Abstract
Cellulosic ethanol production from biomass raw materials involves process steps such as pre-treatment, enzymatic hydrolysis, fermentation, and distillation. Use of streams within cellulosic ethanol production was explored for onsite enzyme production as part of a biorefinery concept. Sixty-four fungal isolates were in plate assays screened for lignocellulolytic activities to discover the most suitable fungal strain with efficient hydrolytic enzymes for lignocellulose conversion. Twenty-five were selected for further enzyme activity studies using a stream derived from the bioethanol process. The filter cake left after hydrolysis and fermentation was chosen as substrate for enzyme production. Five of the 25 isolates were further selected for synergy studies with commercial enzymes, Celluclast 1.5L and Novozym 188. Finally, IBT25747 (Aspergillus niger) and strain AP (CBS 127449, Aspergillus saccharolyticus) were found as promising candidates for onsite enzyme production where the filter cake was inoculated with the respective fungus and in combination with Celluclast 1.5L used for hydrolysis of pre-treated biomass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The need for purchasing commercial enzymes is an economical boundary for feasible second-generation bioethanol production [1]. Therefore, the production of enzymes onsite using low-cost substrates or even process waste streams as a production substrate should be considered.
Evaluating on the overall production cost of bioethanol the price of commercial enzymes is of outmost importance as it typically contributes to a large part of the final bioethanol cost. Other costs related to second-generation ethanol production involve biomass raw material costs as well as transport of the biomass to the ethanol production facility. Operational costs are mainly linked to pre-treatment strategies of the material, enzymes, microorganisms for the fermentation, distillation method, other process options such as discharge or reuse of waste streams, and distribution of the product to the consumer [2].
The bioethanol process in focus is based on the Maxifuel concept, where the main process steps are pre-treatment, hydrolysis, C6-sugar fermentation, separation, C5-sugar fermentation, and anaerobic digestion [3]. The separation step between C6- and C5-sugar fermentation results in a “filter cake” that represents a waste stream that could be used as substrate for enzyme production by solid-state fermentation. The advantages of solid-state fermentation include high volumetric productivity, relatively higher concentration of the products, less cell mass generation, as well as lower energy consumption [4, 5].
For efficient hydrolysis of cellulose, a complete set of cellulase enzymes is needed [6]. Celluclast 1.5L and Novozym 188 represent two commercial enzyme preparations from Novozymes A/S often used in combination for hydrolysis of lignocellulosic biomasses. In order to reduce the cost of commercial enzymes for the hydrolysis of pre-treated lignocellulosic biomasses, introduction of enzymes produced onsite using a slip stream from the bioethanol process as part of the fermentation medium could be an attractive alternative.
For this purpose, a screening strategy was used, using different lignocellulosic, cellulosic, and hemicellulosic substrates to identify fungi able to degrade lignocelluloses, with primary focus on the cellulolytic activities. A predictive cellulase assay or screening is particularly difficult to develop because of the complex nature of the plant biomasses that the enzymes should hydrolyze; the pre-treated substrates are not pure and possible inhibitory compounds may influence the hydrolysis [7]. In this study, pre-treated wheat straw that had been processed in a pilot plant and the filter cake obtained after C6-sugar fermentation were used to screen for and to test potential onsite fungal enzyme producers. Fungal isolates collected for this work were screened for their cellulase activity and compared with the activity of two reference strains, Aspergillus niger and Trichoderma reesei that are commonly known as good enzyme producers [8]. The aim was to identify fungal strains that efficiently can utilize the filter cake stream of the second-generation bioethanol production for enzyme production and to investigate the potential of such onsite enzyme production compared with the use of commercially available enzymes.
Materials and Methods
Biomass and Biomass Characterization
Wet oxidized wheat straw (WO) and the filter cake (FC) obtained after C6-sugar fermentation of wet oxidized straw were kindly provided by Biogasol ApS, Denmark. Total solids (TS), volatile solids, and ash contents were determined according to the NREL procedures “Determination of Total Solids in Biomass version 2005” and “Determination of Ash in Biomass version 2005”, while structural carbohydrates and lignin were determined according to NREL procedure “Determination of Structural Carbohydrates and lignin in Biomass version 2006” [9].
Media
Potato dextrose agar (PDA) was prepared by finely dicing 200 g peeled potatoes, boiling them in 1 l of water for one hour, and letting this mixture pass through a sieve. 1.5% agar, 2% dextrose, and 1-ml trace metals (1 g ZnSO47H2O and 0.5 g CuSO45H2O in 100 ml of water) per liter were added, followed by autoclaving at 121°C for 20 min.
Agar plates of FC (Biogasol ApS), WO (Biogasol ApS), birch wood xylan (Sigma), avicel (Sigma), and carboxymethyl cellulose (CMC; Sigma) were prepared as follows. The FC was washed to decrease the amount of free sugars by adjusting the FC to a TS of 4%, autoclave at 121°C for 30 min, followed by settling for one hour, removing of top liquid, and addition of new water. The procedure was repeated, ending up with a TS of approximately 4%; 2% agar was added to the washed FC, autoclaved at 121°C for 20 min, and pH adjusted to 4.8. The other agar plates were prepared by adding 2% w/v of either WO, birch wood xylan, avicel, or CMC to the basic Czapek (CZ) agar media (3 g/l NaNO3, 1 g/l K2HPO4, 0.5 g/l KCl, 0.5 g/l MgSO47H2O, 0.01 g/l FeSO47H2O, and 15 g/l agar) [10] and adjusting the pH to 4.8.
Fungal Collection
Fungal isolates were obtained from various sources. Samples were isolated from wooden biomass such as decomposed wood in a local swamp, treated hardwood, and rye bread. Additionally, fungal strains were provided from IBT’s Culture Collection of Fungi by selection of Professor Jens C. Frisvad, Centre for Microbiology, Department of Systems Biology, Technical University of Denmark. These included the following strains: IBT25747 (A. niger), IBT3016 (A. “massa”), IBT3945 (Penicillium “pseudofuniculosum”), IBT14668 (P. “rapidoviride”), IBT15094 (P. “rapidoviride”), IBT16756 (A. pseudofumigatus), IBT18366 (P. “pseudoverruculosum”), IBT26808 (P. pulvillorum), and IBT7612 (Trichiderma reesei), where A. niger and T. reesei are used as reference strains.
Fungal Isolation
Initially a small piece of, e.g., wood was placed on PDA plates and incubated at room temperature. Several rounds of culture transfers onto new PDA plates were carried out in order to obtain pure single isolates.
Screening of Fungal Growth on Agar Plates
Five different substrates were used for the initial screening on agar plates: FC, WO, birch wood xylan, avicel, and CMC, prepared as described above. Spores of seven day old fungal cultures were transferred to the center of the different plates using a 1 μl inoculation loop. The plates were incubated at 25°C for 7 days, and growth was graded on a subjective scale 1–5 by visually determining the colony diameters. On the xylan plates an additional subjective grade was introduced: 0–3z, which relates to the clearing zone observed in the medium, often having a larger diameter than the growth of the fungus itself. The screening on different agar media was carried out in single determination.
Activity Studies Using AZCL Plates
Agar plates with CZ medium pH 4.8 containing 0.1% azurine cross-linked substrates: AZCL-HE-cellulose (HE = hydroxyethyl) and AZCL-arabinoxylan (Megazyme), were used for cellulase and xylanase activity studies, respectively. Inoculation was done by placing 1 μl of 106/ml spore suspension in the center of the plates. The activity was indicated by blue color zones resulting from hydrolysis of the substrate. The progress of the blue zones was followed for 7 days at room temperature and the final coloration was used to categorize the strains on a subjective scale from 1 to 5. The activity study on AZCL plates was carried out in double determination.
Activity Studies by Solid-State Fermentation on Filter Cake and Hydrolysis of Wet Oxidized Biomass
FC was autoclaved and freeze dried in order to stabilize the biomass. A mixture of 4.15 ml CZ medium and 0.85 g freeze dried FC (TS = 17%), in total 5 g, was used as growth medium in solid-state fermentation. The medium was inoculated with a spore solution of approximately 106 spores per gram DM in each fermentation and the beakers were incubated at 25°C in high humidity for 7 days. Growth set-up was conducted in triplicates. After incubation, 0.1 M Na-citrate buffer pH 4.8 was added directly to the beaker to give a TS of 3%, followed by blending with a coffee mixer for 30 s to disrupt the fungus/medium complex created by the growth of the fungus. This homogenized fermentation broth (0.85 g DM FC) was then added to wet oxidized straw (containing 2.8 g DM WO) pH 4.8, and the total mixture was brought to a final TS of 2% by addition of 0.1 M Na-citrate buffer pH 4.8. The FC to WO ratio was approximately 1:3 on dry matter basis. Incubation was done in 50 ml vials at 50°C for 4 days with shaking at 160 rpm, and 1 ml samples were taken before and after hydrolysis. The samples were centrifuged and the supernatants were analyzed using HPLC and the reducing sugar assay (see below).
Synergistic Test with Commercial Enzymes
Solid-state fermentation was used for the purpose of evaluating synergistic effects of the fungi and Celluclast 1.5L and Novozym 188 (Novozymes A/S, Denmark). The set-up was as described above, but with commercial enzyme preparations added after the FC with fungus and WO had been mixed. One setup contained the fungus as the only source of enzymatic activity; a second setup contained the fungus and 1 FPU (filter paper unit) Celluclast 1.5L added; while a third contained the fungus, 1 FPU Celluclast 1.5L added, and Novozym 188 (in a ratio of 4:1, respectively).
Activity Assay of Fungal Extracts
Endo-glucanase activity was determined by the use of AZO-CMC, assayed according to the manufacturer’s description (Megazyme) [11]. The enzyme solution was assayed in various dilutions at the conditions stated by the manufacturer. A standard curve was obtained by activity measurements of a pure endoglucanase from A. niger (Megazymes), with an activity of 322 U/ml at 40 °C, pH 4.6, reported by the manufacturer.
Beta-glucosidase activity was assayed with 5 mM p-nitrophenyl-beta-d-glucopyranoside (Sigma) in 50 mM sodium citrate buffer pH 4.8 as substrate. The assay was carried out as described by Flachner et al. (1999) [12]. The activity was expressed in units (μmoles substrate converted per minute).
Sugar Analysis
All samples to be analyzed by HPLC (Hewlet Packard 1100 series) were run on a 300⋅7.8 mm Aminex HPX-87H Column (BioRad) at 60°C with sulfuric acid as eluent at a flow rate of 0.6 ml/min and an injection volume of 10 μl. The components were detected refractometrically on a RI detector.
The reducing sugar assay was carried out according to Ghose (1987) and Miller (1959) [13, 14]. One milliliter of milliQ water and 0.5 ml sample solution were mixed before 3.0 ml dinitrosalicylic acid was added. The mixture was boiled for 5 min; then diluted with MilliQ water, followed by spectrophotometric absorbance measurements at 540 nm (Milton Roy spectronic 301), using MilliQ water as standard. A standard curve was obtained with different concentrations of glucose, thereby expressing absorbance as glucose equivalents.
Results and Discussion
Initial Screening and Biomass Evaluation
A screening program for different new fungal isolates from environmental samples was established in order to identify strains with high hydrolytic activity compared with well-known reference strains. A total of 64 strains were included in the initial screening that comprised grading of growth on the different substrates: FC, WO, xylan, avicel, and CMC (Table 1). The grading was based on colony size (morphology), as it was expected that the better the fungi grew, the better they utilized the substrate. In general, FC and WO were the media that resulted in the best growth of the fungi tested. 25% of the strains were graded with 3 or above on FC, while 35% of the strains were graded 3 or above on WO, yet WO was at the same time the substrate having the greatest number of strains that did not grow at all (Table 1).
FC of hydrolyzed wet oxidized wheat straw represents a fraction from the bioethanol process with low commercial value and could be of great interest to utilize as a substrate for enzyme production for the bioethanol process. The reuse of FC would loop back the un-hydrolyzed biomass into the process and thereby make good value of this fraction. The main components of the FC are relatively high levels of lignin followed by cellulose (Table 2). The cellulose of FC is regarded as poorly available as it remained part of the solid product and thus was not initially hydrolyzed. It is speculated that fungi capable of growing on FC potentially have new interesting enzymes that can facilitate further hydrolysis of FC. Overall, this favors the use of FC for onsite enzyme production and FC was therefore included in the screening. Adding FC as fermentate directly in the hydrolysis will increase the overall lignin concentration in the hydrolysis; this could potentially have a negative impact on the enzyme hydrolysis as lignin is known to enhance enzyme adsorption [15]. However, FC is likely to already be associated with proteins from the previous hydrolysis [16]. This would reduce both the non-productive and productive binding of new enzymes due to low accessibility. During fermentation the close association of the growing fungus with the FC could result in proteolytic metabolic activities that to a certain extent could remove some of the enzymes bound to the FC. This would increase the accessibility of the remaining cellulose in the FC [17].
WO represents another fraction in the ethanol process and could be a potential substrate for enzyme production as it is for ethanol production. Wet oxidized material has previously been reported to contain compounds inhibitory towards enzyme hydrolysis and fermentation [18]. However, poor growth of enzyme producers on this medium does not necessarily relate to a lack of cellulolytic enzymes produced by the fungus. Amongst those strains that did grow on WO, an increased number of strains received good growth grades indicating that the greater levels of free sugars in the WO media facilitated increased growth compared with the FC where washing had removed the free sugars. Free sugars will help initiate fungal growth and perhaps thereby boost the fungi that have the right combination of enzymes to degrade the substrate to continue growth. The lower lignin content of the WO (Table 2) compared with FC might also explain the increased growth on WO.
Three commercial substrates: xylan, avicel, and CMC, were used in the screening. The hemicellulose substrate, xylan, supported good growth of the greatest number of strains (grades of 3 or above), and was the only substrate that supported growth of all strains (Table 1). This clearly shows that xylanases are widespread amongst fungi in general. In contrary, the strains did not grow well on the cellulose substrates, avicel and CMC. On avicel, less than 10% of the strains were given grades of 3 or above, while on CMC it was less than 20% of the strains (Table 1), thus making the strains that did succeed in utilizing these carbon sources outstanding compared with most of the strains tested.
Xylan was included in the screening to determine the ability of the fungus to utilize a hemicellulosic carbon source, as the enzymatic degradation of xylan to xylose could add value to the process in terms of the pentoses later being fermented into ethanol by C5 fermenting microbes. Avicel and CMC represented the cellulose fraction of the biomass, each having different degree of crystalline and amorphous regions, where avicel primarily requires cellobiohydrolase activity and CMC requires endoglucanase activity for their hydrolysis [7].
Of the 64 strains included in the screening, 25 were chosen for further studies (grey colored in Table 1), with the grey colored grades representing the primary reasons for choosing these particular strains. The selection was primarily based on good growth grades (≥3) on FC, avicel, and CMC. A. niger IBT25747 and T. reesei IBT7612 were included as reference strains.
Enzyme Activity Studies of Strains Selected from Screening
Two activity studies were carried out to evaluate the enzyme activity of the 25 strains chosen from the initial screening: one using AZCL medium for simple measurements of cellulase and xylanase activity, and one using the complex filter cake as substrate for enzyme production followed by its use in hydrolysis of WO to determine activity on this complex substrate. AZCL media are commonly used for broad enzyme screening with focus on endo-activites [19]. The use of pre-treated biomass (including wheat straw, wheat bran, rice straw, groundnut shells, and corn fiber) and wastes (including paper pulp, municipal refuse, stillage of sugar cane bagassem and spruce wood hydrolysates) to support fungal growth and enzyme production has previously been reported [20–24]. However, it has not previously been reported to use the waste stream after C6 fermentation and where C5 sugars are removed with the soluble phase. That is, to use the filter cake of the wheat straw bioethanol process for enzyme production and reuse it directly for hydrolysis without separating out the enzymes, thereby adding value to the overall process.
The activity study using AZCL substrates was subjectively graded based on degree of blue color zone and its intensity (Table 3). Comparing these results with the initial growth screening (Table 1), it was found that the grades on CMC and AZCL-cellulose correlate well. Therefore, the ability to grow well on CMC was indeed an indication that the fungi had enzymes for hydrolysis of CMC, shown by the dye-release on AZCL-cellulose. The growth grades on xylan (Table 1) and color grades on AZCL-arabinoxylan (Table 3) were for the most part comparable. However, the clearing zone grades on xylan only partially correspond with the color grades on AZCL-arabinoxylan. This might be due to that the color grades on AZCL-arabinoxylan are based on both color intensity as well as zone diameter, while it for the xylan plates was difficult to judge clearing zone intensity.
The enzyme activities produced by the fungi growing in solid state on FC were evaluated by analysis of the soluble sugars after hydrolysis of WO. Three values were compared to evaluate the results: (1) increase in reducing ends (measured as glucose equivalents), (2) final C6 sugars (glucose) present after hydrolysis of wet oxidized biomass, and (3) final C5 sugars (xylose) present after hydrolysis of wet oxidized biomass (Table 4). It was found that the increase in monomeric sugar concentration only partly correlates with the measured increase in reducing ends of the samples. Two good examples are the strains 11.4 and IBT15094; these are in the top 5 of the reducing ends measurements, but do not even get in top 10 of the monomeric sugar measurements. A high score was, however, given on both AZCL-cellulose and –arabinoxylan, indicating high endo-activity. Great endo-activity will increase the number of reducing ends, but if the fungus does not have good beta-glucosidase/-xylosidase activity, no great monomeric concentration will be detected. To reach a high concentration of sugar monomers, a full enzyme cocktail must work together, consisting of exo-, endo-glucanases/xylanases, and beta-glucosidases/-xylosidases [7]. Accumulation of cellobiose was detected for strain 1.8.1 and 2.3 (data not shown), indicating high cellobiohydrolase activity, but lack of sufficient beta-glucosidase activity. These different observations highlight the importance of multiple screening methods to be able to map the different activities of each fungus.
Of the 25 strains, five were selected for further studies with commercial enzymes. We chose to continue with the strains that had generally performed best in terms of total cellulose degrading abilities in multiple of the assays (ref. strain IBT25747, AP, and 5.1), and also to include some that are thought to specifically possess high endo-activity (IBT15094) or exo-glucanase activity (1.8.1).
Synergies with Commercial Enzymes
The five strains were grown in FC for enzyme production, followed by hydrolysis of WO in combination with the commercial enzymes Celluclast 1.5L and Novozym 188. Low filter paper unit loadings of Celluclast 1.5L and a 4:1 ratio of Novozym 188 were applied to visualize the synergistic effects of the fungi that were tested and the commercial enzymes. The beta-glucosidase and endo-glucanase activities were measured for each fungal or commercial enzyme addition (Table 5).
Of the two commercial enzyme preparations, the beta-glycosidase activity of Novozym 188 helps increase the glucose yields and decrease the cellobiose concentration created by the cellobiohydrolase activity of Celluclast 1.5L that can be self-inhibiting [25, 26]. The glucose concentration after hydrolysis with Celluclast 1.5L and Novozym 188, was slightly higher than the sum of glucose and cellobiose after hydrolysis using just Celluclast 1.5L (data not shown). This could be explained by the higher cellobiose concentration inhibiting the endo-glucanases and cellobiohydrolases resulting in a lower total hydrolysis in the case of just using Celluclast 1.5L [6].
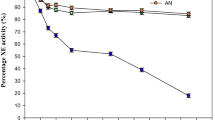
Such synergies were examined in our five selected strains, using their enzymes produced on FC to hydrolyze WO alone, WO in combination with Celluclast1.5L, and WO in combination with Celluclast 1.5L and Novozym 188 (Fig. 1). Evaluations were primarily based on the cellobiose concentration at the end of the hydrolysis. On this basis, synergies were found with the strain AP and IBT25747, as there was no accumulation of cellobiose and therefore sufficient synergies with the enzyme activities of Celluclast 1.5L. With strain 1.8.1, 5.1, and IBT15094, accumulation of cellobiose was seen after hydrolysis when hydrolysis was performed with the test fungus and Celluclast 1.5L and here it is obvious that synergetic activities resulting from growth on FC were insufficient. This correlates well with the measured activities (Table 5), where AP and IBT25747 showed high beta-glucosidase activity while the beta-glucosidase activity of 1.8.1, 5.1, and IBT15094 was low. It was further confirmed by the addition of Novozym 188 that in all cases resulted in a drop in cellobiose concentrations.
The profiles of the strains AP and IBT25747 are very similar with regards to beta-glucosidase activity, while IBT25747 has far greater endo-glucanase activity (Table 5). Strain IBT25747 gives the highest glucose yield when hydrolysis was performed with only this fungus, while AP gives the second highest (Fig. 1). Combining either IBT25747 or AP with Celluclast 1.5L for hydrolysis showed that the beta-glucosidase activity of IBT25747 and AP has synergies with the cellobiohydrolase activity of Celluclast 1.5L, and the endoglucanase activities add to the total hydrolysis. Addition of Novozym 188 has no significant effect on total hydrolysis evaluated by glucose yields, with the enzyme concentrations used here, likely explained by the fact that the fungus contributes with about 5 times the amount of beta-glucosidase activity compared with the Novozym 188 added (Table 5).
Xylose is readily released by all strains (Fig. 1), supporting the fact that xylose is more accessible due to the nature of the hemicellulose structure [27]. It is therefore speculated that enzymes with relatively high xylanase activity are likely to be “part of the package” when using organisms with cellulolytic activities.
Evaluating the enzyme activities (Table 5) together with the hydrolysis results (Fig. 1), it is clear that greater activity results in a higher degree of hydrolysis. The endoglucanase activity of IBT25747 is approximately 10 times higher than the endoglucanase activity of AP, while the beta-glucosidase activity is approximately the same. This resulted in a difference in glucose yield amongst the two strains of 46% when hydrolysis was performed with only the fungus, but only a difference of 17% when hydrolysis was performed with the fungus and Celluclast 1.5L combined. The additional endo-glucanase contribution from Celluclast 1.5L is the most likely reason for this decreased difference in yield.
Strain IBT25747 is A. niger and it is, therefore, not surprising that its enzymes in combination with Celluclast 1.5L are good at hydrolyzing WO. However, besides of this known strain, one of our own isolates, strain AP, showed a very promising profile in terms of onsite enzyme production using FC as growth medium. This strain AP (CBS 127449) has recently been characterized as a novel Aspergillus species: Aspergillus saccharolyticus [28]. Stain AP was able to compete with the reference strain and was sufficient for hydrolysis of WO in combination with Celluclast 1.5L. Addition of Novozym 188 had no extra effect on sugar yields when FC pre-grown with strain AP was added. Eliminating the need for beta-glucosidase addition during hydrolysis will significantly lower the cost of enzyme addition so our results have importance for practical applications.
Conclusions
This work demonstrates the possibility of using a low value stream of the biofuel production, FC, for enzyme production, where the fungus is grown in the FC and the FC with the fungus is used directly during hydrolysis of WO to obtain monomeric sugars for biofuel production. Such onsite enzyme production is valuable in terms of obtaining a complete value chain of the biofuel production. Through a broad screening for onsite enzyme producers as well as testing for synergistic effects, promising candidates were selected. From the use of reference enzymes, e.g., Celluclast 1.5L, that is known to lack important enzyme activities for complete hydrolysis, identification of enzymes that can contribute to synergy and thereby more efficient hydrolysis were found. Here, ref strain IBT25747 (A. niger) and own strain AP (CBS 127449, A. saccharolyticus) were found as promising candidates for onsite enzyme production with FC as growth and production medium. It was showed that the FC grown with these fungi can substitute Novozym 188 in the hydrolysis of WO.
References
NREL National Renewable Energy Laboratory. Biomass Research - Biochemical Conversion Projects 2009. Available at: http://www.nrel.gov/biomass/proj_biochemical_conversion.html. Updated October 2009.
Knauf, M., & Moniruzzaman, M. (2004). Lignocellulosic biomass processing: a perspective. International Sugar Journal, 106, 147–150.
Ahring, B. K., & Westermann, P. (2007). Coproduction of bioethanol with other biofuels. Biofuels, 108, 289–302.
Pandey, A., Selvakumar, P., Soccol, C. R., & Nigam, P. (1999). Solid state fermentation for the production of industrial enzymes. Current Science, 77, 149–162.
Pandey, A. (2003). Solid-state fermentation. Biochemical Engineering Journal, 13, 81–84.
Mansfield, S. D., Mooney, C., & Saddler, J. N. (1999). Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnology Progress, 15, 804–816.
Zhang, Y.-P., Himmel, M. E., & Mielenz, J. R. (2006). Outlook for cellulase improvement: screening and selection strategies. Biotechnology Advances, 24, 452–481.
Mathew, G. M., Sukumaran, R. K., Singhania, R. R., & Pandey, A. (2008). Progress in research on fungal cellulases for lignocellulose degradation. Journal of Scientific and Industrial Research, 67, 898–907.
NREL National Renewable Energy Laboratory. Biomass research - Standard Biomass Analytical procedures 2010. Available at: http://www.nrel.gov/biomass/analytical_procedures.html. Updated September 2010.
Samson, R. A., Hoekstra, E. S., & Frisvad, J. C. (2004). Introduction to Food- and Airborne Fungi. Centralbureau voor Schimmelcultures (7th ed.). Utrecht, Netherlands: American Society Microbiology.
Wood, T. M., & Bhat, K. M. (1988). Methods for Measuring Cellulase Activities. Methods in Enzymology, 160, 87–112.
Flachner, B., Brumbauer, A., & Reczey, K. (1999). Stabilization of beta-glucosidase in Aspergillus phoenicis QM 329 pellets. Enzyme and Microbial Technology, 24, 362–367.
Ghose, T. K. (1987). Measurement of Cellulase Activities. Pure and Applied Chemistry, 59, 257–268.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.
Berlin, A., Gilkes, N., Kurabi, A., Bura, R., Tu, M. B., Kilburn, D., et al. (2005). Weak lignin-binding enzymes—a novel approach to improve activity of cellulases for hydrolysis of lignocellulosics. Applied Biochemistry and Biotechnology, 121, 163–170.
Jorgensen, H., Vibe-Pedersen, J., Larsen, J., & Felby, C. (2007). Liquefaction of lignocellulose at high-solids concentrations. Biotechnology and Bioengineering, 96, 862–870.
Berlin, A., Balakshin, M., Gilkes, N., Kadla, J., Maximenko, V., Kubo, S., et al. (2006). Inhibition of cellulase, xylanase and beta-glucosidase activities by softwood lignin preparations. Journal of Biotechnology, 125, 198–209.
Klinke, H. B., Thomsen, A. B., & Ahring, B. K. (2004). Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Applied Microbiology and Biotechnology, 66, 10–26.
Pedersen, M., Hollensted, M., Lange, L., & Andersen, B. (2009). Screening for cellulose and hemicellulose degrading enzymes from the fungal genus Ulocladium. International Biodeterioration & Biodegradation, 63, 484–489.
Alriksson, B., Rose, S. H., van Zyl, W. H., Sjode, A., Nilvebrant, N., & Jonsson, L. J. (2009). Cellulase production from spent lignocellulose hydrolysates by recombinant Aspergillus niger. Applied and Environmental Microbiology, 75, 2366–2374.
Dien, B. S., Li, X., Iten, L. B., Jordan, D. B., O'Bryan, P. J., & Cotta, M. A. (2006). Enzymatic saccharification of hot-water pretreated corn fiber for production of monosaccharides. Enzyme and Microbial Technology, 39, 1137–1144.
Doppelbauer, R., Esterbauer, H., Steiner, W., Lafferty, R. M., & Steinmuller, H. (1987). The use of lignocellulosic wastes for production of cellulase by Trichoderma reesei. Applied Microbiology and Biotechnology, 26, 485–494.
Gupte, A., & Madamwar, D. (1997). Solid state fermentation of lignocellulosic waste for cellulase and beta-glucosidase production by cocultivation of Aspergillus ellipticus and Aspergillus fumigatus. Biotechnology Progress, 13, 166–169.
Thygesen, A., Thomsen, A. B., Schmidt, A. S., Jorgensen, H., Ahring, B. K., & Olsson, L. (2003). Production of cellulose and hemicellulose-degrading enzymes by filamentous fungi cultivated on wet-oxidised wheat straw. Enzyme and Microbial Technology, 32, 606–615.
Bhat, M. K., & Bhat, S. (1997). Cellulose degrading enzymes and their potential industrial applications. Biotechnology Advances, 15, 583–620.
Beguin, P., & Aubert, J. P. (1994). The biological degradation of cellulose. FEMS Microbiology Reviews, 13, 25–58.
Saha, B. C. (2003). Hemicellulose bioconversion. Journal of Industrial Microbiology & Biotechnology, 30, 279–291.
Sørensen, A., Lübeck, P.S., Lübeck, M., Nielsen, K.F., Ahring, B.K., Teller, P.J., Frisvad, J.C. (2011). Aspergillus saccharolyticus sp. nov., a new black Aspergillus species isolated in Denmark. International Journal of Systematic and Evolutionary Microbiology (in press)
Acknowledgements
The project was financially supported by the Danish Council for Strategic Research. Furthermore, Mette Lübeck, Section for Sustainable Biotechnology, Aalborg University Copenhagen, is acknowledged for revision of the manuscript
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sørensen, A., Teller, P.J., Lübeck, P.S. et al. Onsite Enzyme Production During Bioethanol Production from Biomass: Screening for Suitable Fungal Strains. Appl Biochem Biotechnol 164, 1058–1070 (2011). https://doi.org/10.1007/s12010-011-9194-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9194-2