Abstract
Corynebacterium glutamicum wild type lacks the ability to utilize the xylose fractions of lignocellulosic hydrolysates. In the present work, we constructed a xylose metabolic pathway in C. glutamicum by heterologous expression of the xylA and xylB genes coming from Escherichia coli. Dilute-acid hydrolysates of corn cobs containing xylose and glucose were used as a substrate for succinic acid production by recombinant C. glutamicum NC-2. The results indicated that the available activated charcoal pretreatment in dilute-acid hydrolysates of corn cobs could be able to overcome the inhibitory effect in succinic acid production. Succinic acid was shown to be efficiently produced from corn cob hydrolysates (55 g l−1 xylose and 4 g l−1 glucose) under oxygen deprivation with addition of sodium carbonate. Succinic acid concentration reached 40.8 g l−1 with a yield of 0.69 g g−1 total sugars within 48 h. It was the first report of succinic acid production from corn cob hydrolysates by metabolically engineered C. glutamicum. This study suggested that dilute-acid hydrolysates of corn cobs may be an alternative substrate for the efficient production of succinic acid by C. glutamicum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Considering a low-cost and high-carbohydrate content characteristic [1], a global energy and environmental crisis has stimulated efforts towards converting agricultural and industrial waste or residue into numerous economical products (fuels, chemicals, and other target molecules) [2–4]. Lignocellulosic biomass is a potential source of bioenergy, due to its enormous quantities and renewability [5].
Corn cob is a significant lignocellulosic by-product of corn in industry. It is a kind of potentially useful raw material which is used to obtain added-value products [6]. Such a feedstock contained 32–41 % cellulose, 32–41 % hemicellulose, and 6–21 % lignin [7–9], which can be fractioned in separate streams. Among them, hemicellulose is a heteropolymer of various hexose (glucose, galactose, and mannose) and pentose (xylose and arabinose) sugars. One of the main components in corn cobs is hemicellulose containing xylose, glucose, and arabinose [10].
Unfortunately, relatively few native strains of industrial microorganisms can utilize pentose sugars as fermentable substrates [11]. The lack of pentose-utilizing industrially relevant microorganisms is often a major bottleneck to the implementation of successful industrial processes based on lignocellulosic biomass [12]. Corynebacterium glutamicum is used for the industrial production of various amino acids [13]. Moreover, due to cell growth arrest under oxygen deprivation, it has great potential capacity for efficient production of alanine, ethanol, d-lactate, and succinate [14–17]. It is known that C. glutamicum wild type, which is unable to utilize the pentose sugar xylose, has also been engineered for growth with xylose as the sole carbon source. But C. glutamicum shows remarkable resistance towards inhibitors, derived from hydrolysates like furfural, 5-hydroxymethylfurfural, and 4-hydroxybenzaldehyde, under growth-arrested conditions [18]. Previous work demonstrated that genetically engineered C. glutamicum strains utilized xylose and glucose for succinic acid production based on pure chemicals [19]. Whereas considerable efforts have been made to utilize lignocellulosic hydrolysates or hemicellulose for the production of l-lysine, l-glutamate, and 1,5-diaminopentane[12, 20], succinic acid production using lignocellulosic biomass by C. glutamicum has received much less attention.
In the present work, to develop an efficient process of succinic acid production from corn cob hydrolysates, we constructed a recombinant C. glutamicum strain, which could utilize xylose to grow on corn cob hydrolysates, and investigated the effect of hydrolysates for growth performance in aerobic culture. Moreover, we explored the effect of succinic acid production in corn cob hydrolysates during anaerobic phase conditions. Subsequently, the recombinant strain was tested on corn cob hydrolysates, mainly containing xylose and a small amount of glucose, for succinic acid production in a two-stage process. It is desirable that large-scale processes of succinic acid production make use of non-food carbon sources in order to avoid competition with carbon sources such as starch and sugar in nutrition.
Materials and Methods
Bacterial Strains, Plasmids, and Media
All bacterial strains and plasmids used in this study have been listed in Table 1. The nutrient-rich medium (A medium) was used for aerobic growth: 7 g l−1 casamino acids, 7 g l−1 (NH4)2SO4, 2 g l−1 urea, 2 g l−1 yeast extract, 0.5 g l−1 KH2PO4, 0.5 g l−1 K2HPO4, 0.5 g l−1 MgSO4·7H2O, 6 mg l−1 FeSO4·7H2O, 4.2 mg l−1 MnSO4·H2O, 0.2 mg l−1 biotin, and 0.2 mg l−1 thiamine. The mineral salts medium (MM) including 0.5 g l−1 KH2PO4, 0.5 g l−1 K2HPO4, 0.5 g l−1 MgSO4·7H2O, 6 mg l−1 FeSO4·7H2O, 4.2 mg l−1 MnSO4·H2O, 100 μg l−1 biotin, and 100 μg l−1 thiamine was used for anaerobic fermentation.
Construction of Deletion Mutants, Chromosomal Gene Replacements, and Plasmids
C. glutamicum mutants with in-frame deletions of ldhA (ΔldhA) were constructed in a two-step homologous recombination procedure as described previously [15]. Based on C. glutamicum ATCC 13032 whole genome sequence, two oligonucleotide primers were designed in order to amplify a portion of the ldhA gene. The oligonucleotide primers were used in a PCR with C. glutamicum ATCC 13032 chromosomal DNA as a template. The 1.4-kb PCR product bearing the ldhA gene was digested with HindIII and inserted into the same site of pUC18, yielding pUC18-ldhA (Table 1). The 4.0-kb pUC18-ldhA deleted 149-bp fragment was digested with blunt ends enzyme EcoRV and NaeI and self-ligated yielding pUC18-ΔldhA (Table 1). The 1.2-kb HindIII ΔldhA DNA fragment was inserted into the HindIII-digested pK18mobsacB plasmid DNA yielding pK18mobsacB ΔldhA (Table 1).
Plasmid pTrc99a-xylA was constructed for cloning the Ptrc-xylA fragment. The xylA gene was amplified using the oligonucleotide pair xylA1/xylA2 and chromosomal DNA of Escherichia coli K-12. The PCR product of 1.4 kb was digested with EcoRI/HindIII and cloned into pTrc99a cut with the same enzymes. Similarly, plasmid pTrc99a-xylB was constructed by digesting with EcoRI/HindIII and cloned into pTrc99a cut with the same enzymes. Plasmid pXMJ19-xylA was constructed. The Ptrc-xylA gene was amplified using the oligonucleotide pair trc-xylA1/trc-xylA2 and plasmid of ptrc99a-xylA. The PCR product of 1.6 kb was digested with PstI/XbaI and cloned into pXMJ19. Plasmid pXMJ19-xylA-xylB was constructed for amplifying a 3.3-kb DNA fragment of Ptrc-xylA-Ptrc-xylB. The fragment was amplified using the oligonucleotide pair trc-xylAB1/trc-xylAB2.
Plasmid pK18mobsacB-Δpta-ackA::Ptrc-xylA-Ptrc-xylB was constructed for replacing the chromosomal pta-ackA gene with the xylA-xylB gene from E. coli K-12 under the control of the trc promoter. First, the regions upstream and downstream (0.6 and 0.6 kb, respectively) of the Δpta-ackA deletion region were amplified with the oligonucleotide pairs pta-ackAF1/pta-ackAF2 and pta-ackAR1/pta-ackAR2, respectively (Table 1). The two PCR products served as the templates for an overlap extension PCR with oligonucleotide pair pta-ackAF1/pta-ackAR2 (Table 2). The PCR product of about 1.2 kb, which carried cloning sites, was digested with XhoI/BamHI and cloned into pK18mobsacB cut with the same enzymes.
C. glutamicum ATCC 13032 was transformed by electroporation with plasmids pK18mobsacB ΔldhA and pK18mobsacB-Δpta-ackA::Ptrc-xylA-Ptrc-xylB. The transfer of the resulting deletion plasmids into C. glutamicum and selection for the first and second recombination events were performed as described previously [15].
Preparation of Corn Cob Hydrolysates
Corn cobs were obtained from Yanjin Longfeng Industry and Trade Co. Ltd., China. The corn cobs passed through 80-mesh screens were mixed with 2 % (v/v) sulfuric acid at a ratio of 1:5 (w/v). The suspension was kept in an autoclave at 105 °C for 2 h. The raw hydrolysates were adjusted to pH 6.0 with solid Ca(OH)2 at 50 °C and then filtered through filter paper to remove any solid material. The acid-soluble portion was assayed for carbohydrate composition (glucose, xylose) by high-performance liquid chromatography (HPLC) (Table 3).
Activated charcoal was used for inhibitor removal. Activated charcoal was added to the corn cob hydrolysates by 2 % (w/v), and the mixture was heated at 50 °C for 2 h and then passed through filter paper to remove the activated charcoal.
Fermentation
For aerobic growth, a seed inoculum from an overnight 5-ml culture was added to 100 ml A medium with either glucose or xylose or corn cob hydrolysates as carbon source(s). The culture was incubated at 30 °C for 18 h.
For organic acid production, the cells grown in aerobic-phase cultures were harvested by centrifugation at 4 °C (5,000 rpm, 10 min). The cell pellets were subsequently washed twice with mineral salts medium. Following the second wash, the cells were resuspended in 50 ml mineral salts medium containing 300 mM Na2CO3. Organic acid production was started by adding variable amounts of sugar or corn cob hydrolysates in a rotary shaker (150 rpm) at 30 °C.
Batch fermentation was carried out as follows. C. glutamicum NC-2 cells were precultured with constant agitation at 200 rpm at 30 °C overnight in A medium containing 20 g l−1 xylose. Fifteen milliliters of preculture was inoculated in 300 ml of A medium containing 20 g l−1 xylose. After approximately 12 h of cultivation at 200 rpm and 30 °C, the cells were used to inoculate a 5-l fermenter (BIOTECH-5JG, China) with an initial broth volume of 3 l. The culture was incubated on an agitation speed of 400 rpm for 12 h at 30 °C. The aerobic medium used threefold-diluted hydrolysates containing 1 g l−1 glucose and 16 g l−1 xylose. The pH was automatically controlled at a value of 7.0 by addition of 5 M NaOH. After 12 h of aerobic culture, the cell mass was harvested by centrifugation at 4 °C (8,000 rpm, 10 min) and subsequently washed twice with mineral salts medium; the cell suspension was incubated in 1.5 l mineral salts medium containing undiluted hydrolysates and 300 mM Na2CO3 at 30 °C. A feeding process of 300 mM Na2CO3 was done after 12 h. The pH was kept at pH 8.0 by automated addition of 15 M NaOH.
Analytical Methods
Cell growth was monitored by optical density at 600 nm (OD600) with a spectrophotometer (UV-2800, UNICO, USA) and was transformed into dry cell weight (DCW) using the equation: DCW (g l−1) = 0.4 × OD600.
The culture samples were centrifuged (12,000 rpm, 4 °C, 1 min), and the resulting supernatants were analyzed for the presence of sugars, organic acids, and phenolic compounds. Xylose, glucose, and arabinose were quantified in hydrolysates with an Agilent HPLC on an Aminex HPX-87H column (Bio-Rad) operating at 45 °C with 5 mM H2SO4 as the mobile phase at a flow rate of 0.6 ml/min and detected with an RI detector.
Acetate, succinate, and pyruvate were quantified by HPLC (Agilent, America) equipped with a UV detector and conductivity meter and Grace PrevaiTM column operating at 215 nm with 25 mM KH2PO4 (pH 2.5) mobile phase at a flow rate of 1.0 ml/min.
Hydroxymethylfurfural (HMF), vanillic acid, and p-hydroxybenzoic acid in hydrolysate samples were analyzed with an HPLC system equipped with a Sepax HP-C18 column using a UV detector set on a wavelength of 230 nm and operating at 20 °C. The mobile phase consisted of 40 % (v/v) aqueous methanol, adjusted to pH 3.0 with concentrated HCl and supplied at a flow rate of 0.6 ml/min.
Results and Discussion
xylA–xylB Integrants Construct a Xylose-Utilizing Pathway in C. glutamicum
C. glutamicum usually does not use d-xylose as substrate; however, it harbors a gene encoding the xylulokinase xylB [21]. To construct a metabolic pathway that converts d-xylose into d-xylulose-5-phosphate in C. glutamicum, E. coli xylA and xylB genes were isolated by PCR and were precisely subcloned under the control of the constitutive trc promoter. The xylose-utilizing recombinant strain designated as C. glutamicum NC-2 was selected on agar plates by kanamycin resistance based on a parent strain NC-1 which could produce succinic acid. Strain NC-1 lacked the ldhA gene.
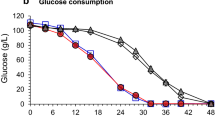
To investigate the efficiency of d-xylose utilization, parent strain and recombinant strain were aerobically grown in mineral medium containing either d-xylose (20 g l−1) or d-glucose (20 g l−1) as sole carbon source. The strain NC-2 can utilize d-xylose to grow, whereas the original strain cannot. But for d-glucose, the growth of two strains was almost the same (Fig. 1). As previously reported, the introduction of both E. coli xylA and xylB under the control of a constitutive promoter could make the xylose catabolism pathway avoid being subject to catabolite repression in corynebacteria [19]. Therefore, we integrated the xylA–xylB gene into C. glutamicum.
Comparative aerobic growth of C. glutamicum strains in mineral medium containing either glucose or xylose. These precultures were used to inoculate to an initial OD600 of 0.5 mineral medium containing either 20 g l−1 glucose or 20 g l−1 of xylose as the sole carbon source. The reported data represent the averages calculated from triplicate measurements
Hydrolysis of Corn Cob
In a subsequent step, the succinic acid production was tested to evaluate its full potential through the utilization of corn cob hydrolysates by the xylose-metabolism strain. Dilute-acid hydrolysis has been investigated as one of the fast and easy operations. Corn cobs were hydrolyzed by dilute acid into fermentative sugars such as xylose and glucose. The original material did not contain a significant amount of monosaccharides, but the concentration of single sugars increased during hydrolysis (Table 3). The major compound released was xylose. Glucose and arabinose were detected at a lower amount. The total concentration reached a constant level after 2 h of hydrolysis reaction; the total sugar concentration was 62.5 g l−1 (Table 3). The corn cob hydrolysates contained a xylose, glucose, and arabinose concentration of 56.3, 4.7, and 1.5 g l−1, respectively. Acetate, 5-HMF, vanillic acid, and p-hydroxybenzoic acid derived from the corn cob hydrolysates were detected as by-product (8.8, 0.8, 0.07, and 0.08 g l−1). The monosaccharides of the final hydrolysates suggested a good potential as substrate for succinic acid production by the mutant xylose-utilizing strain NC-2.
Growth Performance in Corn Cob Hydrolysates Under Aerobic Conditions
In a previous study of biomass pretreatment with dilute acid, the major degradation by-products released included organic acids such as acetate, furans like furfural and 5-HMF, and phenols such as 4-hydroxybenzaldehyde (4-HB), vanillin, and syringaldehyde [22]. Ethanol production by microorganisms was inhibited in the presence of these by-products after pretreatment [23–26]. Therefore, the growth performance in corn cob hydrolysates at different diluted rates was investigated during aerobic culture. As shown in Fig. 2, there was no cell growth on the condition that hydrolysates were diluted below onefold. The growth of the strain increased slightly with a declining concentration of hydrolysates. The relative growth of the strain with twofold dilution was increased to 35 % of that of the control (LB containing 15 g l−1 xylose). The cells were relieved of the decrease when the dilution rate was more than threefold. It appeared that C. glutamicum displays sensitivity to corn cob hydrolysates during aerobic culture, and the cell growth was significantly inhibited by increasing the concentration of hydrolysates. On the one hand, partly the higher osmolality of the hydrolysates-based medium as compared to xylose medium and on the other hand, the presence of inhibitors in lignocellulosic hydrolysates may as well play a role leading to the decrease in growth and production rates in the hydrolysates-based media.
As above described, the complex inhibitor components were contained in the hydrolysates. We investigated the effect of activated charcoal pretreatment for hydrolysates on succinic acid production. During the aerobic culture phase, the growth behavior was no different whether or not the activated charcoal absorbs pigment steps while the hydrolysates were diluted threefold (data not shown). The results suggested activated charcoal pretreatment did not affect the cell growth. However, succinic acid productivity was improved greatly by activated charcoal absorption (Table 4). During the period of succinic acid production, the rates of succinic acid production and xylose consumption were increased 1.76-fold and 1.67-fold, respectively, through the activated charcoal pretreatment process. Similarly, succinic acid production was increased 1.9-fold in such operation. It seemed that the components of corn cob hydrolysates, such as acetic acid, HMF, and vanillic acid, reduced the rate of sugar consumption and succinate productivity. As previously reported, the acid hydrolysates detoxified with overliming plus activated charcoal brought about a maximum decrease in furfural (100 %), acetic acid (62.4 %), and phenolic compounds (96.6 %) [27]. The result indicated that activated charcoal could absorb various pigments and toxins which may repress several key enzyme activities and influence the formation of product. The repression mechanism was unclear, due to the complex inhibitor components. Whereas, the rate of succinic acid production and xylose consumption have been decreased within 30 h. These results may be attributed to the bicarbonate concentration drop below 100 mM, because the rate of bicarbonate consumption was proportional to the rate of succinic acid production in the presence of bicarbonate [17]. However, succinic acid productivity in the activated charcoal absorption pretreatment process was 1.85-fold higher than that of the no detoxification pretreatment.
Effect of Succinic Acid Production in Corn Cob Hydrolysates During Anaerobic Phase Conditions
C. glutamicum cell growth was arrested under oxygen deprivation, but the cells retained the capability to metabolize sugars to succinic acids. This metabolic activity under growth-arrested conditions may be advantageous in so far as they avoid inhibitory effects on growth [18]. The effects of hydrolysate concentration on the succinic acid yield, the rate of succinic acid production, and xylose consumption of C. glutamicum NC-2 under oxygen deprivation were investigated to demonstrate that C. glutamicum was unaffected completely by hydrolysates in succinic acid production phase (Table 5); on the contrary, the rate of total sugar uptake and succinate production was slightly higher compared with the control medium (mineral salt medium containing 30 g l−1 xylose). Similarly, the growth-arrested C. glutamicum strain R retained 98 % of its ethanol productivity with several classical inhibitors of a corn stover model composition, indicating that the strain possesses a high tolerance to the combination of organic acids and furans in succinic acid production. An increasing acetate was found in mineral salt medium containing hydrolysates (HMM) comparative with mineral salt medium containing xylose (XMM), because of the presence of acetic acid in the hydrolysates (Table 3). This result indicated that the method employing growth-arrested C. glutamicum strain NC-2 showed great capacity from corn cob hydrolysates to produce succinic acid.
Succinic Acid Production on Corn Cob Hydrolysates in a Two-Stage Process
During the anaerobic phase of succinic acid production, the cell density of C. glutamicum NC-2 was tested to be 12 g dry cell l−1 (Fig. 3). The recombinant strain successfully utilized the applied hydrolysates for succinic acid production in a two-stage process, completely consuming the major sugars xylose and glucose. There was not a typical simultaneous utilization of xylose and glucose problem, because the glucose was exhausted rapidly. In an aerobic culture process, growth was relatively slow. This might suggest inhibition by toxic substances released during the previous hydrolysis process, though the hydrolysates were diluted threefold. Under oxygen deprivation, C. glutamicum NC-2 produced succinic acid in 48 h with addition of 300 mM sodium carbonate was examined. Succinic acid concentration reached 40.8 g l−1 at 0.85 g l−1 h−1 of the whole volumetric productivity at 48 h, though the rate of succinic acid production and xylose consumption declined gradually. The major by-product acetate reached 11.9 g l−1 (hydrolysates containing 6.2 g l−1 acetate). The yield of succinic acid from total sugar was 0.69 g g−1, while the yield of succinic acid from total sugar was 0.9 g g−1 at 12 h. The higher yield perhaps can be ascribed to an energy-dependent transporter which achieves energy balance [19] by analogy with the xylose uptake mechanism of E. coli. In E. coli cells, under anaerobic conditions, xylose metabolism was estimated to be 0.67 M of ATP per xylose molecule less than half of that produced from glucose (2 M ATP per glucose molecule) [28]. So far, there were no reports about succinic acid production from lignocellulosic biomass by metabolically engineered C. glutamicum. A metabolically engineered E. coli was used in producing succinic acid with a final concentration of 57.81 g l−1 and a yield of 0.87 g g−1 total sugar from corn stalk hydrolysates in two-stage fermentation [1]. Our results demonstrated that C. glutamicum was a feasible host strain for succinic acid production. Therefore, corn cob hydrolysates could be an alternative substrate for the efficient production of succinic acid by C. glutamicum.
Conclusion
In this study, diluted-acid hydrolysates of corn cobs were investigated for the fermentative production of succinic acid by recombinant C. glutamicum NC-2. The results indicated that dilute-acid hydrolysis could be used as a carbon source for efficient production of succinic acid not only during aerobic culture phase but also in an arrested growth condition. The present study demonstrated that the production of succinic acid from lignocellulosic biomass was a realistic possibility. To our knowledge, this was the first attempt to develop an efficient succinic acid production from xylose in corn cobs. A further improved pentose metabolism approach can also be integrated into the hydrolysates process to enhance the utilization of lignocellulosic materials and agricultural residues.
References
Wang, D., Li, Q., Yang, M. H., Zhang, Y. J., Su, Z. G., & Xing, J. M. (2011). Process Biochemistry, 46, 365–371.
Ji, X. J., Huang, H., Du, J., Zhu, J. G., Ren, L. J., Li, S., et al. (2009). Bioresource Technology, 100, 5214–5218.
Dorado, M. P., Lin, S. K., Koutinas, A., Du, C. Y., Wang, R. H., & Webb, C. (2009). Journal of Biotechnology, 143, 51–59.
Lynd, L., & Wyman, C. (1999). Biotechnology Progress, 15, 777–793.
Kim, K. H., & Hong, J. (2001). Bioresource Technology, 77, 139–144.
Rivas, B., Torre, P., Dominguez, J. M., Perego, P., Converti, A., & Parajo, J. C. (2003). Biotechnology Progress, 19, 706–713.
Garrote, G., Cruz, J. M., Dominguez, H., & Parajo, J. C. (2003). Journal of Chemical Technology and Biotechnology, 78, 392–398.
Nabarlatz, D., Farriol, X., & Montane, D. (2004). Industrial and Engineering Chemistry Research, 43, 4124–4131.
Tada, K., Horiuchi, J. I., Kanno, T., & Kobayashi, M. (2004). Journal of Bioscience and bioengineering, 98, 228–230.
Hendriks, A. T., & Zeeman, G. (2009). Bioresource Technology, 100, 10–18.
Jeffries, T. W., & Jin, Y. S. (2000). Advances in Applied Microbiology, 47, 221–268.
Gopinath, V., Meiswinkel, T. M., Wendisch, V. F., & Nampoothiri, K. M. (2011). Applied Microbiology and Biotechnology, 92, 985–996.
Hermann, T. (2003). Journal of Biotechnology, 104, 155–172.
Jojima, T., Fujii, M., Mori, E., Inui, M., & Yukawa, H. (2010). Applied Microbiology and Biotechnology, 87, 159–165.
Inui, M., Kawaguchi, H., Murakami, S., Alain, A. V., & Yukawa, H. (2004). Journal of Molecular Microbiology and Biotechnology, 8, 243–254.
Okino, S., Inui, M., & Yukawa, H. (2005). Applied Microbiology and Biotechnology, 68, 475–480.
Okino, S., Noburyu, R., Suda, M., Jojima, T., Inui, M., & Yukawa, H. (2008). Applied Microbiology and Biotechnology, 81, 459–464.
Sakai, S., Tsuchida, Y., Okino, S., Ichihashi, O., Kawaguchi, H., Watanabe, T., et al. (2007). Applied and Environmental Microbiology, 73, 2349–2353.
Kawaguchi, H., Vertès, A. A., Okino, S., Inui, M., & Yukawa, H. (2006). Applied and Environmental Microbiology, 72, 3418–3428.
Buschke, N., Schroer, H., & Wittmann, C. (2011). Journal of Biotechnology, 6, 306–317.
Blombach, B., & Seibold, G. M. (2010). Applied Microbiology and Biotechnology, 86, 1313–1322.
Klinke, H. B., Thomsen, A. B., & Ahring, B. K. (2004). Applied Microbiology and Biotechnology, 66, 10–26.
Palmqvist, E., Grage, H., Meinander, N. Q., & Hahn-Hagerdal, B. (1999). Biotechnology and Bioengineering, 63, 46–55.
Zaldivar, J., & Ingram, L. O. (1999). Biotechnology and Bioengineering, 66, 203–210.
Zaldivar, J., Martinez, A., & Ingram, L. O. (1999). Biotechnology and Bioengineering, 65, 24–33.
Zaldivar, J., Martinez, A., & Ingram, L. O. (2000). Biotechnology and Bioengineering, 68, 524–530.
Ge, J. P., Cai, B. Y., Liu, G. M., Ling, H. Z., Fang, B. Z., Song, G., et al. (2011). African Journal of Microbiology Research, 5, 1163–1168.
Tao, H., Gonzalez, R., Martinez, A., Rodriguez, M., Ingram, L. O., Preston, J. F., et al. (2001). Journal of Bacteriology, 183, 2979–2988.
Schäfer, A., Tauch, A., Jäger, W., Kalinowski, J., Thierbach, G., & Pühler, A. (1994). Gene, 145, 69–73.
Jakoby, M., Ngouoto-Nkili, C. E., & Burkovski, A. (1999). Biotechnology Techniques, 13, 437–441.
Acknowledgments
We are grateful to Professor Min Jiang at Nanjing University of Technology, China, for providing strains of C. glutamicum ATCC 13032. This work was financially supported by the National Key Basic Research and Development Program of China (no. 2011CBA00807).
Author information
Authors and Affiliations
Corresponding author
Additional information
Chen Wang and Hengli Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, C., Zhang, H., Cai, H. et al. Succinic Acid Production from Corn Cob Hydrolysates by Genetically Engineered Corynebacterium glutamicum . Appl Biochem Biotechnol 172, 340–350 (2014). https://doi.org/10.1007/s12010-013-0539-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0539-x