Abstract
The breakdown of cellulose, the most prevalent carbon resource on Earth, by cellulase is very important for acquiring soluble sugars. Solid-state fermentation (SSF) stands out as a proficient approach for generating economically valuable compounds, facilitating cost reduction in production. The fermentation factors were optimized to enhance cellulolytic enzyme production. Of the various inexpensive and readily accessible lignocellulosic residues, sorghum straw emerged as the utmost appropriate substrate. The maximal cellulase productivity of 14.12 ± 0.06 U/g DS was obtained after 72 h of fermentation using sorghum straw with 10% v/v moisture content at pH 7, 37 °C, and an inoculum volume of 1.5% v/v. The crude cellulase was purified using various methods. The employment of the aqueous two-phase system (ATPS) utilizing a polyethylene glycol 8000/MnSO4 combination demonstrated optimal purification, resulting in a 26.02-fold enhancement in activity, a yield of 48.7%, and a partition coefficient of 1.27. The molecular size of cellulase was approximated to be 84 kDa using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). The optimum pH and temperature for enzyme activity were identified as 7.0 and 50 °C, respectively. The cellulolytic activity exhibited the highest stimulation in the presence of Mn2+. Upon enzymatic saccharification of alkali-treated sorghum feedstock, the highest reducing sugar (30.51 ± 0.13 mg/mL) was obtained after an incubation period of 72 h, with a substrate loading of 4% w/v, enzyme concentration 30 U/g DS, pH 5, and the presence of Tween-80 as a surfactant. These findings may pave the way for a cost-effective and competent process within the framework of the biorefinery concept.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microbes growing on cellulosic materials release a group of enzymes called cellulase. In nature, cellulose is present in abundance. During crop production and harvesting, a large amount of lignocellulosic waste is generated, which can be utilized as a sustainable and economically viable alternative for the production of energy and better-quality products. About 120 × 109 tons of lignocellulosic biomass (LCB) is produced worldwide each year [1]. A large proportion of LCB is subjected to open agricultural burning, especially in developing nations such as India, causing environmental pollution and serious effects on human well-being [2]. Cellulase converts the cellulosic portion of waste biomass to sugars via enzymatic saccharification. Numerous value-added goods, involving ethanol, are obtained from the conversion of these sugars. Cellulases are important in several industrial sectors like food, fuel, textile, animal feed, paper and pulp, and detergents. Other applications include protoplast production, waste management, genetic engineering, and the pharmaceutical industry [3].
Fungal cellulases produced chiefly from Aspergillus and Trichoderma using solid-state fermentation (SSF) are used for industrial purposes. SSF requires minimal moisture. The substrate can be colonized by filamentous fungi (like Aspergillus and Trichoderma) even in water-deficient environments. Crop residues such as sorghum straw can act as a solid substrate for the growth of microbes. SSF is a widely used approach for enzyme production because it recovers more concentrated crude enzymes than submerged fermentation, thus reducing the cost of downstream processing. Additionally, SSF is economical and needs low operative costs and less investment [4, 5].
The most important factor limiting enzyme production is the production cost. The solution to this problem is the optimization of the fermentation medium and physiochemical factors to achieve maximum yield. Optimization of the medium reduces production costs and opens research prospects for many industrial applications. Extracellular enzyme production is impacted by numerous factors, including pH, temperature, inoculum size, and components of the medium (carbon sources, nitrogen sources, and trace elements) [6].
Enzyme purification is a multistep process that involves the removal of unwanted proteins from a protein cocktail and the enrichment of the desired target protein. This leads to an increase in performance parameters [7]. Applications in the industrial sector require cellulase with high stability that can endure extreme temperatures as well as pH levels. The search for tolerant cellulases from microorganisms is conducted to meet this requirement [8]. For cellulases to be industrially suitable, they must be characterized under different conditions. Cellulolytic activity across an extensive array of pH and temperature levels, as well as the occurrence of chemical solvents, has important commercial and biotechnological applications.
The cellulose found within lignocellulosic material presents an appealing reservoir of simple sugar, specifically glucose. This glucose can be extracted through the enzymatic breakdown of cellulose, a procedure termed saccharification [9]. In recent times, there has been increasing global awareness of enzymatically saccharifying cellulose, primarily driven by the potential of glucose as a precursor for producing bioethanol fuel. This offers a promising alternative to finite fossil fuel energy sources.
Thus, the present study comprises the optimization of process parameters to achieve maximum cellulase production under SSF (using low-cost biomass, i.e., sorghum straw). The crude enzyme underwent purification utilizing various approaches, and a relative assessment of the effectiveness of every approach in purification was conducted. This comparative analysis provides valuable insights into the efficiency and practicality of different purification techniques. Subsequently, the cellulase was characterized and analyzed via SDS-PAGE. Characterization adds another layer of novelty by providing detailed information about the enzyme’s molecular properties. Additionally, the study investigated the practical application of cellulase in the saccharification process, uniquely demonstrating both its sustainable potential to convert biomass into fermentable sugars for biofuel production and its practical utility in advancing eco-friendly and cost-effective energy solutions.
1.1 Statement of novelty
Although substantial research has been conducted on cellulase production through SSF, there remains a significant gap in optimizing process parameters specifically for sorghum straw, a low-cost and underutilized biomass. The present study addresses this gap by optimizing these parameters to achieve maximum cellulase production under SSF conditions using sorghum straw. Unlike previous studies that often focus on more conventional substrates, this research explores the potential of sorghum straw, thus contributing to cost-effective and sustainable enzyme production. A key innovative aspect of this study is the use of the novel fungal isolate Aspergillus uvarum. This strain’s unique properties and its performance in SSF conditions using sorghum straw present new insights into microbial enzyme production. The investigation into the practical application of cellulase in the saccharification process demonstrates the enzyme’s potential utility in biofuel production, addressing a crucial step towards the development of sustainable energy sources. This holistic approach not only advances the understanding of cellulase production and application but also promotes the use of sorghum straw as a viable substrate, highlighting the study’s significance in both scientific and practical contexts.
2 Materials and methods
2.1 Microorganism
In this study, a pure culture of A. uvarum CBS 121591, formerly isolated from soil samples collected at Banasthali Vidyapith, Rajasthan, India (latitude 26.4035° N, longitude 75.8745° E), was utilized. The strain was preserved at 4 °C on PDA slants and plates and was recultivated every 2 weeks [10].
2.1.1 Fungal spore formation and harvesting
After 7 days of cultivation, slants containing A. uvarum were utilized to harvest spores. An aqueous spore solution was prepared using sterilized purified water with 0.5% v/v Tween 80. Slants were rinsed with sterile purified water, and the fungal filaments were delicately detached with an inoculating loop to liberate the spores into the suspension. The resulting solution was then preserved at 4 °C for subsequent seeding into the medium.
2.2 Enzyme production
SSF was performed using 5 g of wood shavings as the substrate, moistened with a 30% v/v mineral salt solution [11]. The composition of the mineral salt solution (MSS) was made following the process outlined by Kulkarni et al. [12]. Fungal cells (108 mL−1) were introduced into the flask and nurtured at 28 °C for 4 days. The crude enzyme was procured by adding 50 mM Na-citrate buffer (pH 4.8) to the production flasks, followed by a 2-h nurturing period at 28 °C and 120 rpm. The production medium was then filtered using muslin cloth and centrifuged at 10,000 × g at 4 °C for 10 min, and the subsequent supernatant was procured as the enzyme extract.
2.2.1 Cellulase assay
The total cellulase activity was analyzed using the FPase approach, following the standard procedure [13]. For this, enzyme extracts (0.5 mL) were added to a Whatman filter paper strip (1 × 6 cm; 50 mg) immersed in 1 mL of 50 mM Na-citrate buffer at pH 4.8. After conditioning for 1 h at 50 °C, the reducing sugars were evaluated using the Miller method [14]. Enzyme activity was quantified as one unit, signifying the amount of enzyme necessary to liberate 1 μM of product per minute under the defined analysis parameters. The enzyme units were expressed as units per gram of dry substrate (U/g DS).
2.3 Optimization of fermentation parameter using the one-factor-at-a-time approach (OFAT)
Several production factors were optimized to achieve the utmost enzyme productivity [15]. For screening fermentation time, flasks containing 5 g of wood shavings were seeded with spore inoculum and nurtured for a duration of 168 h (7 days). Individual flasks were sampled every 24 h. Different substrates, including wood shavings, coconut coir, sorghum straw, sawdust, and mixed biomass, were screened. Each 5-g substrate was hydrated with a MSS to attain a moisture level of 30% v/v to determine the optimum substrate. For optimization of moisture content, 5 g of sorghum straw was hydrated with a mineral salt solution at different concentrations (0, 10, 30, 50, 70, 90% v/v). Next, the fermentation medium was exposed to different pH levels (ranging from pH 3 to 9) to determine the optimum pH. The pH of the media was adjusted with 1 N HCl and 1 N NaOH, while the pH of the substrate was maintained via a mineral salt solution. For temperature optimization, the flasks were nurtured at diverse temperatures (28 °C, 37 °C, 44 °C, and 50 °C). Additionally, the production medium was optimized for inoculum volume by inoculating it with various concentrations (0.5–2.5% v/v) of freshly prepared spore suspension. Throughout the optimization process, enzyme extraction was conducted following the procedure outlined in Section 2.2, and crude enzyme extracts were analyzed for cellulolytic potential. All tests in this study were carried out in triplicate.
2.4 Enzyme purification
The enzyme was subjected to ion-separation chromatography employing DEAE-cellulose (Sigma Aldrich), which was pre-equilibrated with 10 mM phosphate buffer (pH 7.5). The targeted enzyme was allowed to adhere to the matrix at 4 °C for 2 h. The bound fractions were then eluted using a gradient of 0.1–0.5 M NaCl. The elutes were procured at a flow rate of 1 mL/5 min, with 2-mL fractions being collected. Protein content and enzyme potential were measured for each fraction [16]. DEAE-cellulose functions as a mild anion exchange resin, allowing the desired protein to remain in the eluate while other proteins bind to the resin. The active fractions were combined and further purified using gel permeation chromatography on a Sephadex G-100 column, which was pre-equilibrated with 10 mM phosphate buffer (pH 7.5). The flow rate was set to 5–6 mL h−1, and 1-mL fractions were harvested individually. Cellulolytic potential and protein concentration were assessed for every fraction. Protein concentration was assessed following the Lowry approach with BSA (bovine serum albumin) as the standard [17].
2.4.1 Aqueous two-phase separation (ATPS)
The ATPS was conducted using the methodology suggested by Lin et al. [18]. Stock solutions of (30% w/w) polymer (PEG4000, 6000, and 8000 MW) and 40% of diverse salt stock solutions (MnSO4, MgSO4) were used to prepare the polymeric phase setup. The setups were arranged in a graduated centrifuge tube. Partition was done at room temperature (22 ± 2 °C) by adding cell extract (up to 2 mL) to the biphasic solution, mixed completely in an orbital shaker at 200 rpm for about 30–40 min, and then allowed to settle undisturbed for 50 min.
Evaluation of relative value, volume ratio, partition coefficient, and yield of cellulase
The relative value of cellulase activity or cell content was calculated as the enzyme activity ratio (U/mL) or the cell content in the test mixture, compared to the reference phase (lacking ATP components) [19]. Additionally, the volume ratio (VR) refers to the proportion of the top phase volume (VT) to the bottom phase volume (VB), as described in Eq. 1:
The cellulase partition coefficient (K) was calculated by the ratio of cellulase potential or content of the cell in the top phase (CT) to the bottom phase (CB), as described in Eq. 2:
The yield (%) of cellulase in the top phase was assessed as described in Eq. 3:
2.4.2 Determination of molecular size
SDS-PAGE was conducted following Laemmli’s protocol [20]. Protein bands were examined using the Coomassie brilliant blue R-250 staining technique.
2.5 Characterization of purified enzyme
2.5.1 Influence of temperature
The impact of temperature on cellulase activity was investigated by exposing the reaction mixture to temperatures ranging from 30 to 90 °C in 10 °C increments. Additionally, the enzyme was kept at 50 °C for 0–120 min to assess its stability. The remaining cellulolytic potential was determined by conducting the process at a temperature of 50 °C and pH 7. The cellulolytic activity was regarded as 100% under specified assay parameters [21].
2.5.2 Influence of pH
The influence of pH on cellulase potential was measured across the pH spectrum of 3–10, using appropriate buffers (3–5, sodium citrate; 6–7, sodium phosphate; 8–9, Tris–HCl; 10, glycine–NaOH) under specified assay parameters. The enzyme’s pH stability was examined by pre-incubating it at 50 °C for 0–24 h at pH 7. Aliquots of the blend were then sampled to assess the remaining cellulolytic potential (%) under the specified assay parameters [21].
2.5.3 Influence of metal ions
The influence of metal ions on the potential of the purified cellulase was evaluated by exposing the enzyme to various salts (NaCl, CaCl2, MnSO4, MgSO4, CoSO4, FeSO4, K2SO4, and ZnSO4) at a concentration of 1 mM for 2 h. The cellulolytic assay was executed at 50 °C for 60 min and pH 7 [22].
2.6 Application of cellulase in saccharification of sorghum straw
The pretreatment of sorghum straw was performed using 2% w/v NaOH, as described in our earlier study [23]. Enzymatic saccharification was performed by adding 5 g of pretreated sorghum straw and cellulase enzyme (20 U/g DS) to a 250-mL Erlenmeyer flask. The flasks were nurtured at 50 °C for 48 h. Sample fractions of 1 mL were taken for centrifugation, and the supernatants were assessed for reducing sugars using the DNS method [14]. The optimization of enzymatic saccharification was conducted with respect to hydrolysis time, pH, enzyme loading, substrate concentrations, and the addition of surfactant [24]. Individually, the factor was optimized within a chosen range (hydrolysis time, 24–96 h; substrate load, 2–10% w/v; enzyme concentration, 10–40 U/g DS; pH, 4.0–6.5; and surfactant, Tween 20, Tween 80, PEG 2000, and Triton X-100).
2.7 Statistical analysis
The standard error ( ±) of the mean was calculated from three measurements. The significance of the variables was assessed using one-way ANOVA, with P < 0.05 considered significant, using IBM-SPSS 21 software.
3 Results and discussion
3.1 Optimization of fermentation factors
3.1.1 Optimization of incubation time
The cellulase activity pattern observed during the fermentation time screening experiments indicates that the highest cellulolytic activity of 11.10 ± 0.06 U/g DS was attained after 72 h of fermentation, as depicted in Fig. 1a. Cellulases are constituent of the primary metabolic processes, with their production commencing during the logarithmic growth phase and declining during the death phase [25]. The outcomes of this investigation indicated that cellulase production was lower during the first 0–24 h, indicating that the strain was using the medium to sustain its own growth and development. Cellulase production was lowest at 144–168 h, which may be attributed to nutrient depletion and the microbial cells entering the death phase. Lodha et al. [4] observed that the combined cultivation of Trichoderma reesei NCIM 1186 and Penicillium citrinum NCIM 768 resulted in peak cellulase production, reaching 6.71 FPU/gds by the sixth day of production.
Influence of (a) incubation time, (b) lignocellulosic biomass as carbon source, (c) moisture content, (d) pH, (e) temperature, and (f) inoculum volume, on cellulase production under solid-state fermentation. Each value is the mean of triplicates ± SD. The mean represented by a different letter is notably dissimilar (p < 0.05)
3.1.2 Optimization of lignocellulosic substrate
Among all the lignocelluloses tested, sorghum straw exhibited the peak cellulolytic activity (11.25 ± 0.05 U/g DS), as observed in Fig. 1b. Sorghum straw had the highest cellulase activity due to the ease of accessibility of cellulose (37.74%) and the lower lignin content (21.48%) in the biomass [26], resulting in efficient cellulose conversion to sugars. Utilization of sorghum husk produced higher cellulolytic and hemicellulolytic enzymes (0.15 U/mL) by Enterobacter sp. SUK-bio [27]. Pramanik et al. [28] reported that Bacillus pseudomycoides depicted maximal cellulase activity (3.5 U/mL) with sugarcane bagasse.
3.1.3 Optimization of moisture content
In SSF, the moisture level significantly influences fermentation and the release of various enzymes, particularly cellulases. Maximum cellulolytic activity (12.41 ± 0.06 U/g DS) was detected with a minimal moisture content of 10% v/v (Fig. 1c). Excessively high moisture levels in solid media reduce substrate porosity and restrict oxygen diffusion within the substrate. Conversely, extremely less water content in the substrate results in inadequate microbial proliferation, limited growth, and restricted access to nutrients [29]. A moisture ratio of 1:2 (w/v) was found to be optimal for the production of cellulase under SSF using coir waste from A. niger [30].
3.1.4 Optimization of pH
A surge in cellulolytic activity was detected as the pH increased from 3 to 7, reaching a maximum of 12.70 ± 0.05 U/g DS at pH 7. Further increases in pH from 7 to 9 led to an insignificant decrease in cellulolytic activity, though substantial activity was still observed at pH 9 (Fig. 1d). The lowest cellulolytic activity (3.68 ± 0.07 U/g DS) was detected at pH 3, indicating that more cellulolytic activity occurred under alkaline conditions compared to acidic pH. Comparable results were attained by Malik et al. [31], which showed optimal cellulolytic activity by Bacillus subtilis at pH 7. El-Hadi et al. [32] observed that Aspergillus hortai demonstrated peak cellulolytic activity (0.160 U/mL) at pH 7.
3.1.5 Optimization of temperature
The incubation temperature, a crucial factor influencing microbial growth, significantly impacts the metabolic functions of microorganisms and should be tailored for specific conditions. The cellulolytic activity profile across various temperatures revealed that A. uvarum achieved peak enzyme production at 37 °C, reaching 13.50 ± 0.04 U/g DS (Fig. 1e). As the incubation temperature rises, the frequency of collisions increases, leading to a higher reaction rate, a phenomenon detected in numerous chemical processes [33]. The outcomes of this investigation align with the outcomes of Ali et al. [34], who documented the optimal cellulolytic yields from the A. terreus and A. niger Z10 strain at 40 °C, respectively. The decline in cellulolytic potential observed past 40 °C and 50 °C may be due to heat-induced enzyme degradation. Bajar et al. [35] observed elevated cellulase activity from A. heteromorphus at an optimum temperature of 32.5 °C.
3.1.6 Optimization of inoculum size
An inoculum size of 1.5% v/v was identified as optimal for achieving the highest cellulase production, reaching approximately 14.12 ± 0.06 U/g DS, as illustrated in Fig. 1f. An elevated concentration of inoculum appears advantageous, likely due to the initial decline being mitigated by a dense inoculum. However, reduced cellulase productivity with higher inoculum volumes may be attributed to the struggle for nutrients among microbial colonies and potentially insufficient nutrients to sustain the vast populace, thereby limiting fungal development. Therefore, an appropriately selected inoculum size or dosage is essential to support robust fungal propagation and enzyme production [36]. A similar study reported that an optimal inoculum size of 1% v/v was required for maximum cellulase activity by Halobacillus sp. [37]. Additionally, a fungal inoculum size of 0.625% v/v was required for the maximum cellulase activity from Aspergillus flavus [2].
3.2 Purification of cellulase
A summary of the cellulase purification process is provided in Table 1. The sample was exposed to ion-separation chromatography using a DEAE-cellulose column adjusted with 10 mM phosphate buffer (pH 7.5). This step achieved a purification fold of 11.01, a specific activity of 6.72 U/mg, and a yield of 5.01%. In the next step of purification using Sephadex G-100 chromatography, the specific activity, yield, and purification fold were 13 U/mg, 1.07%, and 21.31, respectively. Hamdan and Jasim [38] demonstrated anion exchange chromatography of partially purified cellulase from Trichoderma longibrachiatum using a DEAE-cellulose column and reported a purification fold of 7.85, which is less than the purification fold stated in the present investigation. Dehghanikhah et al. [39] investigated the purification of alkalophilic cellulase from Bacillus subtilis using Q-sepharose ion-exchange chromatography and reported a specific activity of 10.86 U/mg, a purification fold of 2.72, and an enzyme yield of 0.2%. Shankar et al. [40] demonstrated the purification of cellulolytic enzyme from Bacillus pumilus using a Sephadex G-100 column and found a specific activity of 1.450 U/mg and a purification fold of 6.62.
A two-phase aqueous system using PEG with molecular weights of 4000, 6000, and 8000, in blend with diverse sulphate salts including MnSO4 and MgSO4, was tested for the purification of crude cellulase extract. Among all tested systems, the combination of PEG 8000 and MnSO4 exhibited maximal purification, with a 26.02-fold increase and a yield of 5.26% for cellulase produced from A. uvarum (Table 1). Thus, among several purification approaches, the ATPS (PEG/MnSO4) system exhibited notable recovery rates, requiring fewer product recovery phases and employing cost-effective phase-forming resources (Table 1). The enzyme’s partition coefficient (K) was 1.27 (Table 2), indicating the system’s potential as an economic and practical substitute method for cellulase purification. Kumar et al. [41] reported that among numerous purification approaches, the ATPS (PEG/MnSO4) system for cellulase from Schizophyllum commune showed a high recovery of 79.5% with a 10.4-fold rise in activity. According to Liu et al. [18], the cellulase derived from Bacillus velezensis achieved its highest enzyme retrieval rate (67.8%) and purification degree (1.14) under conditions of PEG 4000 (20.75% w/w), K2HPO4 (8.5% w/w), and pH 8.5 within an ATPS.
The purified cellulase from A. uvarum exhibited a monomeric band with a molecular size of about 84 kDa (Fig. 2). The cellulase produced by Aspergillus flavus exhibited a sole band of the enzyme at around 55 kDa, a molecular weight lower than that indicated in this investigation [42]. Yasmin et al. [43] stated a cellulase molecular mass of 87 kDa obtained from Trichoderma viride, indicating a notably high molecular mass and highlighting the capability of fungi to produce enzymes with substantial molecular weights.
3.3 Characterization of purified cellulase
3.3.1 Influence of temperature on enzyme activity and stability
The influence of temperature on purified cellulase activity was studied over an extensive array of temperatures (30–70 °C). The optimum potential was observed at 50 °C and decreased subsequently, as shown in Fig. 3a. Regarding temperature stability, the enzyme remained more than 70% stable at an optimum temperature of 50 °C for 60 min, and it retained more than 50% of its relative activity when incubated for 120 min, indicating the thermostable nature of the enzyme (Fig. 3b). The cellulase exhibited activity across a wide temperature spectrum spanning from 30 to 70 °C, showing maximum activity at 50 °C. Even at 70 °C, more than 85% of activity was retained, showing high thermostability. The decline in activity detected beyond the optimal temperature could be attributed to the breakdown of transient interactions, like hydrogen and Van der Waals bonds, which support the enzyme’s three-dimensional structure. Strong heat resistance is an alluring and advantageous feature of an enzyme for numerous biotechnological applications. Cellulase from Acinetobacter junii showed optimal activity and stability at 50 °C [44]. The purified enzyme potential from A. niger was optimum at a temperature of 40 °C [33].
Impact of temperature on activity and stability of purified cellulase (a) optimum temperature and (b) stability at 50 °C for different intervals of time (min). Impact of pH on activity and stability of purified cellulase (c) optimum pH and (d) stability at room temperature and pH 7 for different incubation periods (h). Each value is the mean of triplicates ± SD
3.3.2 Influence of pH on enzyme activity and stability
The purified cellulase’s pH activity was assessed by determining cellulolytic activity across a series of pH values from 3 to 10, utilizing various appropriate buffers (Fig. 3c). The highest cellulolytic potential was detected at pH 7.0. Regarding stability, at the optimal pH of 7, the enzyme retained more than 90% of its activity after 4 h of incubation, and it preserved more than 50% of its activity after 20 h, indicating the enzyme’s alkaline stability (Fig. 3d). Lately, numerous studies have focused on isolating thermostable alkaline enzymes from microbes because of their significant commercial prospect. The high pH and temperature stability exhibited by the purified cellulases could be valuable for rigorous commercial applications. Gad et al. [45] observed maximum cellulase activity from Geotrichum candidum at pH 7. Steiner and Margesin [46] reported that the cellulase from Bacillus mycoides was active over a pH range of 4–6, with optimal potential at pH 5. Bano et al. [42] stated that A. flavus exhibited low cellulolytic activity at pH 5–9, but showed a sharp increase at pH 10, displaying its profound alkaline tolerance.
3.3.3 Influence of metal ions on enzyme activity
The impact of metal ions on the purified cellulase displayed that different metal ions at a 1-mM concentration and pre-incubation for 2 h had varying effects on cellulolytic activity. Specifically, Mn2+, Fe2+, and Mg2+ increased cellulolytic activity, while other metal ions inhibited the enzyme to a certain extent. The maximum relative activity was detected with Mn2+ (136.35%), followed by Mg2+ (125.28%) and Fe2+ (118.78%) (Fig. 4). In contrast, the minimum relative activity was detected with Ca2+ (53.02%). Stimulatory actions of Mn2+ and Co2+ have been reported [47]. The influence of metal ions on purified cellulase derived from Acinetobacter junii revealed that at a concentration of 1 mM, Mn2+, Co2+, and Mg2+ enhanced cellulolytic potential, whereas Ni2+ and Hg2+ significantly suppressed enzyme potential [44]. Okonkwo [22] investigated the influence of numerous metal ions (Cu2+, Pb+, Hg+, Ba2+, Mn2+, Ca2+, Fe2+, Mg2+, Co2+, and Zn2+) on the cellulolytic potential from Aspergillus flavus and found that Fe2+ had a pronounced stimulating impact on the enzyme activity across all substrates at a concentration of 1 mM.
3.4 Enzymatic hydrolysis of pretreated LCB and its optimization
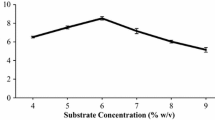
SPSS 21 (statistical Package for the Social Sciences) was utilized to analyze the effects of hydrolysis time, substrate load, enzyme concentration, pH, and surfactant on the production of reducing sugars from the pretreated substrate. ANOVA (analysis of variance) was used to examine the variances in reducing sugar yields attained with individual optimal saccharification factors. As indicated in Table 3, the pretreated biomass yielded a maximum of 30.51 ± 0.13 mg/mL reducing sugars after 72 h of incubation, with a 4% w/v substrate load, 30 U/g DS enzyme concentration, 5 pH, and in the presence of Tween-80 as the surfactant. Further increases in hydrolysis time, substrate load, enzyme concentration, and pH resulted in a decline in reducing sugar production. The reduction in hydrolysis rate beyond a certain threshold may be attributed to substrate inhibition due to excessive substrate addition or enzyme suppression resulting from elevated pH levels [48]. Low pH, near 3, has been shown to reduce fermentation rate [49]. Hari Krishna and Chowdary [50] discovered that pH greatly influences the hydrolysing activity of cellulases. The hydrolysis process occurs only after the formation of enzyme-substrate complexes, and the impacts of pH on both surface binding and breakdown are comparable, typically occurring at approximately pH 4.8. Phuengjayaem et al. [51] reported that pH 5 is optimal for the saccharification of sweet sorghum straw employing cellulase from Coriolus versicolor.
The incorporation of the surfactant Tween-80 showed a positive impact (30.51 ± 0.13 mg/mL) on saccharification. In contrast, the minimum saccharification was observed with PEG 2000 as the surfactant (15.41 ± 0.14 mg/mL). The inclusion of surfactants typically averts the ineffective enzyme binding to the lignin by altering the external characteristics of the feedstock. Overall, surfactants positively influence the saccharification process [52]. Pino et al. [53] studied the effects of non-ionic detergents (Tween 20, Tween 80, Span 80, and PEG 400) as possible enhancers of hydrolysis of hydrothermally pretreated agave bagasse (AGB) solids. Their findings revealed that non-ionic detergents enhanced the translation efficiency of cellulose to glucose, achieving a 100% yield with PEG 400 as a supplement. The incorporation of surfactants facilitates the production of simple sugars, contributing to the advancement of second-generation green refineries.
Following pretreatment, enzymatic hydrolysis becomes imperative for optimizing the production of reducing sugars. The advancement of cost-effective enzyme blends with enhanced efficacy is crucial for the viability of bioethanol making from LCB. Effective hydrolysis of pretreated biomass necessitates the concerted action of diverse lignocellulolytic enzymes, including endoglucanases, exoglucanases, and beta-glucosidases. Additionally, exploring enzyme reuse can contribute to cost reduction. Numerous studies have demonstrated comparable or superior saccharification efficiency when employing the same batch of enzyme blends for multiple cycles. For instance, Kumar et al. [54] utilized bound enzyme cocktails (Celluclast 1.5 L, BGL, and laccase) for the hydrolysis of pretreated rice straw, achieving an 84.6% saccharification rate relative to 77.3% with unbound enzymes. The elevated tolerance of immobilized enzymes to temperature and pH variations might explain the enhanced saccharification efficiency. Enhancements in enzyme formulation, reaction biomass feeding strategies, medium enhancement with additives, designing reactors, and employing methods for stream segregation and decontamination are potential avenues for optimizing enzymatic hydrolysis under high solid loading conditions.
4 Conclusion
In conclusion, this research study has delved into the multifaceted process of optimizing cellulase production under SSF conditions, followed by meticulous purification using strategies such as ion-exchange chromatography, gel permeation chromatography, and ATPS to obtain high yields of biologically active enzymes. Furthermore, the comprehensive characterization of the enzyme facilitates a deeper understanding of its catalytic mechanisms, which is instrumental in enhancing its industrial applicability. The optimization of saccharification processes was aimed towards increased efficiency and cost-effectiveness in bioethanol production.
Data availability
Not applicable.
References
Bhujbal SK, Ghosh P, Vijay VK, Rathour R, Kumar M, Singh L, Kapley A (2022) Biotechnological potential of rumen microbiota for sustainable bioconversion of lignocellulosic waste to biofuels and value-added products. Sci Total Environ 814:152773. https://doi.org/10.1016/j.scitotenv.2021.152773
Singhal A, Kumari N, Ghosh P, Singh Y, Garg S, Shah MP, Jha PK, Chauhan DK (2022) Optimizing cellulase production from Aspergillus flavus using response surface methodology and machine learning models. Environ Technol Innov 27:102805. https://doi.org/10.1016/j.eti.2022.102805
Bhati N, Shreya SAK (2021) Cost-effective cellulase production, improvement strategies, and future challenges. J Food Process Eng 44(2):e13623. https://doi.org/10.1111/jfpe.13623
Lodha A, Pawar S, Rathod V (2020) Optimised cellulase production from fungal co-culture of Trichoderma reesei NCIM 1186 and Penicillium citrinum NCIM 768 under solid state fermentation. J Environ Chem Eng 8(5):103958. https://doi.org/10.1016/j.jece.2020.103958
Darabzadeh N, Hamidi-Esfahani Z, Hejazi P (2018) Improvement of cellulase production and its characteristics by inducing mutation on Trichoderma reesei 2414 under solid state fermentation on rice by-products. Appl Food Biotechnol 5(1):11–8. https://doi.org/10.22037/afb.v5i1.18651
Boro M, Verma AK (2023) Optimization of cellulase production by Cohnella xylanilytica RU-14 using statistical methods. Appl Biochem Biotechnol 30:1–4. https://doi.org/10.1007/s12010-023-04447-4
Gaur R, Tiwari S (2015) Isolation, production, purification and characterization of an organic-solvent-thermostable alkalophilic cellulase from Bacillus vallismortis RG-07. BMC Biotechnol 15:1–2. https://doi.org/10.1186/s12896-015-0129-9
Ibrahim AS, El-diwany AI (2007) Isolation and identification of new cellulases producing thermophilic bacteria from an Egyptian hot spring and some properties of the crude enzyme. Aust J Basic Appl Sci 1(4):473–478
Salehi SA, Karimi K, Behzad T, Poornejad N (2012) Efficient conversion of rice straw to bioethanol using sodium carbonate pretreatment. Energ Fuel 26(12):7354–7361. https://doi.org/10.1021/ef301476b
Bhati N, Shreya SAK (2023) Production and optimisation of cellulase enzyme by Aspergillus uvarum CBS 121591 isolated from soil. Vegetos 36(1):201–209. https://doi.org/10.1007/s42535-022-00450-y
Shruthi K, Yadav PS, Prasad BS, Chandra MS (2019) Cellulase production by Aspergillus unguis in solid state fermentation. J For Res 30:205–212. https://doi.org/10.1007/s11676-018-0619-4
Kulkarni N, Vaidya T, Rathi G (2018) Optimization of cellulase production by Aspergillus species under solid state fermentation. Pharm Inn J 7(1):193–196
Mandels M, Andreotti R, Roche C (1976) Measurement of saccharifying cellulase. Biotechnol Bioeng Symp. 6:21–33
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428. https://doi.org/10.1021/ac60147a030
Darabzadeh N, Hamidi-Esfahani Z, Hejazi P (2019) Optimization of cellulase production under solid-state fermentation by a new mutant strain of Trichoderma reesei. Food Sci Nutr 7(2):572–578. https://doi.org/10.1002/fsn3.852
Bhati N, Shreya SAK (2023) Strain improvement of Aspergillus uvarum CBS 121591 for improved production of cellulase and its immobilization on calcium alginate beads. Biologia 78(8):2233–2243. https://doi.org/10.1007/s11756-023-01354-1
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Liu Y, Guo H, Gu J, Qin W (2019) Optimize purification of a cellulase from Bacillus velezensis A4 by aqueous two-phase system (ATPS) using response surface methodology. Process Biochem 87:196–203. https://doi.org/10.1016/j.procbio.2019.08.017
de Oliveira Júnior SD, de Araújo Padilha CE, de Asevedo EA, de Macedo GR, dos Santos ES (2020) Recovery and purification of cellulolytic enzymes from Aspergillus fumigatus CCT 7873 using an aqueous two-phase micellar system. Ann Microbiol 70:1–2. https://doi.org/10.1186/s13213-020-01573-w
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685. https://doi.org/10.1038/227680a0
Goel N, Patra R, Verma SK, Sharma PC (2019) Purification and characterization of cellulase from Pseudomonas sp. isolated from waste dumping site soil. J Appl Biotechnol Bioeng 6(3):118–24. https://doi.org/10.15406/jabb.2019.06.00183
Okonkwo IF (2019) Effect of metal ions and enzyme inhibitor on the activity of cellulase enzyme of aspergillus flavus. Int J Environ Agric Biotech 4(3). https://doi.org/10.22161/ijeab/4.3.20
Bhati N, Sharma AK (2023) Comparative study of different chemical pretreatments for enhanced enzymatic hydrolysis of sorghum straw. Biomass Convers Bior 12:1–9. https://doi.org/10.1007/s13399-023-05185-7
Aarti C, Khusro A, Agastian P (2018) Carboxymethyl cellulase production optimization from Glutamicibacter arilaitensis strain ALA4 and its application in lignocellulosic waste biomass saccharification. Prep Biochem Biotech 48(9):853–866. https://doi.org/10.1080/10826068.2018.1514513
Lokapirnasari WP, Nazar DS, Nurhajati T, Supranianondo K, Yulianto AB (2015) Production and assay of cellulolytic enzyme activity of Enterobacter cloacae WPL 214 isolated from bovine rumen fluid waste of Surabaya abbatoir, Indonesia. Vet World 8(3):367. https://doi.org/10.14202/vetworld.2015.367-371
Dong M, Wang S, Xu F, Wang J, Yang N, Li Q, Chen J, Li W (2019) Pretreatment of sweet sorghum straw and its enzymatic digestion: insight into the structural changes and visualization of hydrolysis process. Biotechnol Biofuels 12:1–1. https://doi.org/10.1186/s13068-019-1613-6
Waghmare PR, Patil SM, Jadhav SL, Jeon BH, Govindwar SP (2018) Utilization of agricultural waste biomass by cellulolytic isolate Enterobacter sp. SUK-Bio. Agric Nat Resour 52(5):399–406. https://doi.org/10.1016/j.anres.2018.10.019
Pramanik SK, Mahmud S, Paul GK, Jabin T, Naher K, Uddin MS, Zaman S, Saleh MA (2021) Fermentation optimization of cellulase production from sugarcane bagasse by Bacillus pseudomycoides and molecular modeling study of cellulase. Curr Res Microb Sci 2:100013. https://doi.org/10.1016/j.crmicr.2020.100013
Vu VH, Pham TA, Kim K (2011) Improvement of fungal cellulase production by mutation and optimization of solid state fermentation. Mycobiology 39(1):20–25. https://doi.org/10.4489/MYCO.2011.39.1.020
Mrudula S, Murugammal R (2011) Production of cellulase by Aspergillus niger under submerged and solid state fermentation using coir waste as a substrate. Braz J Microbiol 42:1119–1127. https://doi.org/10.1590/S1517-83822011000300033
Malik WA, Khan HM, Javed S (2021) Bioprocess optimization for enhanced production of bacterial cellulase and hydrolysis of sugarcane bagasse. BioEnergy Res 26:1–4. https://doi.org/10.1007/s12155-021-10259-3
El-Hadi AA, El-Nour SA, Hammad A, Kamel Z, Anwar M (2014) Optimization of cultural and nutritional conditions for carboxymethylcellulase production by Aspergillus hortai. J Radiat Res Appl Sci 7(1):23–28. https://doi.org/10.1016/j.jrras.2013.11.003
Sulyman AO, Igunnu A, Malomo SO (2020) Isolation, purification and characterization of cellulase produced by Aspergillus niger cultured on Arachis hypogaea shells. Heliyon 6(12). https://doi.org/10.1016/j.heliyon.2020.e05668
Ali S, Sayed A, Sarker RI, Alam R (1991) Factors affecting cellulase production by Aspergillus terreus using water hyacinth. World J Microbiol Biotechnol 7:62–66
Bajar S, Singh A, Bishnoi NR (2020) Exploration of low-cost agro-industrial waste substrate for cellulase and xylanase production using Aspergillus heteromorphus. Appl Water Sci 10(6):1–9. https://doi.org/10.1007/s13201-020-01236-w
Verma N, Kumar V (2020) Impact of process parameters and plant polysaccharide hydrolysates in cellulase production by Trichoderma reesei and Neurospora crassa under wheat bran based solid state fermentation. Biotechnol Rep 25:e00416. https://doi.org/10.1016/j.btre.2019.e00416
Korany AH, Ali AE, Essam TM, Megahed SA (2017) Optimization of cellulase production by Halobacillus sp. QLS 31 isolated from lake qarun, Egypt. Appl Biochem Biotechnol 183:189–199. https://doi.org/10.1007/s12010-017-2438-z
Hamdan NT, Jasim HM (2018) Purification and characterization of cellulase enzyme from Trichoderma longibrachiatum isolated in Iraqi soil. IOSR J Biotechnol Biochem 4:32–41. https://doi.org/10.9790/264X-04013241
Dehghanikhah F, Shakarami J, Asoodeh A (2020) Purification and biochemical characterization of alkalophilic cellulase from the symbiotic Bacillus subtilis BC1 of the leopard moth, Zeuzera pyrina (L.)(Lepidoptera: Cossidae). Curr Microbiol 77:1254–1261. https://doi.org/10.1007/s00284-020-01938-z
Shankar T, Sankaralingam S, Balachandran C, Chinnathambi A, Nasif O, Alharbi SA, Park S, Baskar K (2021) Purification and characterization of carboxymethylcellulase from Bacillus pumilus EWBCM1 isolated from earthworm gut (Eudrilus eugeniae). J King Saud Univ Sci 33(1):101261. https://doi.org/10.1016/j.jksus.2020.101261
Kumar B, Bhardwaj N, Alam A, Agrawal K, Prasad H, Verma P (2018) Production, purification and characterization of an acid/alkali and thermo tolerant cellulase from Schizophyllum commune NAIMCC-F-03379 and its application in hydrolysis of lignocellulosic wastes. AMB Express 8:1–16. https://doi.org/10.1186/s13568-018-0696-y
Bano A, Chen X, Prasongsuk S, Akbar A, Lotrakul P, Punnapayak H, Anwar M, Sajid S, Ali I (2019) Purification and characterization of cellulase from obligate halophilic Aspergillus flavus (TISTR 3637) and its prospects for bioethanol production. Appl Biochem Biotechnol 189:1327–1337. https://doi.org/10.1007/s12010-019-03086-y
Yasmin S, Mattoo RL, Nehvi FA (2013) Isolation, characterization and molecular weight determination of cellulase from Trichoderma viride. Afr J Biotechnol 12(28):4512–4518. https://doi.org/10.5897/AJB2013.12275
Banerjee S, Maiti TK, Roy RN (2020) Production, purification, and characterization of cellulase from Acinetobacter junii GAC 16.2, a novel cellulolytic gut isolate of Gryllotalpa africana, and its effects on cotton fiber and sawdust. Ann Microbiol 70:1–16. https://doi.org/10.1186/s13213-020-01569-6
Gad AM, Suleiman WB, El-Sheikh HH, Elmezayen HA, Beltagy EA (2022) Characterization of cellulase from Geotrichum candidum strain Gad1 approaching bioethanol production. Arab J Sci Eng 47(6):6837–6850. https://doi.org/10.1007/s13369-021-06391-z
Steiner E, Margesin R (2020) Production and partial characterization of a crude cold-active cellulase (CMCase) from Bacillus mycoides AR20-61 isolated from an Alpine forest site. Ann Microbiol 70:1–8. https://doi.org/10.1186/s13213-020-01607-3
Irfan M, Safdar A, Syed Q, Nadeem M (2012) Isolation and screening of cellulolytic bacteria from soil and optimization of cellulase production and activity. Turk J Biochem 37(3):287. https://doi.org/10.5505/tjb.2012.09709
Wen Z, Liao W, Chen S (2004) Hydrolysis of animal manure lignocellulosics for reducing sugar production. Bioresour Technol 91(1):31–39. https://doi.org/10.1016/S0960-8524(03)00166-4
Hajar N, Elida T (2012) Optimization of ethanol fermentation from pineapple peel extract using response surface methodology (RSM). Int J Nutr Food Eng 6(12):1102–1108. https://doi.org/10.5281/zenodo.1055487
Hari Krishna S, Chowdary GV (2000) Optimization of simultaneous saccharification and fermentation for the production of ethanol from lignocellulosic biomass. J Agric Food Chem 48(5):1971–1976. https://doi.org/10.1021/jf991296z
Phuengjayaem S, Poonsrisawat A, Petsom A, Teeradakorn S (2014) Optimization of saccharification conditions of acid-pretreated sweet sorghum straw using response surface methodology. J Agric Sci 6(9):120. https://doi.org/10.5539/jas.v6n9p120
Das S, Bhattacharya A, Haldar S, Ganguly A, Gu S, Ting YP, Chatterjee PK (2015) Optimization of enzymatic saccharification of water hyacinth biomass for bio-ethanol: comparison between artificial neural network and response surface methodology. Sustain Mater Technol 3:17–28. https://doi.org/10.1016/j.susmat.2015.01.001
Pino MS, Michelin M, Rodríguez-Jasso RM, Oliva-Taravilla A, Teixeira JA, Ruiz HA (2021) Hot compressed water pretreatment and surfactant effect on enzymatic hydrolysis using agave bagasse. Energies 14(16):4746. https://doi.org/10.3390/en14164746
Kumar V, Patel SK, Gupta RK, Otari SV, Gao H, Lee JK, Zhang L (2019) Enhanced saccharification and fermentation of rice straw by reducing the concentration of phenolic compounds using an immobilized enzyme cocktail. Biotechnol J 14(6):1800468. https://doi.org/10.1002/biot.201800468
Acknowledgements
The authors convey gratefulness to the Head of the Department of Bioscience and Biotechnology at Banasthali Vidyapith, Rajasthan, for supplying the essential research facilities required to conduct this study.
Author information
Authors and Affiliations
Contributions
Nikita Bhati: conceptualization, methodology, writing—original draft, formal analysis. Yatika Dixit: carried out some experiments. Preeti Yadav: carried out some experiments. Arun Kumar Sharma: conceptualization, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhati, N., Dixit, Y., Yadav, P. et al. Process optimization, purification, and characterization of cellulase from Aspergillus uvarum and its industrial application in saccharification. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-06122-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-06122-y