Abstract
A 1,080-bp cDNA (CGMCC 2873) encoding of a cold-active lipase of Aspergillus fumigatus (AFL67) was cloned and expressed in Escherichia coli for the first time. The new lipase, AFL67, was one-step purified by 8.30 folds through Ni–NTA affinity chromatography with a recovery of 86.8 %. The specific activity of purified AFL67 was 449 U mg−1 on p-NP hexanoate. AFL67 preferentially hydrolyzed p-nitrophenyl esters of short- and medium-chain fatty acids, with p-nitrophenyl hexanoate the maximum. The optimum temperature and pH was 15 °C and 7.5, respectively. The purified AFL67 was stable at 10–25 °C for 30 min, and in the pH range of 6.0–9.0 for 16 h (at 4 °C). Its activity was increased by 47 and 50 %, in the presence of 10 % (v/v) ethanol and isopropanol, respectively. The new lipase AFL67 highly enantioselectively deacylated (S)-α-acetoxyphenylacetic acid (APA) and o-Cl-APA, m-Cl-APA, and p-Cl-APA to (S)-mandelic acid and its derivates. These features render this cold-active novel lipase AFL67 attractive for biotechnological applications in the field of enantioselective synthesis of chiral mandelic acids, o-acylated mandelic acids, and their derivates and detergent additives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The importance of chirality has constantly increased in recent decades, and it is currently a central issue in the organic and pharmaceutical industry, especially in the development of new drugs. Non-steroidal anti-inflammatory drugs (NSAIDs) of the 2-arylpropionic acid class represent one of the most commercially successful and important classes of analgesic anti-inflammatory drugs. Mandelic acid (MA), the deacylated product of acetoxyphenylacetic acid (APA), is one of the styrene metabolites [1–4] and is considered to be the most important intermediates in the chemical synthesis and pharmaceutical production, together with its derivatives. (R)-MA has played an important role in producing semi-synthetic cephalosporins and penicillins [5], while (S)-MA has been used to synthesize substituted cyclopentenones and commercial drugs like the nonsteroidal anti-inflammatory drugs celecoxib (Celebrex®) and deracoxib [6, 7]. (R)-ο-chloromandelic acid ((R)-o-Cl-MA) is the key active center in the production of clopidogrel (Plavix, clopidogrel bisulfate), the second largest selling medicine with sales in the region of 6.4 billion dollars per year [8, 9]. Enzymatic production of chiral MA is a promising pathway for its features, such as high efficiency, high enantioselectivity, and coenzyme free [10].
Lipases (E.C. 3.1.1.3) are triacylglycerol hydrolases that catalyze hydrolysis reactions at the interface of lipids and water [11] with excellent activity and diversified stereoselectivity towards a wide range of substrates [12–15]. Except for detergent additives and the processing of fats and oils, lipases have been widely used in the production of single isomeric compounds because of their ability to kinetically resolve racemic mixtures or because of their stereoselective hydrolysis of prochiral diesters [3, 16]. Lipase-catalyzed enantioselective hydrolysis is a well-established procedure to access enantiomerically pure NSAIDs; and although many lipases have been identified and characterized, lipase resolving acylation of MA is rare and its reaction rates and enantioselectivity are unsatisfied [17, 18]. The production of (S)-MA via deacylation with (S)-α-acetoxyphenylacetic acid was only carried out by the whole Pseudomonas sp. cells isolated from soil samples in our previous study [19].
Fungi are the preferred resources for lipases, and numerous useful lipases have been isolated, cloned, and characterized from filamentous fungi, such as Geotrichum, Rhizopus, Mucor, Rhizomucor, Aspergillus, and Penicillium [3, 20–22]; however, few genes have been cloned from Aspergillus fumigatus. We have cloned and characterized a lipase AFL1-1 from A. fumigatus (CGMCC 2873). In this paper, a novel lipase, AFL67, which can enantioselectively hydrolyze racemic APA (RS-APA) to enantiopure S-MA and R-APA was cloned, highly expressed in Escherichia coli and characterized.
Materials and Methods
Strains and Materials
A. fumigatus Af 293 (CGMCC 2873) was purchased from the China General Microbiological Cultures Collection Center (CGMCC; Beijing, China); while the host strains E. coli DH5α, E. coli BL21 (DE3), and expression vector pET-28a (+) were obtained from Novagen (Carlsbad, CA, USA). Ex-Taq DNA polymerase, T4 DNA ligase, pMD-18T vector, restriction endonucleases, and DNA marker were purchased from TaKaRa Co. (Dalian, China). All kinds of p-nitrophenyl esters were prepared from p-nitrophenol and corresponding fatty acids [23]. Racemic mandelic acid ((RS)-MA) was purchased from Guangde Chemicals Co. Ltd. (Anhui, China). (S)-MA and (R)-MA were purchased from Sigma-Aldrich (St. Louis, MO, USA). (RS)-α-acetoxyphenylacetic acid (APA) was prepared with mandelic acids and acetyl chloride as previously reported [19]. All other chemicals were available commercially and of analytic reagent grade.
Extraction of A. fumigatus RNA and RT-PCR
A. fumigatus existed predominantly as mycelia and hyphae; 0.2 g mycelia and hyphae were scraped and ground to a fine powder with a pre-frozen mortar and pestle in liquid nitrogen. Total RNA was extracted from the prepared powder using a Trizol reagent (Invitrogen, USA), then cDNA was synthesized from 5 μg total RNA with oligo-dT [18] as the primer, using a 1st Strand Synthesis kit (Fermentas, USA) under the manufacturer’s instructions.
Cloning, Sequencing, and Overexpression of the Lipase Gene
The putative lipase gene (AFL67) was amplified from cDNA by polymerase chain reaction (PCR) with primers F1 (5′-CGCATATGGCAGACTACTCGGAAT-3′, Nde I site) and R1 (5′-CGCAAGCTTCTACTTTGTTTTGATC′, Hind III site), which were designed based on the putative lipase ORF sequence of A. fumigatus Af 293 (GenBank accession number: 3511767). The PCR program was as follows: 1 cycle of 94 °C for 5 min, 30 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 70 s, and a final extension cycle at 72 °C for 10 min. The amplified products were cloned directly into pMD18T to get pMD-afl67, and then sequenced by Majorbio Ltd (Shanghai, China). After digesting pMD18T-afl67 with Nde I and Hind III, afl67 was gel-purified and sub-cloned into pET28a which was previously digested with the same restriction enzymes, resulting in an expression plasmid pET28-afl67.
A single clone of E. coli BL21 (DE3) transformed with pET28-afl67 was inoculated into 30 ml LB media supplemented kanamycin (50 μg/ml) and then grown at 37 °C for 12 h. The culture was then diluted 1:100 into a fresh medium and again grown at 37 °C. When OD600 nm reached about 0.8, isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to a final concentration of 0.2 mM to induce the gene expression. After 15 h of induction at 15 °C, the cells were harvested by centrifugation at 3,000 rpm for 20 min and washed twice with normal saline.
Purification of the Recombinant Lipase and Protein Content
Ni–NTA agarose affinity chromatography was used for the enzyme purification. The cells harvested above were suspended in a 10-ml cold binding buffer (50 mM Tris–HCl, pH 8.0) and lysed by sonication at 400 W using a 50 % pulsed mode for 15 min. The supernatant was collected by centrifugation at 12,000 rpm for 10 min (4 °C) and loaded on Ni–NTA His-Bind resin column (ø1.0 × 10 cm), which was pre-equilibrated with 50 mM Tris–HCl (pH 8.0). After washing with the same buffer, the recombinant proteins were eluted with the solutions of 10, 200, and 500 mM imidazole dissolved in Tris–HCl (50 mM, pH 8.0). The collected protein fractions were analyzed by 13.5 % sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Bradford assay with bovine serum albumin as the standard was used to determine the protein concentration [24].
Lipase Assay
Lipase activity was examined by the spectrometric method using 100 mM 4-nitrophenyl hexanoate (p-NP hexanoate) as a substrate [25]. One unit of lipase activity was defined as the amount of enzyme releasing 1.0 μmol of p-nitrophenol per minute under the condition of 30 °C and pH 7.0 (100 mM KPB) [26]. All the assays were performed in triplicate, the average values were taken and corrections were made for spontaneous hydrolysis of the substrate. Relative activity was defined as the percentage of the maximum enzyme activity value.
Substrate Specificity on p-Nitrophenyl Esters
The substrate specificity of the lipase was studied using p-nitrophenyl fatty acid esters with various acyl chain lengths (C2–C16) under the activity assay conditions as described above.
Effects of Temperature and pH on Lipase Activity
The effect of temperature on the lipase activity was determined by measuring the hydrolytic activity at different temperatures ranging from 10 to 45 °C. The stability of the lipase on temperature was investigated by measuring the residual activity after incubating the purified lipase at 10–45 °C for 30 min.
The optimum pH was determined by measuring the hydrolytic activity in buffers with various pH values. The different pH buffers (50 mM) consisted of: citrate phosphate (pH 3.0–8.0), Tris–HCl (pH 8.5), and glycine–NaOH (pH 9.0–10.0). The effect of pH on lipase stability was determined by analyzing the residual activity after incubating the purified lipase in the buffers with different pH values (pH 4.0–10.0) at 4 °C for 16 h.
Effect of Metal Ions and Chemical Additives on Lipase Activity
After co-incubating the purified lipase with EDTA (2 mM), each of the various metal ions (2 mM) and each of the chemical additives (10 mM) in a 50-mM Tris–HCl buffer (pH 7.0) at 30 °C for 30 min, respectively, the effects on the hydrolytic activity were determined by measuring the residual activity. A sample without any additives under the same conditions was set as the control.
Effect of Organic Solvents on Lipase Activity
The enzyme was incubated in the presence of 10 % various organic solvents (methanol, ethanol, isopropanol, 1-butanol, isoamyl alcohol, n-hexane, isooctane, and trichloromethane) at 30 °C, 900 rpm for 1 h. The control was the sample without organic solvents under the same experimental conditions. Residual activity was measured with p-NP hexanoate as the substrate at pH 7.0, 30 °C.
Enantioselectively Deacylation of APA and HPLC Assay
The procedures of enantioselectively deacylation and high-performance liquid chromatography (HPLC) analysis have been described by Ju et al. [19]. Briefly, the resting recombinant cells (wet 4 g) were suspended in a 10-ml KPB buffer (100 mM, pH 7.0) containing 5 mM APA in a flask (50 ml) at 30 °C, 180 rpm for 3.5 h until yielding (S)-MA with the reaction reaching approximately 50 % conversion. The reaction was terminated with H2SO4 and extracted twice with ethyl acetate. Subsequently, the combined extract was dried over Na2SO4 for a high-performance liquid chromatography analysis.
HPLC assay was performed on HPLC (Shimadzu SPD-10A), equipped with a chiral column (Chiracel OD-H, Daicel Co., Japan). Hexane/isopropanol/trifluoroacetic acid (94:6:0.2 (v/v)) was used as the mobile phase at a flow rate of 1.0 ml/min, and the separation was monitored at 228 nm [19]. The retention time of (S)-APA, (R)-APA, (S)-MA, and (R)-MA were 10.8, 12.3, 20.3, and 23.9 min, respectively. The conversion (c) of APA, the enantiomeric excesses of APA (ee s) and MA (ee p) were calculated as previously reported [19].
Substrate Specificity on Cl-APA
Considering that AFL67 can catalyze APA with high enantioselectivity, the derivates of APA including o-Cl-APA, m-Cl-APA, and p-Cl-APA were also employed as a substrate. The method was as described above, with the retention times of the compounds shown in Table 1.
Results
Cloning of the Lipase Gene and Construction of the Expression Plasmids
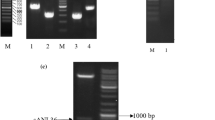
A DNA fragment (afl67) of about 1,000 bp was PCR amplified with primers described above (Fig. 1). Sequence alignment by DNAMAN software showed that the amplified sequence was 99.91 % similar to the putative lipase gene (Gene ID: 3511767). The sequence of afl67 was submitted to GenBank with an accession number of HQ231778.
After introducing the coding region of AFL67 into pET28a at Nde I and Hind III sites, the expression plasmid pET28-afl67 was constructed and then sequenced to verify its correctness and authenticity.
Expression and Purification of the Recombinant Lipase AFL67
Lipase AFL67 was overexpressed as an N-terminal (His)6 tag fusion protein in E. coli BL21 (DE3) harboring pET28-afl67. SDS-PAGE analysis showed that AFL67 was almost expressed in the inclusion body at 37 °C (data not shown). Lowering the induction temperature to 15 °C was used to enhance the soluble expression. Under optimized conditions (0.2 mM IPTG, 15 °C for 15 h.), maximal soluble AFL67 and maximal lipase activity were reached (Fig. 2a). The hydrolysis activity against 4-nitrophenyl hexanoate was about 4.74 × 104 U/L broth. The molecular weight of recombinant AFL67 was about 41 kDa (Fig. 2a), which coincided with the expected molecular weight.
SDS-PAGE analysis of expression (a) and purification (b) of AFL67 lipase. a SDS-PAGE analysis of the expression of AFL67 lipase. Lane 1 protein molecular weight markers, lane 2 lysates of uninduced recombinant cell, lane 3 lysates of induced recombinant cell, lane 4 supernatant of the recombinant cell extract, lane 5 inclusion body of the recombinant cell extract. b SDS-PAGE analysis of the purification of AFL67 lipase. Lane 1 protein molecular weight markers, lane 2 supernatant of the cell extract, lane 3 collection of flow-through, lane 4 elute with 50 mM Tris–HCl buffer (pH 8.0), lane 5 eluate with 50 mM Tris–HCl buffer containing 20 mM imidazole (pH 8.0), lane 6 elute with 50 mM Tris–HCl buffer containing 200 mM imidazole (pH 8.0)
The recombinant AFL67 was successfully one-step purified with Ni–NTA affinity chromatography using (His)6 tag portion. A single protein band with a molecular weight of 41 kDa was observed (Fig. 2b). As summarized in Table 2, the activity recovery was 86.8 %, the purification fold was 8.30, and the specific activity of the purified enzyme was 449 U mg−1 protein, respectively.
Specificity on p-Nitrophenyl Esters of AFL67
Substrate specificity of AFL67 towards acyl chain length was tested using various p-nitrophenyl esters including p-NP acetate (C2), p-NP butyrate (C4), p-PN hexanoate (C6), p-PN caprylate (C8), p-PN decanoate (C10), p-NP laurate (C12), and p-NP palmitate (C16). Figure 3 reveals that AFL67 was preferred to hydrolyze p-nitrophenyl esters with short and medium chains of fatty acids, and exhibited the highest activity with p-PN hexanoate (C6) at 30 °C, pH 7.0. There was almost no activity on long-chained acyl ester, such as p-NP laurate (C12) and p-NP palmitate (C16).
Effect of Temperature on Lipase AFL67 Activity
The temperature profiles of the pure lipase activity were studied in the ranges of 10 to 45 °C. The optimum temperature for AFL67 was 15 °C (Fig. 4). The activity dropped sharply above the optimum temperature and remained less than 40 % of its original activity at 30 °C. AFL67 was stable at 15 °C, but was almost inactivated at temperatures higher than 40 °C after 30 min incubation.
Effects of temperature on activity and stability of the recombinant lipase. Temperature-activity profile was determined by measuring the relative activity at different temperatures at pH 7.0 (black diamond). Temperature-stability profile was determined by measuring the residual activity at pH 7.0 and 30 °C after incubating the purified lipase at different temperatures for 30 min (white diamond). Values are means ± SD (n = 3)
Effect of pH on Lipase AFL67 Activity
The optimum pH for AFL67 was 7.5 using p-PN hexanoate as the substrate, and the activity dropped dramatically at pH of below 7.0 (Fig. 5). Above pH 9.0, the spontaneous hydrolysis of p-PN hexanoate was severe and the activity could not be detected. More than 60 % of the maximum activity remained at pH 6.0–9.0 after 16 h incubation at 4 °C (Fig. 5).
Effects of pH on activity and stability of the recombinant lipase. Purified lipase samples were assayed in various buffers from pH 6.0 to pH 9.0 using p-PN hexanoate (100 mM) as substrate at 30 °C (black diamond). Purified lipase samples were diluted in buffers with different pH values, pH adjusted, and incubated for 16 h at 4 °C and the residual activity was measured at pH 7.0, 30 °C (white diamond). Values are means ± SD (n = 3)
Effect of Metal Ions and Chemical Additives on Lipase AFL67 Activity
Effects of metal ions and chemical additives on the recombinant lipase activity were tested in a 50-mM Tris–HCl buffer (pH 7.0; Table 3). When 2 mmol/L Cu2+ or Zn2+ was added in the reaction mixture, enzyme activity was almost completely abolished, indicating that the catalytic active site might combine with Cu2+ or Zn2+ inhibiting substrate binding. Ni2+, Co2+, and Fe3+ strongly decreased lipase activity by 81.2, 81.5, and 50 %, respectively. Mn2+ and other metal ions also slightly decreased lipase activity. However, Ca2+ and EDTA did not significantly affect AFL67 activity.
As shown in Table 3, serine proteinase inhibitor (PMSF) and anionic surfactant SDS strongly inhibited the lipase activity, whereas β-ME increased the lipase activity by 17 %.
Effect of Organic Solvents on the Stability of Lipase AFL67
The sensitivity of lipases to organic solvents was diverse. Stability and activity in organic solvents are important characteristics of lipases used in organic synthesis. Table 4 shows the residue activity of AFL67 in the presence of different organic solvents (10 % v/v) after incubation at 30 °C for 1 h at 900 rpm. The rAF167 was activated in the presence of ethanol and isopropanol, not affected by methanol, and inactivated to some extent in the presence of 1-butanol, isoamyl alcohol, n-hexane, and trichloromethane. The observed highest activities to control were 147 % in ethanol and 150 % in isopropanol. In contrast, the activity decreased to 1.1, 0.85, or 0.15 % when 1-butanol, isoamyl alcohol, or trichloromethane were involved.
Enantioselectivity of AFL67
The best enantioselective catalytic effect of lipase AFL67 was observed in the deacylating of (S)-APA from a racemic mixture to produce (S)-MA and (R)-APA (Scheme 1). Hydrolysis of APA was conducted by whole recombinant AFL67 E. coli cells because they were more stable than the crude cell extracts.
Figure 6 shows the deacylation of 5 mM APA by 4 g recombinant cells at 30 °C. In the beginning, the amount of (R)-APA and (S)-APA was almost the same (Fig. 6a). After incubation for 1 h, about 7/9 (S)-APA was converted to (S)-MA, but (R)-APA was unreacted, the conversion was 38.6 %, the enantiomeric excess (ee) of the residual (R)-APA was 69.1 %, and ee of produced (S)-MA was 88.7 % (Fig. 6b). After incubation for 2 h, the conversion was 47 %, ee of residual (R)-APA was 93.9 %, and ee of (S)-MA was 89.5 % (Fig. 6c). After incubation at 30 °C for 3.5 h, the conversion was 48 %, ee of the residual (R)-APA was more than 97.2 %, and ee of produced (S)-MA was 91.9 % (Fig. 6d). AFL67 biocatalytically deacylated (S)-APA with high enantioselectivity of E = 64.
Substrate Specificity on Cl-APA
Considering that AFL67 can catalyze APA with high enantioselectivity, the derivates of APA, including o-Cl-APA, m-Cl-APA, and p-Cl-APA, were also employed as a substrate. As shown in Table 5, all derivates were enantioselectively deacylated, while the ee of o-Cl-MA, m-Cl-MA, and p-Cl-MA were 91.3, 88.5, and 99.5 %, respectively. That is to say, compared with m-Cl-APA, AFL67 was preferred to catalyze o-Cl-APA and p-Cl-APA.
Discussion
Lipases (EC 3.1.1.3) are enzymes originally characterized by their ability to hydrolyze triglycerides at the oil–water interface. They also function in a non-aqueous environment and catalyze a number of useful reactions, such as esterification, transesterification, acidolysis, alcoholysis, aminolysis, and resolution of racemic mixtures [21]. Filamentous fungi are one of the best lipase producers and the preferred source for lipase [27, 28]. The Aspergillus sp. is a ubiquitous kind of filamentous fungi that covers an evolution of 200 million years, and which is recognized as a beneficial species to produce foodstuffs and industrial enzymes [27]. In this study, we have cloned, expressed, purified, and characterized AFL67, a novel lipase, from A. fumigates. Unlike most reported lipase, AFL67 had no dependence on calcium for activity [21, 29]. It showed the maximum activity and stability at 15 °C, similar to the characteristics of a cold-active lipase from Acinetobacter sp. XMZ-26 (15 °C at pH 9.0) [30], but much different from the normal fungal lipases which are generally active and stable at 40–50 °C [31]. Also, in contrast with previous reported majority lipases from Aspergillus with acidic pH optima (5.0–6.0) [20], AFL67 showed an optimum pH of 7.5 and was almost inactivated below pH 6.5. Stability in the presence of different organic solvents is an essential characteristic of lipases when they are used in organic solvents. It was showed that AFL67 activity was increased by 47 % with ethanol and 50 % with isopropanol, respectively, in the reaction mixture, in similarity to the lipase from Pseudomonas fluorescens JCM5963 [32].
Lipase-catalyzed kinetic resolution (KR) of racemates is regarded as a clean and green technique. An enzyme efficient for enantioselectively conversion would be a valuable resource, and the enhancement of the production could greatly reduce the cost of lipase. Here, AFL67 was highly expressed in E. coli and showed enantioselective resolution of various substrates including methyl 3-(4-methoxyphenyl) glycidate, methyl 3-phenylglycidate, and α-acetoxyphenylacetic acid, among which APA was the best. The high E value and enantiomeric excess of the product in the KR of APA suggest the application of AFL67 in the production of chiral mandelic acids and o-acylated mandelic acids—the high value intermediates in chemical industry.
Though enantioselective resolution of (R,S)-methyl mandelate or o-butyrylated mandelate to (R)-MA has been reported [33, 34], production of (S)-MA by deacylation with an S-specific lipase was only found in our previous report using whole cells esterase of Pseudomonas sp. (PsE) [19], and the KR process was restricted by its relatively low enzyme production. AFL67 was the first reported recombinant lipase with high deacylation activity from (S)-APA to (S)-MA, and might make the hydrolysis resolution process more competitive in chiral mandelic acid production. In addition, AFL67 could also enantioselectively hydrolyze o-Cl-APA, m-Cl-APA, and p-Cl-APA to the derivatives of mandelic acid with high enantiomeric excess of the product. The enantioselective resolution of other derivatives of APA by AFL67 is ongoing.
In conclusion, AFL67, a cold-active lipase that can enantioselectively deacylate racemic α-acetoxyphenylacetic acid to S-mandelic acid was cloned, expressed, and characterized for the first time. Recombinant AFL67 has the advantage not only in enzyme production, but also in facilitating the approaches of protein engineering to improve the bioprocess of kinetic resolution of APA. The novel cold-active lipases like AFL67 are potentially important biocatalysts for various industrial applications, due to their high catalytic activity at low temperatures and their thermal stability at low temperatures.
References
Linhart, I. (2001). Stereochemistry of styrene biotransformation. Drug Metabolism Reviews, 33, 353–367.
Reetz, M. T. (2002). Lipases as practical biocatalysts. Current Opinion in Chemical Biology, 6, 145–150.
Santaniello, E., Ferraboschi, P., Grisenti, P., & Manzocchi, A. (1992). The biocatalytic approach to the preparation of enantiomerically pure chiral building blocks. Chemical Reviews, 92, 1071–1140.
Utkin, I. B., Yakimov, M. M., Matveeva, L. N., et al. (1991). Degradation of styrene and ethylbenzene by Pseudomonas species Y2. FEMS Microbiology Letters, 77, 237–241.
Ghanem, A., Aboul-Enein, M. N., El-Azzouny, A., & El-Behairy, M. F. (2010). Lipase-mediated enantioselective kinetic resolution of racemic acidic drugs in non-standard organic solvents: direct chiral liquid chromatography monitoring and accurate determination of the enantiomeric excesses. Journal of Chromatography. A, 1217, 1063–1074.
Blay, G., Fernandez, I., Molina, E., et al. (2006). Diastereoselective Michael addition of (S)-mandelic acid enolate to 2-arylidene-1,3-diketones: enantioselective diversity-oriented synthesis of densely substituted pyrazoles. Tetrahedron, 62, 8069–8076.
Mateo, C., Chmura, A., Rustler, S., et al. (2006). Synthesis of enantiomerically pure (S)-mandelic acid using an oxynitrilase-nitrilase bienzymatic cascade: a nitrilase surprisingly shows nitrile hydratase activity. Tetrahedron-Asymmetry, 17, 320–323.
Ema, T., Ide, S., Okita, N., & Sakai, T. (2008). Highly efficient chemoenzymatic synthesis of methyl (R)-o-chloromandelate, a key intermediate for clopidogrel, via asymmetric reduction with recombinant Escherichia coli. Advanced Synthesis and Catalysis, 350, 2039–2044.
Grimley, J. (2006). Pharma challenged. Chemical & Engineering News, 84, 17–28.
Wang, P. Y., Tsai, S. W., & Chen, T. L. (2008). Improvements of enzyme activity and enantioselectivity via combined substrate engineering and covalent immobilization. Biotechnology and Bioengineering, 101, 460–469.
Kim, E. K., Jang, W. H., Ko, J. H., et al. (2001). Lipase and its modulator from Pseudomonas sp strain KFCC 10818: proline-to-glutamine substitution at position 112 induces formation of enzymatically active lipase in the absence of the modulator. Journal of Bacteriology, 183, 5937–5941.
Gill, I. I., Das, J., & Patel, R. N. (2007). Enantioselective enzymatic acylation of 1-(3 ′-bromophenyl)ethylamine. Tetrahedron-Asymmetry, 18, 1330–1337.
Ismail, H., Lau, R. M., van Rantwijk, F., & Sheldon, R. A. (2008). Fully enzymatic resolution of chiral amines: acylation and deacylation in the presence of Candida antarctica lipase B. Advanced Synthesis and Catalysis, 350, 1511–1516.
Pilissao, C., Carvalho, P. D., & Nascimento, M. D. (2009). Enantioselective acylation of (RS)-phenylethylamine catalysed by lipases. Process Biochemistry, 44, 1352–1357.
Wang, Y. H., Li, Q. S., Zhang, Z. M., Ma, J. T., & Feng, Y. (2009). Solvent effects on the enantioselectivity of the thermophilic lipase QLM in the resolution of (R, S)-2-octanol and (R, S)-2-pentanol. Journal of Molecular Catalysis B: Enzymatic, 56, 146–150.
Ghanem, A., & Aboul-Enein, H. Y. (2005). Application of lipases in kinetic resolution of racemates. Chirality, 17, 1–15.
Cabrera, Z., Fernandez-Lorente, G., Fernandez-Lafuente, R., Palomo, J. M., & Guisan, J. M. (2009). Enhancement of Novozym-435 catalytic properties by physical or chemical modification. Process Biochemistry, 44, 226–231.
Volpato, G., Filice, M., Rodrigues, R. C., et al. (2009). Modulation of a lipase from Staphylococcus warneri EX17 using immobilization techniques. Journal of Molecular Catalysis B: Enzymatic, 60, 125–132.
Ju, X., Yu, H.-L., Pan, J., Wei, D.-Z., & Xu, J.-H. (2009). Bioproduction of chiral mandelate by enantioselective deacylation of α-acetoxyphenylacetic acid using whole cells of newly isolated Pseudomonas sp. ECU1011. Applied Microbiology and Biotechnology, 86, 83–91.
Mhetras, N., Bastawde, K., & Gokhale, D. (2009). Purification and characterization of acidic lipase from Aspergillus niger NCIM 1207. Bioresource Technology, 100, 1486–1490.
Rajan, A., Kumar, D. R. S., & Nair, A. J. (2011). Isolation of a novel alkaline lipase producing fungus Aspergillus fumigatus MTCC 9657 from aged and crude rice bran oil and quantification by HPTLC. International Journal of Biological Chemistry, 5, 116–126.
Shi, B. L., Zeng, L. Q., Song, H. L., et al. (2010). Cloning and expression of Aspergillus tamarii FS132 lipase gene in Pichia pastoris. International Journal of Molecular Sciences, 11, 2373–2382.
Nishikawa, M., Gomi, H., & Kishimoto, F. (1993). Purification and some properties of carboxyl esterase from a Arthrobacter globiformis: stereoselective hydrolysis of ethyl chrysanthemate. Bioscience, Biotechnology, and Biochemistry, 57, 594–598.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry, 72, 248–254.
Wang, Y., Zhao, J., Xu, J.-H., et al. (2010). Significantly improved expression and biochemical properties of recombinant Serratia marcescens lipase as robust biocatalyst for kinetic resolution of chiral ester. Applied Biochemistry and Biotechnology, 162, 2387–2399.
Becker, P., Abu-Reesh, I., Markossian, S., Antranikian, G., & Markl, H. (1997). Determination of the kinetic parameters during continuous cultivation of the lipase-producing thermophile Bacillus sp. IHI-91 on olive oil. Applied Microbiology and Biotechnology, 48, 184–190.
Contesini, F. J., Lopes, D. B., Macedo, G. A., Nascimento, M. D. G., & Carvalho, P. D. O. (2010). Aspergillus sp. lipase: potential biocatalyst for industrial use. Journal of Molecular Catalysis B: Enzymatic, 67, 163–171.
de Oliveira Carvalho, P., Contesini, F., Bizaco, R., & Alves Macedo, G. (2005). Kinetic properties and enantioselectivity of the lipases produced by four Aspergillus species. Food Biotechnology, 19, 183–192.
Gupta, R., Gupta, N., & Rathi, P. (2004). Bacterial lipases: an overview of production, purification and biochemical properties. Applied Microbiology and Biotechnology, 64, 763–781.
Zheng, X., Chu, X., Zhang, W., Wu, N., & Fan, Y. (2011). A novel cold-adapted lipase from Acinetobacter sp. XMZ-26: gene cloning and characterisation. Applied Microbiology and Biotechnology, 90, 971–980.
Hiol, A., Jonzo, M. D., Rugani, N., et al. (2000). Purification and characterization of an extracellular lipase from a thermophilic Rhizopus oryzae strain isolated from palm fruit. Enzyme and Microbial Technology, 26, 421–430.
Zhang, A., Gao, R., Diao, N., et al. (2009). Cloning, expression and characterization of an organic solvent tolerant lipase from Pseudomonas fluorescens JCM5963. Journal of Molecular Catalysis B: Enzymatic, 56, 78–84.
Cabrera, Z., Fernandez-Lorente, G., Fernandez-Lafuente, R., Palomo, J. M., & Guisan, J. M. (2009). Novozym 435 displays very different selectivity compared to lipase from Candida antarctica B adsorbed on other hydrophobic supports. Journal of Molecular Catalysis B: Enzymatic, 57, 171–176.
Palomo, J. M., Fernandez-Lorente, G., Mateo, C., et al. (2002). Modulation of the enantioselectivity of lipases via controlled immobilization and medium engineering: hydrolytic resolution of mandelic acid esters. Enzyme and Microbial Technology, 31, 775–783.
Acknowledgments
This research was financially supported by China National Special Fund for State Key Laboratory of Bioreactor Engineering (no. 2060204) and Shanghai Leading Academic Discipline Project (no. B505).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jiao-Jiao Shangguan and Li-qiang Fan contributed equally to this work
Rights and permissions
About this article
Cite this article
Shangguan, JJ., Fan, Lq., Ju, X. et al. Expression and Characterization of a Novel Enantioselective Lipase from Aspergillus fumigatus . Appl Biochem Biotechnol 168, 1820–1833 (2012). https://doi.org/10.1007/s12010-012-9899-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9899-x