Abstract
Cellulases can be used for biofuel production to decrease the fuel crises in the world. Microorganisms cultured on lignocellulosic wastes can be used for the production of cellulolytic enzymes at large scale. In the current study, cellulolytic enzyme production potential of Aspergillus fumigatus was explored and optimized by employing various cultural and nutritional parameters. Maximum endoglucanase production was observed after 72 h at 55 °C, pH 5.5, and 70 % moisture level. Addition of 0.3 % of fructose, peptone, and Tween-80 further enhanced the production of endoglucanase. Maximum purification was achieved with 40 % ammonium sulfate, and it was purified 2.63-fold by gel filtration chromatography. Endoglucanase has 55 °C optimum temperature, 4.8 optimum pH, 3.97 mM K m, and 8.53 μM/mL/min V max. Maximum exoglucanase production was observed at 55 °C after 72 h, at pH 5.5, and 70 % moisture level. Further addition of 0.3 % of each of fructose, peptone, and Tween-80 enhances the secretion of endoglucanase. It was purified 3.30-fold in the presence of 40 % ammonium sulfate followed by gel filtration chromatography. Its optimum temperature was 55 °C, optimum pH was 4.8, 4.34 mM K m, and 7.29 μM/mL/min V max. In the case of β-glucosidase, maximum activity was observed after 72 h at 55 °C, pH 5.5, and 70 % moisture level. The presence of 0.3 % of fructose, peptone, and Tween-80 in media has beneficial impact on β-glucosidase production. A 4.36-fold purification was achieved by 40 % ammonium sulfate precipitation and gel filtration chromatography. Optimum temperature of β-glucosidase was 55 °C, optimum pH was 4.8, K m was 4.92 mM, and V max 6.75 μM/mL/min. It was also observed that fructose is better than glucose, and peptone is better than urea for the growth of A. fumigatus. The K m and V max values indicated that endoglucanase, exoglucanase, and β-glucosidase have good affinity for their substrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rising costs of raw materials and environmental issues have diverted the attention of researchers towards the exploration of alternate sources of energy and materials. In this context, the application of biological systems for the production of enzymes, vaccines, fuels, chemicals, etc. has been quite successful during the recent years. Bioconversion of biomass has significant advantages over other alternative energy strategies because biomass is the most abundant and also the most renewable biomaterial. Bioconversion of lignocellulosic residues is initiated primarily by microorganisms such as fungi and bacteria which are capable of degrading lignocellulolytic materials [1, 2].
Enzymatically hydrolyzed products can act as raw material for the production of other important chemicals like ethanol, methanol, and other fuels [3]. These biofuels can replace currently used petroleum-based fuels and can help to reduce fuel crises. Utilization of biofuels can also cause the reduction of greenhouse gas emission that causes global warming [4].
Cellulose is a biopolymer consisting of linear chain of d-glucose linked through β (1 → 4) glycosidic linkage [5]. Most of the hydrolysis of a lignocellulosic material is due to a synergistic action of a group of cellulolytic enzymes; the most important are endoglucanase, exoglucanase, and β-glucosidase [6, 7].
Cellulases are the hydrolytic enzymes produced by many types of microorganisms. Fungi and bacteria are the major natural agents that produce cellulases. Fungal strains secrete more cellulases than the bacterial strains and are considered an excellent protein secretor [8, 9]. Studies showed that the presence of extra nutrients in the growing media of Aspergillus fumigatus increased cellulase production comparable to other higher cellulase-producing strains [10]. Another study conducted in 2013 reported that cellulases of A. fumigatus produced under optimized conditions increased the brightness of office paper more than 80 [11]. Various cellulases act in synergistic manner which enhances their hydrolytic efficiency [12]. A. fumigatus has the ability to secrete many other extracellular enzymes including lipases and xylanase [13].
Cellulases and hemicellulases have numerous applications and biotechnological potential for various industries including chemicals, fuel, food, brewery and wine, animal feed, textile and laundry, pulp and paper, and agriculture [6, 14, 15]. Therefore, the current study was designed for investigating economic cellulase production that can be a good agent for industrial applications.
Materials and Methods
Lignocellulosic Substrate Wheat straw collected from the surroundings of Rawalpindi and Islamabad was used as lignocellulosic substrate for fungus. It was air dried for 10 days, and in order to remove any remaining moisture contents, the substrate was oven dried for 24 h at 60 °C. It was then ground to powder at the Department of Soil Science, PMAS Arid Agricultural University Rawalpindi. The substrate was then packed in air-tight plastic jars for subsequent use in fermentation process.
Fermentative Organism Thermophilic A. fumigatus was isolated from the temperate region of Pakistan and was used for the production of cellulases. Fungus was identified on morphological basis from the fungi culturing laboratory of the Plant Pathology Department, PMAS Arid Agriculture University Rawalpindi. The colonies of A. fumigatus were picked on loop and maintained on potato dextrose agar (PDA) media to get pure cultures [16]. Slants were prepared from these pure cultures. The slants and pure cultures of fungus were preserved at 4 °C in a refrigerator for later use.
Inoculum Preparation The flask containing inoculum media was adjusted at pH 5.5 with the help of 1 M HCl/NaOH and autoclaved at 121 °C and 15 psi for 15 min. The flask was then inoculated aseptically with a loopful of fungal spores from preserved slants and placed in a shaking incubator at 180 rpm and 55 °C for 72 h. The conidial (spores) suspension was adjusted at 107–108 conidia/mL with the help of a hemocytometer and a biomass monitor (ABER 220 UK) [17].
Fermentation Process Solid-state fermentation (SSF) process was used for the production of cellulases by A. fumigatus using wheat straw as a substrate. Flasks containing 5 g of ground wheat straw were moistened with 3.5 mL (70 % of dry contents) of distilled water, having pH 5. Each flask, after autoclaving, was inoculated aseptically with 2 mL of A. fumigatus inoculum. These flasks were then incubated in duplicate at 55 °C for a specific day.
Crude Enzyme Harvesting After the specified day, the flasks were harvested for the extraction of cellulases by contact method. In each of the flasks, 50 mL of distilled water (pH 5) was added. These flasks were shaken at 120 rpm for 1 h in a shaking incubator. In this way, all the extra cellular enzymes dissolved in the water, which were then filtered with the help of a filter paper. Filtered enzyme extracts were then centrifuged at 10,000 rpm for 10 min at 4 °C to remove spores and other impurities. Supernatant was stored as a crude enzyme at 4 °C before performing the assay [18, 19].
Optimization of Parameters The enzyme production was optimized by maintaining different conditions in order to get maximum production of cellulases from A. fumigatus. These conditions were different growth factors as well as nutritional factors like carbon and nitrogen sources. After optimizing one factor, its optimized concentration was included in the next experiment for the optimization of the next parameter.
Cellulase Assay The activity of the cellulases was checked by adding 1 mL of crude enzyme into 1 mL of substrate solution (1 %) in a test tube. The pH of the mixture was maintained with the help of 1 mL of phosphate buffer having pH 5 [18]. The test tubes were then incubated at 55 °C for 30 min in the incubator. After that, 3 mL of dinitrosalicylic acid (DNS) was added into each test tube to stop the reaction, and tubes were placed in boiling water for 15 min. During boiling, DNS reacts with enzymatically digested products and forms complexes. The concentration of these complexes was detected by measuring the absorbance at 540 nm in a spectrophotometer.
Cellulase Enzyme Substrates Carboxymethyl cellulose, Avicel, and Salicin were used as substrate of endoglucanase, exoglucanase, and β-glucosidase, respectively.
Enzyme Activity One unit of enzyme activity in each case was defined as the amount of enzyme which released 1 μmol of product per minute.
Calculation of Cellulase Activity Enzyme activity would be calculated by using the following formula:
where
Protein Estimation Protein contents were estimated in a crude enzyme sample according to the biuret method.
Purification of Crude Enzymes The enzyme produced under optimized conditions was purified for further characterization. The following methods were used for the purification of cellulase.
Ammonium Sulfate Precipitation Ammonium sulfate causes the precipitation of proteins into the solution by decreasing their solubility. The crude cellulases were partially purified by adding different concentrations of (NH4)2SO4, e.g., 20, 30, 40, 50, and 60 % in 10 mL of enzyme. Partially purified enzymes were subjected to activity and biuret assay in order to find out the protein concentration.
Gel Filtration Chromatography Ammonium sulfate precipitation-purified enzyme was then subjected to gel filtration chromatography for further purification; 5 % of silica gel column was used for the purification of cellulases, dissolved in sodium citrate buffer having pH 5. Elutions were maintained at a linear flow rate 30 cm/h [20]. The different elutions were subjected to enzyme activity assay and biuret assay to detect the concentration of protein. Elution having a maximum activity was further used for characterization of different kinetic parameters [21].
Characterization of Cellulases Cellulases purified through ammonium sulfate precipitation and gel filtration chromatography was subjected to the characterization of different kinetic parameters, to study on the following:
-
1.
effect of pH on cellulases
-
2.
effect of temperature
-
3.
effect of substrate concentration for the determination of K m and V max
Results and Discussion
Optimization of Fermentation Conditions A. fumigatus was grown on wheat straw, and various growth parameters were optimized to obtain maximum cellulase production.
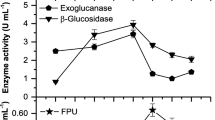
All the cellulases have a maximum activity after 72 h of growth of A. fumigatus on growing media in controlled conditions (Fig. 1). pH plays a vital role in the fermentation process by maintaining suitable ionic strength; during the current study, all three cellulases gave a maximum activity at pH 5.5 of the growth media (Fig. 2). A. fumigatus being thermophilic showed higher growth and cellulase activity at 55 °C (Fig. 3). Beta-glucosidase has higher activity than the other two cellulases. The presence of a suitable moisture level in solid-state fermentation is very important for fungal growth; it was found that in the presence of 70 % moisture level (including 2 mL inoculum), A. fumigatus gave more cellulase production [22].
Optimization of Nutritional Conditions Availability of carbon source and other nutrients in the vicinity is necessary for the growth of fungus. The presence of additional readily available nutritional sources enhanced the growth as well as production of associated enzymes [23]. The presence of 0.3 % fructose (% of total dry weight) is the most suitable than the other concentrations and presence of glucose (Fig. 4). Peptone as an additional nitrogen source increases all cellulase production at a concentration of 0.3 % than urea and ammonium sulfate (Fig. 5). Tween-80 acts as a better surfactant than Tween-20 and SDS; it increases the degradation of substrate. The presence of 0.3 % of Tween-80 (% of total dry weight) in growth media was found more suitable for cellulose enzyme production by A. fumigatus.
Partial Purification of Cellulases
Ammonium Sulfate Purification Cellulases produced at optimized conditions were partially purified by adding different concentrations of ammonium sulfate. Ammonium sulfate causes the precipitation of protein by salting out process, and it increases the ionic strength of the solution and decreases the solubility of protein. In 10 mL of crude enzyme sample, 20, 30, 40, 50, and 60 % were added overnight. Enzyme samples were then centrifuged at 10,000 rpm for 10 min, and supernatants were used for activity assay. Maximum precipitation was observed at 40 % of (NH4)2SO4 for all cellulases, and further addition of (NH4)2SO4 decreased the precipitation (Table 1) [24].
Gel Filtration Chromatography Partially purified cellulases were further purified by gel filtration chromatography, using 5 % silica gel column. Enzymes were run in citrate buffer having pH 4.8, and different elutions obtained were subjected to activity assay. It was observed that there was an increase in enzymatic activity and reduction in total protein concentration after gel filtration chromatography. It was due to purification of our desired proteins and exclusion of unnecessary proteins in crude sample. Elutions having a maximum activity were further used for protein estimation and enzyme characterization [25].
Characterization of Cellulases Partially purified cellulases were characterized for optimum temperature, pH, and kinetic parameters like K m and V max. Characterization of enzymes enhances their efficiency and feasibility for industrial processes.
Temperature and pH The results of pH and temperature characterization of cellulases produced by A. Fumigatus indicated that these enzymes are thermophilic and have a maximum activity at 55 °C (Fig. 6). pH characterization indicates that all cellulases are functional in acidic pH range with optimum pH 5.5 (Fig. 7). These results made these enzymes suitable for high-temperature industrial processes taking place in acidic conditions.
Study on Enzyme Kinetics
To find out the effect of substrate concentration on enzyme activity and K m and V max, solutions of substrate of different concentrations were prepared. These were 2, 4, 6, 8, and 10 mM of CMC, Avicel, and Salicin for endoglucanase, exoglucanase, and β-glucosidase, respectively. With increasing concentration of substrate, the velocity of enzyme increases, but after a certain concentration, the rate becomes constant. This is due to the nonavailability of the binding sites for the substrate on enzyme. K m and V max were found from the Lineweaver-Burk plot between 1/[S] on the X-axis and 1/[V0] on the Y-axis; these were 3.97 mM and 8.53 μM/mL/min for endoglucanase and 4.34 mM and7.29 μM/mL/min for exoglucanase [26], while K m was 4.92 mM and Vmax was 6.75 μM/mL/min for β-glucosidase. All the three cellulases gave a maximum activity at the temperature of 55 °C and pH 5.5 [27].
Conclusion
Partially purified cellulases produced from A. fumigatus under optimum conditions having activity around 4 U/mL can be a suitable agent for industrial processes. Characterization studies indicate that these enzymes remain active for a range of pH and temperature values. Cellulases of A. fumigatus have higher affinity for their substrate and have good enzymatic activity. Further purification through affinity chromatography and characterization would more explore their potential [28].
References
Carere, C. R., Sparling, R., Cicek, N., & Levin, B. D. (2008). Third Generation biofuels via direct cellulose fermentation. International Journal of Molecular Sciences, 9, 1342–1360.
Liu, R. G., Yu, H., & Huang, Y. (2005). Structure and morphology of cellulose in wheat straw. Cellulose, 12, 25–34.
Levin, D. B., Islam, R., Cicek, N., & Sparling, R. (2006). Hydrogen production by Clostridium thermocellum 27405 from cellulosic biomass substrates. International Journal of Hydrogen Energy, 31, 1496–1503.
Dashtban, M., Schraft, H., & Qin, W. (2009). Fungal bioconversion of lignocellulosic residues: opportunities and perspectives. International Journal of Biological Sciences, 5118, 578–595.
Jarvis, M. (2003). Cellulose stacks up. Nature, 426, 611–612.
Bhat, M. K. (2000). Cellulases and related enzymes in biotechnology. Biotechnology Advances, 18, 355–383.
Malherbe, S., & Cloete, T. E. (2002). Lignocellulose biodegradation: fundamentals and applications: a review. Environmental Science and Biotechnology, 1(2), 105–114.
Amouri, B., & Gargouri, A. (2006). Characterization of a novel β-glucosidase from a Stachybotrys strain. Biochemical Engineering Journal, 32, 191–197.
Beauchemin, K. A., Colombatto, D., Morgavi, D. P., Yang, W. Z., & Rode, L. M. (2003). Use of exogenous fibrolytic enzymes to improve animal feed utilization by ruminant. Journal of Animal Science, 81(2), 37–47.
Stewart, J. C., & Parry, J. B. (1981). Factors influencing the production of cellulase by Aspergillus fumigatus (Fresenius). Journal of General Microbiology, 125(1), 33–39.
Das, A., Paul, T., Halder, S. K., Jana, A., Maity, C., Dasmohopatra, P. K., et al. (2013). Production of cellulolytic enzymes by Aspergillus fumigatus ABK9 in wheat bran-rice straw mixed substrate and use of cocktail enzymes for deinking of waste office paper pulp. Bioresource Technology, 128, 290–296.
Wnag, D., Sun, J., Yu, L. H., Li, C. X., Bao, J., & Xu, J. H. (2012). Maximum saccharification of cellulose complex by an enzyme cocktail supplemented with cellulase from newly isolated Aspergillus fumigatus ECU0811. Applied Biochemistry and Biotechnology, 166, 176–186.
Shangguan, J. J., Fan, L. Q., Ju, X., Zhu, Q. Q., Wang, F. J., Zhao, J., et al. (2012). Expression and characterization of a novel enantioselective lipase from Aspergillus fumigatus. Applied Biochemistry and Biotechnology, 168, 1820–1833.
Sun, Y., & Cheng, J. (2002). Hydrolysis of lignocellulosic material from ethanol production. A review. Bioresource Technology, 83, 1–11.
Wong, K. K. Y., & Seddler, J. N. (1992). Application of hemicellulases in food, feed and pulp and paper industries. In P. P. Coyghlan & G. P. Hazlewood (Eds.), Hemicellulose and hemicellulases (pp. 127–143). London: Portland.
Sherief, A. A., Al-Tanash, A. B., & Atia, N. (2010). Cellulase production by Aspergillus fumigatus grown on mixed substrate of rice straw and rice bran. Research Journal of Microbiology, 199–211.
Zerofonetis. (1959). Approved laboratory techniques. Appleton. Century Craft Inc. New York. 5: 54–59.
Shafique, S., Asgher, M., Sheikh, M. A., & Asad, M. J. (2004). Solid state fermentation of banana stalk for exoglucanase production. International Journal of Agriculture and Biology, 3, 488–491.
Latifian, M., Hamidi, E. Z., & Barzegar, M. (2007). Evaluation of culture conditions for cellulase production by two Trichoderma reesei mutants under solid-state fermentation conditions. Bioresource Technology, 98, 3634–3637.
Ahmed, S., Bashir, A., Saleem, H., Saadia, M., & Jamil, A. (2009). Production and purification of cellulose degrading enzymes from a filamentous fungus Trichoderma harzianum. Pakistan Journal of Botany, 41(3), 1411–1419.
Kocher, G., Kalra, K., & Banta, G. (2008). Optimization of cellulase production by submerged fermentation of rice straw by Trichoderma harzianum Rut-C 8230. International Journal of Microbiology, 5.
Brijwani, K., Oberoi, H. S., & Vadlani, P. V. (2010). Production of cellulolytic enzyme system in mixed culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochemistry, 45, 120–128.
Bisaria, V. S., & Mishra, S. (1989). Regulatory aspects of cellulase biosynthesis and secretion. Critical Reviews in Biotechnology, 9, 61–103.
Mahmood, R. T., Asad, M. J., Mehboob, N., Mushtaq, M., Gulfraz, M., Hadri, S. H., et al. (2013). Production, purification and characterization of exoglucanase by Aspergillus fumigatus. Applied Biochemistry and Biotechnology, 170(4), 895–908.
Han, Y., & Chen, H. (2010). Biochemical characterization of maize stover β-exoglucanase and its use in lignocellulose conversion. Bioresource Technology, 101, 6111–6117.
Jabbar, A., Rashid, M. H., Javed, M. R., Perveen, R., & Malana, M. A. (2008). Kinetics and thermodynamics of a novel endoglucanase (CMCase) from Gymnoascella citrine produced under solid state condition. Journal of Industry and Microbiology Biotechnology, 33, 515–524.
Iqbal, H. M. N., Ahmed, I., Muhammad, A. Z., & Muhammad, I. (2011). Purification and characterization of the kinetic parameters of cellulases produced from wheat straw by Trichoderma viride under SSF and its detergent compatibility. Advances in Bioscience and Biotechnology, 2, 149–165.
Cen, P., & Xia, L. (1999). Production of cellulase in solid state fermentation. In: Scheper T, editor. Recent progress in bioconversion of lignocellulosics. Advances in Biochemical Engineering Biotechnology, 65, 69.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehboob, N., Asad, M.J., Asgher, M. et al. Exploring Thermophilic Cellulolytic Enzyme Production Potential of Aspergillus fumigatus by the Solid-State Fermentation of Wheat Straw. Appl Biochem Biotechnol 172, 3646–3655 (2014). https://doi.org/10.1007/s12010-014-0796-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0796-3