Abstract
Procerain B is a novel cysteine protease isolated from Calotropis procera by our group and published recently. We have further characterized the enzyme by N-terminal sequencing and peptide mass fingerprinting. Procerain B showed maximum sequence similarity (80%) with Asclepain. Moreover, the characteristic VDWR motif of cysteine proteases is present in procerain B. The N-terminal and peptide mass fingerprinting analysis showed a distinct nature of the enzyme. Various applications of the enzyme were also evaluated. Procerain B is very effective in milk-clotting and may be a potential candidate for this process in the cheese industry. Additionally, the enzyme has potential application as dietary supplement to aid digestion. Effects of various metal ions on milk-clotting activity were also studied. The milk-clotting activity was increased in case of few metals while others have a negative effect. It is worth mentioning that the easy availability of plant material and simple purification method makes industrial production of the enzyme feasible. A protease with easy purification and suitable properties for application is always desired.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many plant proteases have been purified and characterized in terms of function and folding [1, 2]. However, not much study has been done to explore industrial applications of plant proteases. Calotropis procera, a wild growing plant of family Asclepiadaecae is known to possess multifarious medicinal properties. The plant is used in Ayurveda (Indian traditional medicine) for the treatment of skin diseases, rheumatism, and aches (CSIR Publication and Information Wealth of India: Raw materials III. New Delhi, India: CSIR Publication and Information Directorate; 1992. pp. 78–84). Various extracts of the plant have been reported to have antiinflammatory, analgesic, and antipyretic activities [3–7]. Recently, antioxidant and antibacterial activities of the methanolic extract of the plant have also been published [8]. We have recently reported a cysteine protease, procerain B, from the latex of the plant which is distinct from another protease, procerain, reported from the same source [9, 10]. Purification as well as biochemical properties of procerain B in comparison with other proteases have been reported by our group [10]. Several medicinal properties of the plant may be mediated through proteases present in the plant latex. Moreover, proteases have several applications in food, detergent, leather, and pharmaceutical industries [1].
Proteases are used as an additive in various formulations such as detergents, dehairing, contact lens cleansing agents, the cheese industry, and dietary supplement to aid digestion. Several useful proteases have been obtained from animal sources and used as digestive aids and for predigestion of baby foods. However, large-scale production of animal proteases is difficult. Chymosin is a milk-clotting enzyme from the fourth stomach of the calf and used in the cheese industry. The enzyme is difficult to obtain from a natural source in bulk. Therefore, the search for a new enzyme with chymosin-like milk-clotting action and simple purification method is always on. Similarly, proteases have immense applications in improving digestive function of humans. There is a gradual decrease in the production of digestive enzymes after 50 years of age. In addition, poor eating habits like less chewing and alcohol consumption also cause inadequate production of digestive enzymes. Additionally, the digestive function of an individual gets impaired in several diseased conditions and a digestive supplement is needed. Use of human innate digestive enzymes as digestive agents at large scale is not possible. Alternative sources of proteases could be plants or microbes. We here report procerain B as a potential candidate for milk clotting in the cheese industry and digestive agent in the food industry. The easy availability of plant material and simple purification method makes industrial production of the enzyme possible [10].
Material and Methods
Materials
Superficial incisions on the C. procera yielded milk like latex. Fresh latex of the plant was collected and procerain B was purified using the method of Singh et al. [10]. All other chemicals were of the highest purity, commercially available from Sigma or Merck.
Digestion of Food Protein
Food protein digestion experiment was performed with egg white albumen protein. Two cubical pieces of boiled egg albumen, 500 mg each, were incubated in 15 ml test tubes with 0.75 ml of Tris–Cl buffer pH 7.5 containing 50 mM of β-mercaptoethanol at 37 °C for overnight after addition of 500 μg of enzyme. A control was used for comparison in which enzyme was replaced with distilled water.
Milk Coagulation Assay
The clotting activities of procerain B were determined according to the method of Berridge [11]. A 10% (w/v) solution of skim milk powder (Sabarkantha District cooperative milk products, India) was prepared in Tris–Cl buffer at pH 7.5 containing 0.01 M CaCl2. Then, 500 μg of enzyme was added to 25 ml of this milk solution and the mix was incubated at 37 °C ± 0.5. The time required for the appearance of the clot was observed. A control was used by replacing the enzyme with distilled water. One unit (1 U) was defined as the quantity (milligrams) of enzyme needed to coagulate 1 mL of reconstituted skim milk powder in 1 min. at 37 °C.
Effect of Different Metal Ions on Milk-Clotting Activity of Procerain B
The effect of different metal ions in a range of concentration on the milk-clotting activity of enzyme was studied. The metals used were Cu2+ (CuSO4.5H2O), Co2+ (COCl2.6H2O), Ni2+ (NiSo4.6H2O), Zn2+ (ZnSO4.H2O), Mn2+ (MnCl2.4H2O). A control assay was done for the milk-clotting activity without any metal ion and the activity is considered 100%. A 10% (w/v) solution of skim milk was prepared in 50 mM Tris–Cl buffer, pH 7.5 containing 0.01 M CaCl2 and 5 ml of this solution in 15-ml test tubes were mixed with a range of concentration (10 to 10,000 μM) for each metal. Then 50 μl (2 mg/ml) of enzyme was added in each tube and incubated at 37 °C. Control experiments without protease did not show any effect.
N-Terminal Sequencing
The N-terminal of the procerain B was sequenced to understand homology and evolutionary relationship with other cysteine proteases of its class. Purified protein was transferred to PVDF membrane using the procedure of Choli et al. [12]. N-terminal sequencing was performed using the PROCISE Protein Sequencing System from Applied Biosystems by the method of Matsudaria [13].
Peptide Mass Fingerprinting
In-gel digestion by trypsin and peptide, mass fingerprinting was done by the method of Katayama et al. [14] using Ultraflex matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) (Bruker Daltonics). During gel digestion, the proteins are reduced in 10 mM dithiothrietol for 45 min at 60 °C. The samples are then treated with iodoacetamide (55 mM) in the dark for 45 min to alkylate the newly reduced disulfide bonds. MASCOT search tool (URL, http://www.matrixscience.com) was used for identification of tryptic maps.
Results and Discussion
Proteins are one of the major components of our daily diet and proteases are the enzymes responsible for the digestion of proteins. Impaired digestive function and depletion of digestive proteases may result in bloating, bowel disorders, abdominal cramping and food allergies. A digestive enzyme supplement is generally recommended in such conditions. We have selected the egg white albumen protein as a model protein to test the efficiency of procerain B to digest food proteins. After overnight incubation of egg white albumen protein with procerain B at 37 °C and pH 7.5 nearly 60% of protein get digested (Fig. 1), resulting in a decrease in weight of cubical piece from 500 to 195.5 mg in test sample while there was no decrease in weight of control. This property of procerain B makes it a very important enzyme for food and therapeutic industries.
Proteases are also used in dairy industry for the preparation of cheese and other milk products. Due to the increased demand of rennet in the cheese industry, there is a shortage of the enzyme. This has encouraged the search for alternative milk coagulants. We have studied the milk-clotting efficiency of procerain B to explore its application in the cheese industry. After 14 min of incubation at 37 °C, the clotting of milk has started in test which resulted in the precheese formation after 40 min. There was no clotting in the control experiment. The milk-clotting activity of procerain B was several folds higher than many commonly used proteases in the cheese industry [15]. The high milk-clotting activity of procerain B makes it a potential candidate for the dairy industry.
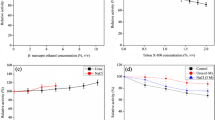
The effect of various metal ions on the milk-clotting efficiency of the enzyme was studied. Some metals increase the milk-clotting efficiency while others decrease. Mn2+, Ni2+, and Co2+ have shown increased milk-clotting efficiency of procerain B. Procerain B showed better milk-clotting property in all Mn2+ concentration range (10–10,000 μM) (Fig. 2) while Ni2+ showed increased clotting activity beyond 100 μM. Co2+ is only marginally effective up to 1,000 μM. However, it showed a 75.23% increase in clotting activity compared to the control at 10,000 μM concentration (Fig. 2a).
Peptide fragment spectra of procerain B. In-gel digestion of the protein was performed by trypsin, and spectra were collected by using Ultraflex MALDI-TOF as explained in “Material and Methods”
Cu2+ and Zn2+ showed inhibition of milk-clotting activity. Cu2+ inhibited the milk-clotting activity 57% at 10 μM concentration, 98.95% at 100 μM, and about 100% at 1,000 μM concentration (Fig. 3b) (but milk gets clotted after overnight incubation at 37 °C). At 10,000 μM concentration, Cu2+ completely inhibited the clotting activity and there was no clotting even after overnight incubation. Zn2+ inhibited nearly 41.3% and 60.5% at 10 μM and 100 μM concentrations, respectively (Fig. 2b) and about 100% inhibition was observed at 1,000 μM and 10,000 μM concentration (but after overnight incubation the milk gets clotted). Although the mechanism of inhibition by Cu2+ and Zn2+ ions are not clear at this point, but it might get coordinated with active site residues, His 57 and Ser 195 to inhibit protease enzyme [15].
The first 15 N-terminal amino acid sequence of procerain B in comparison with other common plant proteases are summarized in Table 1. The sequence alignment shows the distinct nature of procerain B. It is important to mention that another protease, procerain, purified form the same source with distinct biochemical–biophysical properties has N-terminal blocked [9]. It is worth mentioning that many proteins cannot be directly sequenced by Edman degradation because they have an N-terminal amino group that is modified by N-acetylserine (which occurs frequently in eukaryotic proteins) or N-acetylthreonine. Procerain B, from the same source does not show modified N-terminal. Among plant cysteine proteases, proline was highly conserved at position 2 which is reported to be necessary to prevent unwanted proteolysis [15]. The characteristic VDWR motif was also present in procerain B. Procerain B showed maximum sequence similarity (80%) with Asclepain. In most of the other cases, the sequence similarity was above 50%, except for ervatamin C, heynein, and bromelain. However, in the case of ervatamin C, heynein, and bromelain N-terminal sequences, there are few unidentified residues (X) which was not considered as a match with procerain B at respective position in our sequence similarity calculation. Among all plant cysteine proteases, proline was highly conserved at position 15 while in the case of procerain B it was histidine.
Conventional methods to differentiate proteins like protein sequencing and enzymatic characterization is time consuming. Peptide mass fingerprinting is reported to be an excellent tool to differentiate, peptidases even with highly similar properties, in a fast and unequivocal way. The method has been used for differentiation of the isoenzymes of the latex of Asclepias curassavica [16], as well as for the identification of rAfCP, the recombinant enzyme of Acalypha fruticosa [17]. MALDI-TOF mass spectra of peptide fragments after in-gel digestion are shown in Fig. 3. The peptide mass fingerprinting data analysis using MASCOT search tool (http://www.matrixscience.com), even with high peptide mass tolerance of 2 Da, did not yield any hit with acceptable statistics. The result shows a unique sequence of the protease. The mass spectrometrical analysis of tryptic fragments produces a list of molecular weights which does not match with other proteases. This further confirms the distinct nature of procerain B.
References
Dubey, V. K., Pande, M., Singh, B. K., & Jagannadham, M. V. (2007). African Journal of Biotechnology, 6, 1077–1086.
Nallamsetty, S., Dubey, V. K., Pande, M., Ambasht, P. K., & Jagannadham, M. V. (2007). Biochimie, 89, 1416–1424.
Kumar, V. L., & Basu, N. (1994). Journal of Ethnopharmacology, 44, 123–125.
Arya, S., & Kumar, V. L. (2005). Mediators of inflammation, 4, 228–232.
Sangraula, H., Dewan, S., & Kumar, V. L. (2002). Inflammopharmacology, 9, 257–264.
Dewan, S., Sangraula, H., & Kumar, V. L. (2000). Journal of Ethnopharmacology, 73, 307–311.
Dewan, S., Kumar, S., & Kumar, V. L. (2000). Antipyretic effect of latex of Calotropis procera. Indian Journal of Pharmacology, 32, 252.
Yesmin, M. N., Uddin, S. N., Mubassara, S., & Akond, M. A. (2008). American-Eurasian Journal of Agricultural and Environmental Science, 4, 550–553.
Dubey, V. K., & Jagannadham, M. V. (2003). Phytochemistry, 62, 1057–1071.
Singh, A. N., Shukla, A. K., Jagannadham, M. V., & Dubey, V. K. (2010). Process Biochemistry, 45, 399–406.
Berridge, N. J. (1952). The Journal of Dairy Research, 9, 328–329.
Choli, T., Kapp, U., & Wittmann-Liebold, B. (1989). Journal of Chromatography, 476, 59–72.
Matsudaria, P. (1987). The Journal of Biological Chemistry, 262, 10035–10038.
Katayama, H., Nagasu, T., & Oda, Y. (2001). Rapid Communications in Mass Spectrometry, 15, 416–421.
Rawlings, N.M. & Barrett, A.J. (2004). In: Barrett, A. J., Rawlings, N. M., & Woessner, J. F. (Eds.), Handbook of proteolytic enzymes, 2nd edition, vol. II (pp. 1051–1071). London: Elsevier Academic Press.
Obregón, W. D., Liggieri, C. S., Trejo, S. A., Avilés, F. X., Vairo-Cavalli, S. E., & Priolo, N. S. (2009). Biochimie, 91, 1457–1464.
Trejo, S. A., López, L. M. I., Caffini, N. O., Natalucci, C. L., Canals, F., & Avilés, F. (2009). Planta, 230, 319–328.
Lynn, K. R., Yaguchi, M., & Roy, C. (1980). Biochimica et Biophysica Acta, 624, 579–580.
Taylor, M. A., Baker, K. C., Briggs, G. S., Connerton, I. F., Cummings, N. J., Pratt, K. A., et al. (1995). Protein Engineering, 8, 59–62.
Cavalli, S. V., Cortadi, A., Arribere, M. C., Conforti, P., Caffini, N. O., & Priolo, N. (2001). Biological Chemistry, 382, 879–883.
Mitchel, R. E. J., Chaiken, I. M., & Smith, E. L. (1970). The Journal of Biological Chemistry, 245, 3485–3492.
Revell, D. F., Cummings, N. J., Baker, K. C., Collins, M. E., Taylor, M. A., Sumner, I. G., et al. (1993). Gene, 127, 221–225.
Sequeiros, C., Torres, M. J., Trejo, S. A., Esteves, J. L., Natalucci, C. L., & López, L. M. I. (2005). The Protein Journal, 24, 445–453.
Kundu, S., Sundd, M., & Jagannadham, M. V. (2000). Journal of Agricultural and Food Chemistry, 48, 171–179.
Sundd, M., Kundu, S., Pal, G. P., & Medicherla, J. V. (1998). Bioscience, Biotechnology, and Biochemistry, 62, 1947–1955.
Patel, B. K., & Jagannadham, M. V. (2003). Journal of Agricultural and Food Chemistry, 51, 6326–6334.
Goto, K., Takahashi, N., & Murachi, T. (1980). International Journal of Peptide Research, 15, 335–341.
Acknowledgments
Research fellowship to ANS by the Indian Institute of Technology Guwahati is acknowledged. Authors acknowledge Proteomics Facility and Protein Sequencing facility of the Indian Institute of Science for help in the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, A.N., Dubey, V.K. Exploring Applications of Procerain B, a Novel Protease from Calotropis procera, and Characterization by N-Terminal Sequencing as well as Peptide Mass Fingerprinting. Appl Biochem Biotechnol 164, 573–580 (2011). https://doi.org/10.1007/s12010-011-9158-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9158-6