Abstract
Effective wastewater treatment and electricity generation using dual-chamber microbial fuel cell (MFC) will require a better understanding of how operational parameters affect system performance. Therefore, the main aim of this study is to investigate the bioelectricity production in a dual-chambered MFC-operated batch mode under different operational conditions. Initially, platinum (Pt) and mixed metal oxide titanium (Ti-TiO2) electrodes were used to investigate the influence of the electrode materials on the power generation at initial dissolved organic carbon (DOC) concentration of 400 mg/L and cycle time of 15 days. MFC equipped with Ti-TiO2 electrode performed better and was used to examine the effect of influent DOC concentration and cycle time on MFC performance. Increasing influent DOC concentration resulted in improving electricity generation, corresponding to a 1.65-fold increase in power density. However, decrease in cycle time from 15 to 5 days adversely affected reactor performance. Maximum DOC removal was 90 ± 3 %, which was produced at 15-day cycle time with an initial DOC of 3,600 mg/L, corresponding to maximum power generation of about 7,205 mW/m2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial fuel cell (MFC) is an innovative technology that can directly convert the energy stored in chemical bonds of organic biodegradable compounds into electrical energy by means of the catalytic activity of anaerobic microorganisms [1, 2]. MFC technology has particular advantages such as environment-friendly energy generation and simultaneous wastewater treatment in a single step, mild reaction conditions, high conversion efficiency, and sludge minimization [3]. Although MFC technology has been investigated since 1900s, practical applications of the MFCs are still limited because of their low-power generation [4].
It is well known that improvement of MFC performance is dependent on many important factors such as operating conditions (ionic strength, feeding regime, cycle time, microorganism and carbon concentration, substrate type, pH, temperature, etc.), reactor configuration, electrode material, electrode surface area, membrane type, and external resistance. So far, a variety of electrode materials and configurations has been examined to improve MFC performance and to decrease material cost. It has been known that the characteristics of anode or cathode electrode such as surface area, conductivity, bio-compatibility, and surface roughness allow improvement of bacterial attachment, substrate oxidation, and electron transfer from bacteria to the electrode surface [5, 6]. Carbon-based electrodes are among the most frequently investigated electrode materials in MFC systems [5, 7]. However, these carbon-based electrodes generally are non-superior to metal-based electrodes (Pt, Co, TiO2, etc.) because of their low electrical conductivity, poor bio-compatibility, and surface properties [8]. For instance, in the previous study [9], the Ti-TiO2 electrode material was compared with a graphite electrode. It was observed that the power generation in MFC equipped with Ti-TiO2 electrode was approximately 250 times higher than that in MFC with graphite electrode.
Substrate concentration is also an important parameter on the MFC performance since the electricity generation depends upon the substrate conversion rate. Tremouli et al. [10] studied the effect of different influent concentrations of diluted filter-sterilized cheese whey as substrate on the MFC electricity generation, ranging from 0.35- to 6.7-g chemical oxygen demand (COD)/L. The highest power density was obtained at the maximum influent concentration (6.7 g COD/L) of cheese whey, corresponding to 46 mW/m2. Logan [11] obtained a maximum power density (38 mW/m2) at an acetate concentration of 770 mg/L. In another study, Gonzalez del Campo et al. [12] used a dual-chamber air cathode MFC and graphite plate as anode and cathode electrode. They observed that the increased influent COD concentration results in increasing current and activity of the microorganisms, corresponding to a current of about 0.019 mA.
In this study, initial performances of Ti-TiO2 and Pt electrode materials were compared in a fed-batch dual-chambered MFC in respect to power generation and dissolved organic carbon (DOC) removal efficiency. Afterward, the influence of substrate concentration (400–3,600 mg/L as DOC) and cycle time (5–15 days) on MFC performance was studied with the best performed electrode material.
Materials and Methods
MFC Setup and Operation
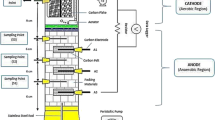
The MFC was consisted of two equal chambers (anode and cathode) separated by a proton exchange membrane (UltrexTM CMI7000, Membranes International Inc., USA) as shown in Fig. 1.The working volume of each chamber was 365 mL, and the membrane was sandwiched between these chambers. Two electrode materials were examined. Initially, MFC was operated with Pt as both anode and cathode electrode material which has been widely used in MFC technology. Further, the Ti-TiO2 electrode material was used for both anode and cathode chambers which has been recently used as a new metal-based electrode in MFC. In order to minimize the internal resistance related with ohmic loses, electrodes were placed in direct contact with the proton exchange membrane. The electrodes were connected with copper wire through 10-Ω external resistors (R) throughout the experiment.
The MFC was operated in a batch mode at room temperature and uncontrolled pH during the experiments. The abiotic cathode chamber was filled with distilled water daily and aerated continuously using air pumps to provide oxygen as a terminal electron acceptor. Formation of a biofilm on the air cathode is undesired during the abiotic cathode operation, which results in decreasing power output due to the increased cathodic resistance and limited proton transfer rate. Thereby, great care was taken to prevent microbial contamination in the cathode chamber by refreshing distilled water daily.
Anode chamber was inoculated with a bulk sediment sample taken from a deep sludge of Golden Horn in Istanbul, Turkey and acclimatized to the described simulated effluent for 90 days under anaerobic conditions. The anode chamber was constantly purged with nitrogen gas at low flow rates to provide the anaerobic conditions.
The performance of the fed-batch MFC was investigated on five different periods (Table 1). First, MFC was performed with Pt (period I) and Ti-TiO2 electrode materials (period II) at an initial DOC concentration of 400 mg/L and a cycle time of 15 days. Ti-TiO2 was found optimum for high electricity generation and organic matter removal yields. In period III, cycle time was kept constant at 15 days, and the substrate reduction ability of the MFC was assessed by increasing the glucose concentration in the synthetic feed. Further cycle time was decreased to 10 days (period IV) and 5 days (period V). All assays were run in triplicate, and the data illustrated in all figures are the mean values of the measurements for three cycles.
Synthetic Wastewater
The synthetic effluent (Table 1) fed to the anode chamber of the MFC reactor was composed of micro- and macronutrients required for microbiological growth. All inorganic nutrients were provided in excess not to limit biological activity. The anode chamber was fed with synthetic wastewater containing per liter of distilled water: 4-g NaHCO3, 0.6-g NH4Cl, 9.3-g NaH2PO4.H2O, 3.2-g Na2HPO4, 0.125-g K2HPO4, 0.1-g MgCl2.6H2O, 0.11-g CaCl2.2H2O, 0.5-g cysteine-HCl, and minor amounts of metal ions (Fe, Zn, Co, Cu, Mn, and Ni) and vitamins [13]. Glucose (contributed to DOC of 400–3,600 mg/L) was used to provide readily biodegradable carbon source and electron donor source during study periods.
Analysis
Liquid samples were directly taken from MFC chambers at regular time intervals and immediately centrifuged and filtered through a sterile syringe 0.45-μm filter (Sartorius AG, Gottingen, Germany) to separate the bacterial cells from the liquid. DOC concentrations of samples were measured by a total organic carbon analyzer (Shimadzu TOC-VCPN, Kyoto, Japan). The voltage (V) across the resistor was continuously recorded to the computer at an interval of 3 h using an online data acquisition system. In addition, this voltage was used to calculate power output (P) and current (I) according to Ohm’s law. The pH and conductivity of each chamber were measured using multimeter (WTW340I Weilheim, Germany).
Results and Discussion
Selection of Electrode Material on Bioelectricity Generation
The electrode material has been known as one of the limiting factors on MFC performance. In this part of the study (periods I and II), Pt and Ti-TiO2 electrode materials were compared for effective MFC operation. Power generation and DOC removal performances of MFC equipped with different electrode materials are shown in Fig. 2a, b, respectively.
In the first period, MFC chambers were equipped with Pt electrodes. The MFC was operated at 15-day cycle time and an initial DOC concentration of 400 mg/L. It was observed that most of the carbon was removed rapidly in the first hours of the cycle time in which maximum power generation took place simultaneously. In this period, maximum power density and voltage reached to 1,250 ± 10 mW/m2 and 200 ± 5 V, respectively (Fig. 2a). In period II, MFC equipped with Ti-TiO2 electrode was operated, and results were compared to period I. In this period, the maximum power density of the MFC reached 4,365 ± 10 mW/m2, which was about 3.5 times higher than that of MFC equipped with Pt electrode. However, there was no significant difference observed in the final carbon removal efficiency in operational cycles during periods I and II (Fig. 2b). Results of percentage carbon reduction at the end of the anaerobic reaction phase were similar for both runs, giving an overall reduction of 80 ± 5 %. It is important to note that generation of different power outputs during periods I and II despite the similar DOC removal efficiencies clearly shows the efficiency of the Ti-TiO2 electrode material. This may be due to the Ti-TiO2 electrode properties for bacterial attachment despite the lower surface area. Additionally, the much higher power output achieved by MFC with Ti-TiO2 indicates that the Ti-TiO2 electrode material can effectively increase the fraction of electrons generated from oxidation of organic carbon recovered as current.
Ozkaya et al. [9] reported that Ti-TiO2 electrodes enable high treatment and bioelectricity generation performance. It was previously demonstrated that the nanostructured polyaniline (PANI)/TiO2 composite as anode electrode material can be used and significantly improves the electricity generation in comparison to MFCs with the carbon nanotube-PANI composite [14, 15].
The influent pH of the anode chamber was about 6.8 throughout the MFC operation due to the characteristic of wastewater. However, pH was decreased due to the release of hydrogen ions and formation of organic acids during anode reaction, corresponding to pH of 6.03 and 6.12, respectively (Table 2). The effluent pH value of the cathode chamber was similar at varying electrode materials. However, pH values in the cathode chamber were higher than that of anode pH. The effluent cathode conductivity gradually increased parallel to the increase in current production.
Effect of Substrate Concentration on Bioelectricity Generation
The substrate concentration has a great influence on the performance of MFC, since the electrons for electricity generation originated from the substrate in the anode chamber. Therefore, the effect of the initial substrate concentration on electricity generation was studied in this part of the study by using MFC with Ti-TiO2. Glucose was supplemented as carbon and electron donor source for microorganisms into the anodic chamber. The electrons released from anaerobic oxidation of the organic matter (glucose) were transferred from anode to air cathode, thereby resulting in carbon removal and electricity production. This part of study was performed by using MFC equipped with Ti-TiO2 electrode, as it was found more effective in the previous part. MFC was fed with synthetic wastewater containing glucose, corresponding to 400 and 3,600 mg/L influent DOC content in periods II and III, respectively (Table 1).
The profile of power generation and substrate degradation at different initial glucose concentrations within cycle time of 15 days is shown in Fig. 3a, b, respectively. A large proportion of the soluble DOC was consumed during the first hours of the cycle for both periods; however, the substrate conversion rate was decreased throughout the cycle time. As seen in Fig. 3b, overall DOC removal efficiency at the end of the cycle was 85 ± 3 and 90 ± 3 %, corresponding to about 59 and 331 mg/L effluent DOC concentration in periods II and III, respectively. In this case, the total concentration of removed DOC in period III (initial DOC of 3,600 mg/L) was about ninefold higher than that obtained in period II (initial DOC of 400 mg/L), corresponding to the maximum value obtained during all study periods. This result may indicate that microorganisms enhance their enzyme synthesis to degrade more organic matter for power generation at the high carbon loading. Therefore, the DOC consumption capacity of anaerobic microorganisms enhanced, which increased the cell voltage [12].
As glucose was oxidized at the beginning of the cycle time, a sharp increase in the voltage and power density values was observed, and when the DOC in the reactor was consumed, voltage and power density values were decreased. This may be resulted from decreasing DOC concentration in the reactor and DOC consumption rate throughout anaerobic cycle, thereby lacking the necessary electron donor for the power generation process. Voltage and power density values were always high when the glucose oxidation reactions in the anode chamber were dominant, especially at the beginning of the reaction time, which indicates the electron flow from anode to the cathode chamber. An increase in the substrate concentration in the anode from 400 (period II) to 3,600 mg DOC/L (period III) resulted in increasing of the cell voltage and power density (Fig. 3a). The maximum power density obtained during period III was about 7,205 mW/m2, corresponding to a 1.65-fold increase in power density compared to period II. Similarly, the cell voltage increased to around 370 mV when the initial substrate concentration was increased. The results showed that increasing substrate concentration has a great effect on the bioelectricity generation of MFC. Similar to our results, Gil et al. [16] reported that there was a linear relationship between the electric current and the substrate concentration up to 400 mg COD/L. Gonzalez del Campo et al. [12] reported that the electric current increased with a stepwise rise of influent COD concentrations from 100 to 3,000 mg/L. Additionally, in a study performed by Zhang et al. [17], it was observed that the substrate concentration could affect the power generation behavior through substrate degradation and an increase in the substrate concentrations promotes the substrate degradation, and consequently it results in a higher power generation. Wei et al. [2] reported that the increasing sucrose concentration from 1 to 3.5 g/L resulted in increasing the voltage outputs of the MFC. However, at the higher sucrose concentrations (≥5 g/L), voltage outputs decreased due to high osmotic pressure arising from concentrated substrate source, accumulated organic acids, and low anode pH value.
The pH of the reactor was regulated with two half-reactions consisted of glucose oxidation in the anode chamber and oxygen reduction in the cathode chamber. The pH value in the anode chamber increased from 6.12 to 6.71 with increasing DOC concentration (Table 3). The reason for this rise is probably due to the increase of proton production as a result of increasing carbon concentration; thereby, the amount of protons passing to the cathode side through the membrane was increased. pH values in the cathode chamber were higher than anode pH, while pH values were theoretically expected to be equal [18]. This obvious pH difference between the anode and cathode chambers was probably that the proton production rate as a result of glucose oxidation in the anode chamber was higher than that of proton diffusion through the membrane [16].
Effect of Cycle Time on Bioelectricity Generation
Cycle time is an important factor for economic feasibility and optimizing of the electricity generation characteristics in the MFC reactor. In this part of the study cycle, time was decreased from 15 days (period III) to 10 days (period IV) and 5 days (period V), respectively. The system performance was determined by total DOC removal, voltage, and power density generation in a fed-batch reactor. As seen in Fig. 4, as glucose was oxidized quickly at the beginning of the reaction time, a rapid increase in the power density output was obtained as observed in previous periods. Maximum power density observed in period III (15-day cycle time) was 7,205 mW/m2 which is 10.5 times greater than that obtained in period V (5-day cycle time). Wei et al. [2] obtained about 4 times lower cell voltage compared to this study’s results, corresponding to 100-mV voltage output. Gálvez et al. [19] recorded a power density of 1,822 μW/cm2 with the COD and BOD5 removals of 31 and 84.4 %, respectively. Ozkaya et al. [20] recorded a power density of 900 mW/m2 in a dual-chambered MFC, which was 8 times lower than the maximum value obtained during this study. Greenman et al. [21] found that maximum current density reached 3.24 mA/m2 in the MFC reactor with activated sludge, corresponding to about 5 times lower current density compared with this study.
Figure 4 represents the DOC removal efficiency of the MFC reactor under 15-day (period III), 10-day (period IV), and 5-day cycle times (period V), respectively. Glucose was used as a readily biodegradable carbon source, corresponding to 3,600 mg/L influent DOC. Effluent DOC value was associated with the cycle length of the MFC. In the period III (cycle time of 15 days), DOC removal performance of the MFC reactor reached 90 ± 3 % at the end of the cycle time, and about 77 ± 3 % of the DOC removal was achieved in the first 8 days of reaction time. In the rest of the reaction time, the effluent DOC concentration slightly decreased because of the insufficient amount of remained readily biodegradable carbon source. DOC removal efficiency was adversely affected by decreasing total cycle time from 15 days to 10 days and 5 days in periods IV and V, respectively. The percentage carbon reduction at the end of the anaerobic reaction in the anode chamber was 56 ± 3 and 51 ± 3 % for the 10- and 5-day cycle times, respectively.
Glucose oxidation, oxygen reduction, and proton exchange membrane property in the air cathode MFC are the main factors responsible for variance in pH. Anode effluent pH for cycle time of 15 days was around 6.71; however; it was lower than the 5-day cycle time being around 6.42 (Table 4). The effluent cathode conductivity gradually increased in contrast with decreasing in current production with decreasing cycle time and reached 94 μS/cm at the last period.
Conclusion
In this study, the effects of different electrode materials, substrate concentration, and cycle time on fed-batch MFC reactor performance were investigated. The results of the present study showed that simultaneous wastewater treatment and bioelectricity production were possible with two different electrode materials. However, the experimental data demonstrated that the Ti-TiO2 electrode exhibited better performance in terms of power generation and DOC removal efficiency. The increasing influent DOC concentration enhanced microorganisms activity and provided opportunity to form the best performance which means the maximum power and voltage outputs corresponding to 7,205 mW/m2 and 370 mV, respectively. Total cycle time length has an important effect on electricity generation and DOC removal. Decreasing cycle time resulted in decreasing DOC removal efficiency and low-power density production. The results indicated that the best performance of MFC equipped with Ti-TiO2 in terms of carbon removal and power generation was achieved from a total cycle time of 15 days and an influent DOC of 3,600 mg/L.
References
Kim, M. H., Iwuchukwu, I. J., Wang, Y., Shin, D., Sanseverino, J., & Frymier, P. (2011). Journal of Power Sources, 196, 1909–1914.
Wei, L. L., Yuan, Z. L., Cui, M. J., Han, H. L., & Shen, J. Q. (2012). International Journal of Hydrogen Energy, 37, 1067–1073.
Digman, B., & Kim, D. S. (2008). Environmental Progress, 27(4), 524–537.
Logan, B. E., & Elimelech, M. (2012). Nature, 48, 313–319.
Logan, B., Hamelers, B., Rozendal, R., Schroeder, U., Keller, J., Freguiac, S., et al. (2006). Environmental Science and Technology, 40(17), 5181–5192.
Zhou, M., Chi, M., Luo, J., He, H., & Jin, T. (2011). Journal of Power Sources, 196, 4427–4435.
Zhao, Y., Watanabe, K., Nakamura, R., Mori, S., Liu, H., Ishii, K., et al. (2010). Chemistry A European Journal, 16, 4982–4985.
Zhang, G. D., Zhao, Q. L., Jiao, Y., Zhang, J. N., Jiang, J. Q., Ren, N. Q., et al. (2011). Journal of Power Sources, 196(15), 6036–6041.
Ozkaya, B., Akoglu, B., Karadag, D., Aci, G., Taksan, E., & Hasar, H. (2012). Bioprocess and Biosystems Engineering, 35, 1219–1227.
Tremouli, A., Antonopoulou, G., Bebelis, S., & Lyberatos, G. (2013). Bioresource Technology, 131, 380–389.
Logan, B. E. (2005). Water Science Technology, 52(1–2), 31–37.
Gonzalez Del Compo, A., Lobato, J., Canizares, P., Rodrigo, M. A., & Fernandez Moralez, F. J. (2013). Applied Energy, 101, 213–217.
Karadag, D. (2011). International Journal of Hydrogen Energy, 36, 458–465.
Qiao, Y., Li, C. M., Bao, S. J., & Bao, Q. L. (2007). Journal of Power Sources, 170(1), 79–84.
Qiao, Y., Bao, S. J., Li, C. M., Cui, X. Q., Lu, Z. S., & Guo, J. (2008). Acs Nano, 2(1), 113–119.
Gil, G. C., Chang, I. S., Kim, B. H., Kim, M., Jang, J. K., Park, H. S., et al. (2003). Biosensors and Biolelectronics, 18, 327–334.
Zhang, J., Zheng, P., Zhang, M., Chen, H., Chen, T., Xie, Z., et al. (2013). Bioresource Technology, 149, 44–50.
Ge, Z., Ping, Q., Xiao, L., & He, Z. (2013). Desalination, 312, 52–59.
Gálvez, A., Greenman, J., & Ieropoulos, I. (2009). Bioresource Technology, 100(21), 5085–5091.
Ozkaya, B., Cetinkaya, A. Y., Cakmakcı, M., Karadag, D., & Sahinkaya, E. (2013). Bioprocess and Biosystems Engineering, 36, 399–405.
Greenman, J., Gálvez, A., Giusti, L., & Ieropoulos, I. (2009). Enzyme and Microbial Technology, 44(2), 112–119.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cirik, K. Optimization of Bioelectricity Generation in Fed-Batch Microbial Fuel Cell: Effect of Electrode Material, Initial Substrate Concentration, and Cycle Time. Appl Biochem Biotechnol 173, 205–214 (2014). https://doi.org/10.1007/s12010-014-0834-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0834-1