Abstract
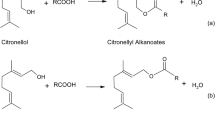
Enzymatic esterification of citronella essential oil towards the production of geranyl and citronellyl esters may present great scientific and technological interest due to the well-known drawbacks of the chemical-catalyzed route. In this context, this work reports the maximization of geranyl and citronellyl esters production by esterification of oleic and propionic acids in a solvent-free system using a commercial immobilized lipase as catalyst. Results of the reactions showed that the strategy adopted for the experimental design proved to be useful in evaluating the effects of the studied variables on the reaction conversion using Novozym 435 as catalyst. The operating conditions that maximized the production of each ester were determined, leading, in a general way, to conversions of about 90% for all systems. New experimental data on enzymatic esterification of crude citronella essential oil for geranyl and citronellyl esters production in solvent-free system are reported in this work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Production of flavors and fragrances through natural substances as starting raw materials has been under intense development over the last three decades. Biotechnology as an emerging science allowed the development of new aromas, making basis and constituting the backbone of what would be named lately as bio-industries. Despite the huge and fast development of the biotechnology field, several years later, the chemical synthesis has still been largely employed to produce such kind of compounds [1]. To take a glance at the flavor and fragrance worldwide market, until recently, the synthetic compounds found wide application in food, drink, and pharmaceutical industries move around US $22 billion in 2010 [2].
In spite of the fact that most of these compounds are obtained through chemical synthesis or by extraction from plants, application of new biotechnological processes has enhanced over the last decades [3, 4]. In this sense, biocatalysis may represent a potential alternative to chemical synthesis, especially in cases where the production of compounds with high stereo or regioselectivities purity is involved. Furthermore, the growing preference for natural products by the consumers has impelled the flavor and fragrance market based on the biotechnological route.
The use of hydrolytic enzymes as esterases, proteases, and peptidases in organic synthesis has become a common practice in the last years, both in scientific and industrial environment. In a general way, the main advantages of using these biocatalysts rely on the high activities exhibited in water and organic solvents and the ability to convert a broad number of substrates with high stereospecificity [5].
Solvent-free systems present as main advantage the easy of purification steps for products. Furthermore, the elimination of solvents offers reduction in the cost of process and minimizes the environment impact [6]. Taking into account these statements, several investigations have focused on the study of enzymatic reactions in solvent-free systems [7–12].
Some other factors should also influence the yields of esterification reactions; one of them is the structure of the alcohol (primary, secondary or tertiary) and the length of aliphatic chain of the fatty acid, used as substrates [13]. Examples concerning the application of primary alcohols as substrates for synthesis reactions catalyzed by lipases can be found in the literature [6, 7]. However, a full inspection of available literature permitted us to note a lack of information regarding the use of oleic and propionic acids and crude essential oils as substrates for this important class of reaction, leading to the production of important flavor compounds, with widespread application in the cosmetic, pharmaceutical, and food industries.
In this context, the present work aims at contributing to this field of knowledge by investigating operational conditions that conduct to interesting conversions using low-cost substrates as crude essential oils. Here, the main objective is to develop a bioprocess for the production of a bioflavor from citronella essential oil (Cymbopogon winterianus). The effect of process variables (substrates molar ratio, enzyme concentration, and temperature) was investigated by the experimental design technique.
Material and Methods
Substrates
The citronella (C. winterianus) essential oil, obtained by hydrodistillation, was purchased from Naturbios Óleos Essenciais e Vegetais (São Paulo, Brazil). Oleic and propionic acids (Quimex, 97% purity) were used as substrates for the esterification reactions. Molecular sieves of 4 Å were purchased from Sigma-Aldrich (USA). The commercial lipase used in this work was Candida antarctica (Novozym 435) immobilized on a macroporous anionic resin, purchased from Novozymes Brazil (Araucária, PR, Brazil).
Chemical Characterization of Essential Oil
The analysis of chemical composition of citronella essential oil was carried out by gas chromatography coupled to mass spectrometry (Shimadzu, Model QP 5050A). A capillary column DB-5 (30 m × 0.25 mm × 0.25 μm) was used. Helium was the carrier gas at a flow rate of 1.0 mL/min; the detector at 1.0 kV, split mode (1:20), and the injector at 250 °C were employed. The initial temperature of 40 °C (4 min) was programmed by 3 °C/min until 180 °C and 30 °C/min until 250 °C. The total time of analysis was 60 min. The sample of essential oil was diluted in dichloromethane (Merck, Rio de Janeiro, Brazil) until a final concentration of 5,000 ppm. The compounds' peaks were integrated in manual mode and compared to the literature and data bank present in the equipment (Wiley, São Paulo, Brazil). The compounds were listed by retention index and peak area.
Experimental Procedure for Enzymatic Esterification
The esterification reactions were performed by preparing a reaction mixture of essential oil, oleic or propionic acid (at preestablished molar ratio), and molecular sieves (20 mg/mL of substrates) in a 50-mL Erlenmeyer. After complete dissolution of the substrates, the enzyme, previously activated in oven at 40 °C and 60 min, was added to the mixture. All experiments were carried out in an orbital shaker at constant agitation of 150 rpm. After the reaction time completion, the biocatalyst was filtered and the reaction conversion was determined as described below.
Experimental Design
The experimental design technique was used for the determination of conditions that maximize the reaction conversion. A central composite design (CCD) 23 was employed in this step to maximize the process conversion for each system studied: essential oil and propionic acid and essential oil and oleic acid. Reaction time and agitation were fixed at 6 h and 150 rpm, respectively [14, 15], and experiments were carried out as described before. The variables studied were temperature (40–70 °C), acid/oil molar ratio (1:1–1:5), and enzyme concentration (1–10%, based on the total mass of substrates). Results, for each system, are expressed in terms of peak area percent of the major constituents of essential oil. The software Statistica® 6.0 (Statsoft Inc.) was used to assist the design and the statistical analysis of experimental information, adopting in all cases studied a confidence level of 95% (p < 0.05).
Determination of Reaction Conversion
The analyses of the reaction conversion in terms of geranyl and citronellyl esters were conducted in a gas chromatography (Shimadzu GC-2010) equipped with data processor, using a capillary column of fused silica INOWAX (30 m × 250 μm × 0.25 μm), flame ionization detector, with the following temperature program: 40–180 °C (3 °C/min), 180–230 °C (20 °C/min), 230 °C (20 min), injector temperature 250 °C, detector at 275 °C, injection in the mode split, ratio of split 1:100, H2 (56 KPa) as carrier gas, injected volume of 0.4 μL of sample diluted in n-hexane (1:10). Reaction conversion was calculated based on the reduction of area of limitant reagent on the basis of reaction stoichiometry [14, 15].
Results and Discussion
Chemical Characterization of Essential Oil
The chemical composition of C. winterianus essential oil, used as substrate for enzymatic esterification, is presented in Table 1. The oil presented citronelal (35.28%) as major compound, followed by geraniol (21.99%) and citronelol (10.90%). The chromatographic analysis also showed that the major fraction of the oil is constituted by oxygenated monoterpenes, corresponding to 84.45% of total. As esterification reactions are favored by primary alcohols (in this case, geraniol and citronelol), the concentration of these compounds was monitored during the experiments.
Enzymatic Production of Citronellyl and Geranyl Oleate
To assess the effects of oil to acid molar ratio, enzyme concentration and temperature on citronellyl and geranyl oleate production, a CCD 23 was adopted. The matrix of the experimental design, with coded and real values and the responses in terms of citronellyl and geranyl oleate production, is presented in Table 2. Results are expressed in terms of these two alcohols since they are two of the major constituents of citronella essential oil. From this table it can be observed that after 6 h of reaction, it was possible to convert the monoterpene alcohols (geraniol and citronelol) present in the crude essential oil in the correspondent esters of oleic acid. Very distinct conversions were obtained as a function of the variables levels studied. The highest values were achieved at higher temperatures, substrates molar ratio of 1:1, and enzyme concentration of 10 wt.%, reaching conversions of 98.9% and 82.5% for citronellyl and geranyl oleate, respectively.
Results obtained in the experimental design presented in Table 2 were statistically analyzed. After 6 h of reaction, one can notice that the enzyme concentration presented a positive significant effect (p < 0.05) on citronellyl oleate conversion, while the substrates molar ratio presented a negative effect, considering a confidence level of 95%. Data were analyzed by analysis of variance (ANOVA), and the correlation coefficient of 0.98 and the calculated F (19.94) higher than the listed one (6.16) permitted to validate an empirical coded optimized model for citronellyl oleate production in terms of substrates molar ratio (MR), temperature (T), and enzyme concentration (E), as represented by Eq. 1. It can be observed that substrates molar ratio was the independent variable that presents the higher main effect in the citronellyl oleate production, followed by the enzyme concentration and temperature, respectively. In terms of interaction effect, it can be noticed that only temperature versus substrates molar ratio was significantly important, showing that when the ranges of these two variables decrease, higher conversions could be obtained.
The same statistical procedure was adopted to treat the experimental data obtained for the system constituted by geraniol and oleic acid. Here, considering a confidence level of 95%, all variables presented significant effects on the reaction conversion. Data were analyzed by ANOVA and the coefficient of correlation (0.93) and the calculated F (9.34) higher than the listed one (6.16) also permitted to validate an empirical coded optimized model (Eq. 2) for geranyl oleate production. Analyzing this equation is possible to see that in terms of main effects, a similar behavior was obtained when comparing this system with the one for citronellyl oleate production. A quite difference could be seen in terms of interaction effects, where one can observe that all of them were of similar magnitude.
Enzymatic Production of Citronellyl and Geranyl Propionate
The effects of oil to acid molar ratio, enzyme concentration, and temperature on citronellyl and geranyl propionate production were evaluated based on the results presented in Table 3. Results are also expressed in terms of citronellyl and geranyl propionate, as mentioned before. From this table it can be noticed that after 6 h of reaction, it was possible to convert the monoterpene alcohols into the correspondent esters of propionic acid. Distinct conversions were obtained as a function of the variables levels studied. Higher yields, for both reaction systems, were obtained in run 6 of the experimental design, related to temperature of 70 °C, substrates molar ratio of 1:1, and 10 wt.% of enzyme, leading to 92.95% and 96.51% of conversion in citronellyl and geranyl propionate, respectively.
Results obtained in the experimental design presented in Table 3 were statistically analyzed by ANOVA and the coefficient of correlation (0.99) and the calculated F higher than the listed ones permitted to validate an empirical coded optimized models for citronellyl and geranyl propionate production in terms of substrates MR, temperature (T), and enzyme concentration (E), represented by Eqs. 3 and 4, respectively. Both equations show that aiming at increasing citronellyl and geranyl propionate production, the enzyme concentration is the independent variable that presents the higher positive effect, followed by substrates molar ratio and temperature, respectively. However, it is important to mention that when the enzyme concentration is evaluated in terms of interaction effects, negative model parameters are found, showing the importance of evaluating the effects of interaction between the three evaluated variables, which is one of the great advantages of using experimental design methodology.
Considering the chemical composition of citronella essential oil, presented in Table 1, it is possible to observe that the total concentration of alcohols is about 40%. The primary alcohols citronelol and geraniol (10.9% and 21.9%, respectively) are the major compounds and presented the ability of esterifying the propionic and oleic acids. Castro et al. [13] evaluated the specificity of a commercial immobilized lipase in terms of the acid and alcoholic molecules of the substrate, by the synthesis of several terpene alcohols using n-heptane as solvent. The authors verified that the commercial enzyme Lipozyme IM presented limitations for catalyzing reactions with secondary and tertiary alcohols, leading to good conversions when primary alcohols (citronelol, geraniol, and nerol) were used as substrates.
High reaction rates in the system for the production of citronellyl oleate is obtained using low substrates molar ratio, favoring the economy of reagents. As high conversions were obtained both at 70 and 40 °C, one can suggest the use of mild temperatures with the aim of reducing the energy costs.
One should notice that from the available literature, we have not found any works directly related to the enzymatic esterification of crude essential oils in solvent-free system. Some works presented in the literature report the enzymatic esterification of different substrates in solvent-free system. Here, special attention is given to the enzymatic production of geraniol and citronelol esters. Note that the substitution of commercial alcohols by essential oils can sometimes make the process economically viable.
As an example, Kumar et al. [16] investigated the ethyl palmitate synthesis and obtained conversions of around 97%. Güvenç et al. [6] evaluated the enzymatic production of isoamyl acetate using Novozym 435 as catalyst (5 wt.% of substrates), acid to alcohol molar ratio of 1:2, 30 °C and 150 rpm in 6 h of reaction, with a reported conversion of 80%. Santos et al. [17], in the enzymatic production of butyl esters, obtained at the best tested experimental condition the conversion of 49% using butyric acid as acyl group donator and Lipozyme IM 77 as catalyst. Chang et al. [18] optimized the hexyl laurate enzymatic production (Novozym 435) as 69.7%, at 40.6 min, 58.2 °C, enzyme concentration of 25.4 mg/volume, and pH of 5.9.
Synthesis of geranyl and citronellyl esters from alcoholysis of coconut oil with a commercial lipase from Rhizomucor miehei was investigated by De et al. [19]. The optimized experimental conditions were 50 °C, 5 h of reaction time, and enzyme concentration of 10 wt.%. Yields higher than 50% were achieved for both tested alcohols. Ikeda and Kurokawa [12] used lipase from C. antarctica immobilized on cellulose acetate–TiO2 gel fiber to produce geranyl acetate. After 100 h of reaction, conversions of 85% were achieved.
Bartling et al. [20] investigated the use of membranes for removing water on the enzymatic synthesis of geranyl acetate catalyzed by C. antarctica lipase in n-hexane organic system. The authors concluded that the use of membrane permitted to enhance the reaction yield from 94% to 100% at 30 °C and acetic acid to geraniol molar ratio of 1:1.
The promising results in two previous works by our research group guide the present work. The first one [14] had as main objective the optimization of geranyl propionate production by esterification of geraniol and propionic acid in a solvent-free system using a commercial lipase as catalyst. The operating conditions that optimized geranyl propionate production were determined to be 40 °C, geraniol to propionic acid molar ratio of 3:1, 150 rpm, and 10 wt.% of enzyme, with a resulting reaction conversion of about 93%. Paroul et al. [15] also reported the maximization of geranyl oleate production by esterification of geraniol and oleic acid in a solvent-free system using a commercial lipase as catalyst. For this purpose, a sequential strategy of experimental designs was carried out. The operating conditions that maximized geranyl oleate production were determined to be 40 °C, geraniol to oleic acid molar ratio of 5:1, 150 rpm, and 10 wt.% of enzyme, reaching a conversion of about 90%. After determining the best reaction parameters, a kinetic study was performed evaluating the influence of substrates molar ratio, enzyme concentration, and temperature on geranyl oleate conversion. Results obtained in this step allow to conclude that an excess of alcohol (alcohol to acid molar ratio of 1:5), relatively low enzyme concentration (5 wt.%) and temperature of 50 °C afforded nearly complete reaction conversion after 1 h of reaction.
Immobilized lipase from Pseudomonas sp. was used for the transesterification of citronellyl butyrate and geranyl caproate in n-hexane. Reaction parameters as enzyme concentration, substrates concentration, water content, temperature, reaction time, and cycles of use of the enzyme were evaluated by Yee et al. [21]. The authors reached high yields (96% and 99%) for citronellyl butyrate and geranyl caproate, respectively, using lipase Amano PS (15 wt.%, in relation to the mass of substrates), 30 °C, 24 h of reaction, and 2 wt.% (based on the mass of substrates) of water.
Finally, it may be relevant to mention that measurements of enzyme activity before (fresh) and after (used) reaction experiments revealed no important changes in residual lipase activity, thus suggesting possible enzyme reuse. In this sense, the enzyme was tested for ten consecutive cycles, at the optimized experimental condition established in the experimental design. After this procedure, the residual activity (data not shown) was defined as the ratio of final activity/initial activity × 100, was about 90% (initial activity of around 60 U/g), hence a warranty that the biocatalyst could be used for successive batches without important loss of activity. In attempt to better understand the esterification reaction in solvent-free system using crude essential oil as substrate, further kinetic experiments using other commercial and noncommercial enzymes are underway within our working group.
Conclusions
New experimental data on enzymatic esterification of citronella essential oil and oleic and propionic acids for geranyl and citronellyl esters in solvent-free system are reported in this work, showing a promising perspective of the technique to overcome the well-known drawbacks of the chemical-catalyzed route. Results of the reactions showed that the strategy adopted for the experimental design proved to be useful in evaluating the effects of the studied variables on the reaction conversion using Novozym 435 as catalyst. The operating conditions that maximized the production of each ester were determined, leading, in a general way, to conversions of about 90% for all systems.
References
Malcata, F. X., Reyes, H. R., Garcia, H. S., Hill, C. G., & Amundson, C. H. (1990). Journal of the American Oil Chemists’ Society, 67, 890–910.
http://www.leffingwell.com/top_10.htm Accessed 16 Feb 2011.
He, X.-L., Chen, B.-Q., & Tan, T.-W. (2002). Journal of Molecular Catalysis B: Enzymatic, 18, 333–339.
Tan, T., Chen, B.-Q., & Ye, H. (2006). Biochemical Engineering Journal, 29, 41–45.
Treichel, H., Oliveira, D., Mazutti, M. A., Di Luccio, M., & Oliveira, J. V. (2010). Food and Bioprocess Technology, 3, 182–196.
Güvenç, A., Kapucu, N., & Mehmetoglu, Ü. (2002). Process Biochemistry, 38, 379–386.
Karra-Chaabouni, M., Pulvin, S., Touraud, D., & Thomas, D. (1996). Biotechnology Letters, 18, 1083–1088.
Karra-Châabouni, M., Ghamghi, H., Bezzine, S., Rekik, A., & Gargouri, Y. (2006). Process Biochemistry, 41, 1692–1698.
Gryglewicz, S., Jadownicka, E., & Czerniak, A. (2000). Biotechnology Letters, 22, 1379–1398.
Converti, A., Del Borghi, A., Gandolfi, R., Molinaripalazzi, F., Perego, E., & Zilli, M. (2002). Enzyme and Microbial Technology, 30, 216–223.
Irimescu, R., Saito, T., & Kato, K. (2004). Journal of Molecular Catalysis B: Enzymatic, 27, 69–73.
Ikeda, Y., & Kurokawa, Y. (2001). Journal of the American Oil Chemists’ Society, 78, 1099–1103.
Castro, H. F., Oliveira, P. C., & Soares, C. A. F. (1997). Ciência e Tecnologia de Alimentos, 17, 197–205.
Paroul, N., Grzegozeski, L. P., Chiaradia, V., Treichel, H., Cansian, R. L., Oliveira, J. V., et al. (2010). Journal of Chemical Technology and Biotechnology, 85, 1636–1641.
Paroul, N., Grzegozeski, L. P., Chiaradia, V., Treichel, H., Cansian, R. L., Oliveira, J. V., et al. (2010). Bioprocess and Biosystems Engineering, 33, 583–589.
Kumar, R., Modak, J., & Madras, G. (2005). Biochemical Engineering Journal, 23, 199–202.
Santos, J. C., Bueno, T., Ros, P. C. M., & Castro, H. F. (2007). Journal of Chemical Technology and Biotechnology, 82, 956–961.
Chang, S. W., Shaw, J. F., Shieh, C. H., & Shieh, C. J. (2006). Journal of Agricultural and Food Chemistry, 54, 7125–7129.
De, B. K., Chatterjee, T., & Bhattacharyya, D. K. (1999). Journal of the American Oil Chemists’ Society, 76, 1501–1504.
Bartling, K., Thompson, J. U. S., Pfromm, P. H., Czermak, P., & Rezak, M. E. (2001). Biotechnology and Bioengineering, 75, 676–681.
Yee, L., Akoh, C. C., & Phillips, R. S. (1997). Journal of the American Oil Chemists’ Society, 74, 255–260.
Acknowledgments
The authors thank CNPq, CAPES, FAPERGS, and URI-Campus de Erechim for the financial support and scholarships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paroul, N., Grzegozeski, L.P., Chiaradia, V. et al. Solvent-Free Production of Bioflavors by Enzymatic Esterification of Citronella (Cymbopogon winterianus) Essential Oil. Appl Biochem Biotechnol 166, 13–21 (2012). https://doi.org/10.1007/s12010-011-9399-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9399-4