above results are somewhat puzzling: sulfite was used as the agent of selection to generate a ‘high-glycerol’ mutant. The strategy was successful, but interestingly, the mutant generated did not require the ‘high-glycerol’ phenotype for tolerance to sulfite. However, it should be remembered that this phenotype was assessed under non-selective conditions. When assessed in the presence of sulfite at high pH, by the end of fermentation all four spores (H1–H4) produced higher levels of glycerol than BY4742, but less than B2-c3. This data is also indicative of a multigenic trait.
Another factor to take into account is that sodium sulfite has osmotic potential and therefore will generate a level of osmotic stress which, in S. cerevisiae, would be expected to induce glycerol production (Hohmann 2002) and potentially select for high glycerol-producing mutants. However, Petrovska et al. (1999) compared the impacts of 40 g/l NaCl (pH 7.0) with 40 g/l sodium sulfite (pH 7.0) in S. cerevisiae and found that the latter was much more effective at inducing glycerol production, and this was associated with acetaldehyde being ‘consumed’ by sulfite. The authors concluded that it was sulfite’s ability to bind acetaldehyde, thereby driving increased glycerol production to maintain redox balance, that was responsible for its high glycerol-inducing potential, rather than glycerol’s role as a compatible solute. Nonetheless, this does not exclude possibility that the high glycerol phenotype of B2-c3 was, at least in part, an adaptation to osmotic stress.
To further test the relationship between glycerol production and sulfite tolerance, BY4742 was GM to overexpress GPD1. The resultant strain, BY4742 FBA-GPD1, produced 3.5 times more glycerol than BY4742, but did not have an increased tolerance to sulfite; in fact, this strain grew less well in sulfite containing medium than its non-engineered parent. Thus, increased glycerol production on its own is not sufficient to render a strain sulfite-tolerant.
Nonetheless, when grown in the presence of sulfite at high pH, relative to BY4742, the four spores (H1–H4) generated from backcrossed B2-c3 produced increased levels of glycerol, and all four had some level of increased tolerance to sulfite. Thus, there is perhaps some link between sulfite tolerance and glycerol production. The huge increase in production of glycerol under non-selective conditions might be regarded as a fortuitous outcome.
In an attempt to identify mutations that confer the high-glycerol and sulfite tolerance phenotypes, the most likely candidate genes in B2-c3 and BY4742 were sequenced and compared. These candidates included: alcohol dehydrogenase genes, mutations in which have been shown to have a negative impact on ethanol production, leading to increased glycerol production (Drewke et al. 1990; Johansson and Sjöström 1984); pyruvate decarboxylase genes PDC1, PDC5 and PDC6 (Geertman et al. 2006), and the transcription factor gene PDC2 (Nevoigt and Stahl 1996), mutations in which have been shown, to be associated with enhanced glycerol production; the triose phosphate isomerise gene, TPI1, which, when disrupted (Compagno et al. 1996), led to a substantial increase in glycerol production; glycerol-3-phosphate dehydrogenase genes, GPD1 and GPD2, which, when overexpressed, generated increased glycerol production (de Barros Lopes et al. 2000; Eglinton et al. 2002; Nevoigt and Stahl 1996). In addition to the above, we also sequenced two genes that are known to impact on sulfite tolerance: SSU1 and its transcription factor, FZF1-4 (Avram and Bakalinsky 1997; Park and Bakalinsky 2000).
Sequence data for all of the above candidate genes including 350 bp upstream and 200 bp downstream of the ORF uncovered no mutations. Thus, the ‘high-glycerol’ and sulfite tolerance phenotypes of B2-c3 cannot be explained by simple nucleotide variations in these genes. However, it is possible that other mutations affecting, for example, gene copy number or mutations in transcription factor genes that regulate expression of the sequenced genes, might confer the observed phenotypes. Further work will be required to identify the genetic determinants of these traits.
In general, our work introduces a novel, non-GMO strategy, which in our view has the potential to increase glycerol production in wine yeast strains. It is important to recognize, however, this is still at the proof-of-concept stage. Further work is required to evaluate its usefulness for the development of commercial wine yeast.
References
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (2007) Current protocols in molecular biology. Wiley, New York
Avram D, Bakalinsky AT (1997) SSU1 encodes a plasma membrane protein with a central role in a network of proteins conferring sulfite tolerance in Saccharomyces cerevisiae. J Bacteriol 179:5971–5974
Brown SW, Oliver SG (1982) Isolation of ethanol-tolerant mutants of yeast by continuous selection. Eur J Appl Microbiol 16:119–122
Burke D, Dawson D, Stearns T (2000) Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Cadiere A, Ortiz-Julien A, Camarasa C, Dequin S (2011) Evolutionary engineered Saccharomyces cerevisiae wine yeast strains with increased in vivo flux through the pentose phosphate pathway. Metab Eng 13:263–271
Cakar ZP, Seker UO, Tamerler C, Sonderegger M, Sauer U (2005) Evolutionary engineering of multiple-stress resistant Saccharomyces cerevisiae. FEMS Yeast Res 5:569–578
Cambon B, Monteil V, Remize F, Camarasa C, Dequin S (2006) Effects of GPD1 overexpression in Saccharomyces cerevisiae commercial wine yeast strains lacking ALD6 genes. Appl Environ Microbiol 72:4688–4694
Chambers PJ, Bellon JR, Schmidt SA, Varela C, Pretorius IS (2009) Non-genetic engineering approaches to isolating and generating novel yeast for industrial applications. In: Kunze G, Satyanarayana T (eds) Yeast biotechnology: Diversity and applications. Springer Science + Business Media, pp 433–457
Compagno C, Boschi F, Ranzi BM (1996) Glycerol production in a triose phosphate isomerase deficient mutant of Saccharomyces cerevisiae. Biotechnol Prog 12:591–595
de Barros Lopes M, Rehman AU, Gockowiak H, Heinrich AJ, Langridge P, Henschke PA (2000) Fermentation properties of a wine yeast overexpressing the Saccharomyces cerevisiae glycerol 3-phosphate dehydrogenase gene (GPD2). Aust J Grape Wine Res 6:208–215
Drewke C, Thielen J, Ciriacy M (1990) Ethanol formation in adh0 mutants reveals the existence of a novel acetaldehyde-reducing activity in Saccharomyces cerevisiae. J Bacteriol 172:3909–3917
Eglinton JM, Heinrich AJ, Pollnitz AP, Langridge P, Henschke PA, de Barros Lopes M (2002) Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldehyde dehydrogenase gene. Yeast 19:295–301
Eustace R, Thornton RJ (1987) Selective hybridization of wine yeasts for higher yields of glycerol. Can J Microbiol 33:112–117
Freeman GG, Donald GM (1957) Fermentation processes leading to glycerol: III. Studies on glycerol formation in the presence of alkalis. Appl Microbiol 5:216–220
Gawel R, Van Sluyter S, Waters EJ (2007) The effects of ethanol and glycerol on the body and other sensory characteristics of Riesling wines. Aust J Grape Wine Res 13:38–45
Geertman JM, van Maris AJ, van Dijken JP, Pronk JT (2006) Physiological and genetic engineering of cytosolic redox metabolism in Saccharomyces cerevisiae for improved glycerol production. Metab Eng 8:532–542
Goto-Yamamoto N, Kitano K, Shiki K, Yoshida Y, Suzuki T, Iwata T, Yamane Y, Hara S (1998) SSU1-R, a sulfite resistance gene of wine yeast, is an allele of SSU1 with a different upstream sequence. J Ferment Bioeng 86:427–433
Grossmann M, Kiessling F, Singer J, Schoeman H, Schröder MB, von Wallbrunn C (2011) Genetically modified wine yeasts and risk assessment studies covering different steps within the wine making process. Ann Microbiol 61:103–115
Higgins VJ, Bell PJL, Dawes IW, Attfield PV (2001) Generation of a novel Saccharomyces cerevisiae strain that exhibits strong maltose utilization and hyperosmotic resistance using nonrecombinant techniques. Appl Environ Microbiol 67:4346–4348
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol R 66:300–372
Huxley C, Green ED, Dunham I (1990) Rapid assessment of S. cerevisiae mating type by PCR. Trends Genet 6:236
Iverson WP (1967) Yeast sporulation on two commonly available media. Appl Microbiol 15:966–967
Johansson M, Sjöström JE (1984) Enhanced production of glycerol in an alcohol dehydrogenase (ADH1) deficient mutant of Saccharomyces cerevisiae. Biotechnol Lett 6:49–54
Kutyna DR, Varela C, Henschke PA, Chambers PJ, Stanley GA (2010) Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci Technol 21:293–302
Kuyper M, Toirkens MJ, Diderich JA, Winkler AA, van Dijken JP, Pronk JT (2005) Evolutionary engineering of mixed-sugar utilization by a xylose-fermenting Saccharomyces cerevisiae strain. FEMS Yeast Res 5:925–934
Michnick S, Roustan JL, Remize F, Barre P, Dequin S (1997) Modulation of glycerol and ethanol yields during alcoholic fermentation in Saccharomyces cerevisiae strains overexpressed or disrupted for GPD1 encoding glycerol 3-phosphate dehydrogenase. Yeast 13:783–793
Ness F, Lavallee F, Dubourdieu D, Aigle M, Dulaub L (1993) Identification of yeast strains using the polymerase chain reaction. J Sci Food Agr 62:89–94
Nevoigt E, Stahl U (1996) Reduced pyruvate decarboxylase and increased glycerol-3-phosphate dehydrogenase [NAD+] levels enhance glycerol production in Saccharomyces cerevisiae. Yeast 12:1331–1337
Nissen T, Schulze U, Nielsen J, Villadsen J (1997) Flux distributions in anaerobic, glucose-limited continuous cultures of Saccharomyces cerevisiae. Microbiology 143:203–218
Noble AC, Bursick GF (1984) The contribution of glycerol to perceived viscosity and sweetness in white wine. Am J Enol Vitic 35:110–112
Park H, Bakalinsky AT (2000) SSU1 mediates sulphite efflux in Saccharomyces cerevisiae. Yeast 16:881–888
Petrovska B, Winkelhausen E, Kuzmanova S (1999) Glycerol production by yeasts under osmotic and sulfite stress. Can J Microbiol 45:695–699
Pretorius IS (2000) Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16:675–729
Rainieri S, Pretorius IS (2000) Selection and improvement of wine yeasts. Ann Microbiol 50:15–31
Remize F, Barnavon L, Dequin S (2001) Glycerol export and glycerol-3-phosphate dehydrogenase, but not glycerol phosphatase, are rate limiting for glycerol production in Saccharomyces cerevisiae. Metab Eng 3:301–312
Remize F, Roustan JL, Sablayrolles JM, Barre P, Dequin S (1999) Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase. Appl Environ Microbiol 65:143–149
Sonderegger M, Sauer U (2003) Evolutionary engineering of Saccharomyces cerevisiae for anaerobic growth on xylose. Appl Environ Microbiol 69:1990–1998
Stanley D, Fraser S, Chambers PJ, Rogers P, Stanley GA (2010) Generation and characterisation of stable ethanol-tolerant mutants of Saccharomyces cerevisiae. J Ind Microbiol Biot 37:139–149
Storici F, Resnick MA (2006) The delitto perfetto approach to in vivo site-directed mutagenesis and chromosome rearrangements with synthetic oligonucleotides in yeast. Method Enzymol 409:329–345
Taherzadeh MJ, Adler L, Liden G (2002) Strategies for enhancing fermentative production of glycerol — a review. Enzyme Microb Technol 31:53–66
Taylor SL, Higley NA, Bush RK (1986) Sulfites in foods: uses, analytical methods, residues, fate, exposure assessment, metabolism, toxicity, and hypersensitivity. Adv Food Res 30:1–76
Zeyl C (2005) The number of mutations selected during adaptation in a laboratory population of Saccharomyces cerevisiae. Genetics 169:1825–1831
Acknowledgements
Research at the Australian Wine Research Institute is financially supported by Australia’s grapegrowers and winemakers through their investment body the Grape and Wine Research Development Corporation, with matching funds from the Australian Government. The AWRI is part of the Wine Innovation Cluster.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kutyna, D.R., Varela, C., Stanley, G.A. et al. Adaptive evolution of Saccharomyces cerevisiae to generate strains with enhanced glycerol production. Appl Microbiol Biotechnol 93, 1175–1184 (2012). https://doi.org/10.1007/s00253-011-3622-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3622-7