Abstract
The influence of two key environmental factors, pH and oxygen transfer coefficient (k La), was evaluated on the lactic acid production as the main answer and, on the size of cell pellets of the fungal strain Rhizopus oryzae KPS106, as second dependant answer by response surface methodology using a central composite design. The results of the analysis of variance and modeling demonstrated that pH and k La had a significant effect on lactic acid production by this strain. However, no interaction was observed between these two experimental factors. pH and k La had no significant influence on the pellet size. Optimal pH and k La of the fermentation medium for lactic acid production from response surface analysis was 5.85 and of 3.6 h−1, respectively. The predicted and experimental lactic acid maximal values were 75.4 and 72.0 g/l, respectively, with pellets of an average of 2.54 ± 0.41 mm. Five repeated batches in series were conducted with a mean lactic acid production of 77.54 g/l. The productivity was increased from 0.75 in the first batch to 0.99 g/l h in the last fifth batch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid (2-hydroxypropionic acid) and its salts are widely applied in food, pharmaceutical, leather, textile industries, and as a chemical feed stock. Lactic acid is also used as a basic component for the elaboration of biodegradable polylactate polymer, an environmentally friendly plastic which is a substitute for plastic derived from petrochemicals [1, 2]. In 2005, global market for lactic acid production ranges at about 120,000 tons per year and shows an annual 15% growth rate [3, 4]. Driven by the propitious business climate in the cosmetic and biodegradable plastics end-use industries, the world market for lactic acid is projected to reach over 200,000 metric tons by the year 2012. Lactic acid is produced both chemically by the hydrolysis of lactonitrile and fermentation using lactic acid bacteria or strains of the fungus Rhizopus oryzae. The filamentous fungus, R. oryzae, has been extensively studied as a commercial producer of optically l(+)-lactic acid. It has the advantages of synthesizing only l(+)-lactic acid, does not require organic nitrogen sources and specific nutrients, and needs little pH maintenance. In addition, the fungal mass can be easily separated from the culture broth at the beginning of the process recovery leading to a cheap and easy downstream process [5, 6]. R. oryzae can grow as extended filamentous form, mycelial mats, pellets, or clumps. Pellets are spherical or ellipsoidal masses of hyphae ranging from loosely packed hyphae to compact bodies. The different morphological forms can have a significant effect on the rheology of the fermentation, on the oxygen supply, and on the level of lactic acid production. The pH is another key factor influencing the synthesis of lactic acid in competition to ethanol and therefore, required a control. Thus, several researchers attempted to use immobilization techniques for l(+)-lactic acid production with R. oryzae [7, 8]. This technique is time-consuming for the entrapment of fungal cells on matrixes and is limited in volume. Therefore, fungal growth in little pellets is the preferable morphology for industrial fermentation process because it improves rheology and mass transfer in culture broth in comparison to extended mycelial growth or large clumps. Such small pellets can be utilized for continuous operations using repeated batches [9].
Response surface methodology (RSM) is an efficient technique for the optimization of processes containing multivariable. It can reduce the number of experiment runs compared with the conventional “one factor at a time” and allow a modeling and the detection of real optima [10]. Central composite design (CCD) is an experimental design based on factorial design which are added axial and center points. It is the most frequently used RSM design. This technique has been successfully applied to optimize fermentation processes, for instance, the production of lovastin by Monascus ruber [11], the formation of conidia of the fungal bioherbicide Septoria polygonorum [12], or for the determination of kinetic constants (V max and K m values) of glucose oxidase [13].
The effect of medium composition on the lactic acid production by R. oryzae KPS 106 was reported in our previous study [14]; however, physical parameters, such as pH and oxygen transfer efficiency (k La), which play important role on conversion of pyruvate to lactic acid, remain to be investigated. In this study, lactic acid production by R. oryzae KPS 106 that has been reported to produce lactic acid using both filamentous form and immobilized cell [14, 15] was grown as mycelial pellets in a 3-L airlift bioreactor. The CCD of two factors was applied to evaluate the effect of pH and oxygen transfer efficiency (k La) of the fermentation media on lactic acid fermentation and pellet formation and to determine their optimal values. Finally, five repeated batch fermentation in series was used to decrease the total fermentation time and to enhance lactic acid productivity.
Materials and Method
Fungal Strain and Media
R. oryzae KPS106 was used throughout this study. The strain was cultivated at 35 °C on potato dextrose agar slants for 5 days and maintained at room temperature. The pre-culture medium consisted of 100 g glucose, 1.35 g (NH4)2SO4, 0.25 g MgSO4·7H2O, 0.3 g KH2PO4, and 0.04 g ZnSO4·7H2O per liter [5]. The fermentation medium consisted of 120 g glucose, 3.2 g (NH4)2SO4, 0.25 g MgSO4·7H2O, 0.20 g KH2PO4, and 0.04 g ZnSO4·7H2O per liter [14]. All medium were autoclaved at 121 °C for 15 min.
Lactic Acid Production
Spore suspension containing 2 × 107 spores/ml, obtained from a 7-day-old culture on agar, was inoculated into a 250-ml flask containing 50 ml of pre-culture medium. This starter-culture was incubated on a rotary shaker at a speed of 150 rpm, at 35°C for 24 h and then was used as inoculum for the production.
Lactic acid fermentation was performed in a 3-L airlift bioreactor with 2-L working volume. The experimental setup was according to [16]. The fermenter has a diameter of 185 cm, a height of 632 mm, and is surrounded by a water jacket for temperature control (35 °C). Air was distributed by an air sparger with multi-porous plate with 10 mm in diameter, located at a bottom of the fermenter. The dissolved oxygen probe, pH probe, and antifoam sensor were positioned at the top of the fermenter and controlled automatically. The pH medium was controlled using 10% NH4OH. A 10% (v/v) pre-culture was inoculated into the airlift bioreactor containing the production medium, and the duration of the fermentation was 96 h.
Repeated batch culture was performed with optimized batch cultivation. Cells from a previous fermentation were used to start the next production directly. The pellets were settled after aeration was stopped; then, the fermentation medium was withdrawn, and 2-L fresh sterilized medium was added aseptically for the next batch.
Experimental Design
The effect of pH and k La on lactic acid production by R. oryzae was evaluated according to CCD of two factors at five different levels. The CCD contains an imbedded factorial matrix with center points and “star points” around the center point that allow estimation of the curvature. A total of 11 combinations (four factorial points, four axial points, and center point in triplicate) were carried out (Table 1). Lactic acid concentration was used as the main dependent output variable. The secondary answer was the diameter of the fungal pellets. Statistic analysis and modeling were conducted with the Design-Expert 7.1.6 software.
Analytical Method
Cell-free fermentation broth was used for determination of residual glucose and lactic acid concentration by HPLC using Aminex® HPX-87H ion exclusion column with refractive index detector (Shimadzu, Japan). Sulfuric acid (0.05 N) was the elution solvent. The temperature of the column was maintained at 40 °C with a flow of 0.8 ml/min. The diameter of the pellets was measured using an Olympus microphotograph system (Tokyo, Japan). Fungal growth in fermenter was measured by the oxygen uptake rate (OUR) using dynamic method [17].
Results
The matrix design with the coded and the real values of the two experimental factors (pH and k La) are given in Table 1 with the corresponding values of the two responses, lactic acid production, and size of pellets. Combination numbers 9–11 were the same experiments under the same conditions (triplicates). The low variation at the center point of the results indicates a low experimental error and the accuracy of the experiments.
The quadratic models explaining the relationship between the experimental variables and the lactic acid production (Y L) and the pellet size (Y P) are given below:
Table 2 presents the analysis of variance of the quadratic models and their levels of significance. The validity of the model for the lactic acid production [Y L (g/l)] is excellent as demonstrated by the high confidence level of the F test and the low value of the term “lack of fit”. The R 2 coefficient of correlation is a statistical measure of how well the regression line approximates the real data points. The value should be close to 1.0. The high value (0.962) of this coefficient demonstrates the fitness between developed model and experimental data; only 3.8% of the total lactic acid is not explained by the quadratic model. The quadratic model with the second answer, size of mycelial pellets, is of insignificant validity as shown by the F test for regression and the low value of R 2. Such a quadratic model cannot explain the result “size of pellets” obtained and has to be rejected.
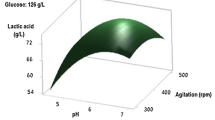
Table 3 gives the value of the various coefficients of the two models with their statistical significance. The independent variables, pH and k La, have a significant effect on the lactic acid production. The ANOVA analysis of the optimization study indicates that the model terms X 1, X 2, \( X_1^2,X_2^2 \) are significant in terms of lactic acid production. The high negative value of the quadratic terms for this response demonstrates the strong curvature and the presence of a maximum in the experimental domain studied. The term of interaction, X 1 X 2, has little value and is statistically insignificant. That means that the effects of pH and aeration on the lactic acid production are only additive; the level of one factor does not affect significantly the influence of the other one on the lactic acid production by this strain of Rhizopus under the chosen media conditions. All coefficients of the quadratic model for the response “pellet diameter” are insignificant. It is impossible to predict accurately this second answer in response to pH and k La. The smallest pellets were obtained at pH 7.0 and k La of 1.8 h−1 but with lowest lactic acid production. The optimal lactic acid production is achieved with relatively large pellets (around 2.7 mm)
Resolution of the quadratic model allows the determination of the position of the optima. The maximum predicted lactic acid yield, 75.2 g/L, occurred at an optimal pH and k La of 5.85 and 3.9 h−1, respectively. The pellet diameter is 2.73 mm. The optimal values are close to the center point.
Figure 1 presents 3-D response surface as well as the contour plots of lactic acid production and pellet formation. As shown in Fig. 1a, at pH 6 and k La of 3.6 h−1, the maximum of lactic acid with average 74.3 g/l was produced and average pellet size of 2.68 ± 0.21 mm in diameter was observed. The R. oryzae KPS 106 formed smooth pellets under tested conditions but different in size.
Repeated Batch Fermentation
Results of repeated batch production performed using recovered pellets were shown in Fig. 2. The five repeated batches were successfully operational with a total fermentation duration of 444 h and a total lactic acid production of 387.72 g/l. Kinetic characteristics of each batch cycle summarized in Table 4 showed that the lactic acid concentration (P), product yield (Y P/S), productivity (Q P), and substrate consumption rate (Q S) were enhanced during the process. Three distinct stages of growth were divided based on OUR measurement; lag, exponential, and decrease phases. In each batch fermentation, OUR was increased in the first 48 h of cultivation and then gradually declined until ending of cultivation. The highest lactic acid production rate was observed at the late exponential phase. In a comparison between each batch cultivation, the highest OUR gradually declined up to the 3rd repeated batch and then was consistent throughout this experiment.
In the last three batches, fermentation time became shorter in 84 h. Substrate consumption rate reached to 1.5 g/l h in the fourth cycle associated with high lactic acid concentration, yield, and production rate which were 82.8 g/l, 0.7 g/g, and 0.99 g/l h, respectively.
Discussion
The influences of pH and k La of fermentation media on lactic acid and size of pellet were investigated using response surface analysis. The response surface method with CCD and regression analysis was effective to find the optimum conditions of pH and aeration for lactic acid production by R. oryzae. However, it was not possible to obtain a clear and significant model for the size of the mycelia pellets in relation to pH and k La. The optimum condition was estimated as pH 5.85 with a k La of 3.9 h−1 for lactic acid production. pH is one of the most crucial factors affecting lactic acid production. It was found that production of lactic acid decreased as pH decreased from 6.0 to 4.0 [18] and that the optimal pH is located between 6.0 and 6.5 [19, 20]. Our results confirm that the optimal pH is located near 6.0.
The pH of media had been reported as an important factor for various fungi in the formation of pellets. However, in the present study of R. oryzae KPS 106, smooth pellets were formed in the whole range pH tested (4.59–7.41). This is in agreement with the observations of [21]; they reported that a pH range of 3.0–7.0 does not induce significant differences on pellet formation of R. oryzae. The smallest pellets were observed at pH 7.0 with k La of 1.8 h−1, but even if it is a prerequisite for adequate mass and heat transfer [22], the titer of lactic acid produced was only 20.3 g/l.
Fermentation by Rhizopus strains is an aerobic process, and thus, oxygen supply plays a key role in lactic acid production. The aeration rate impacted fungal morphology and product yield by improving the mass transfer which is beneficial to microbial growth and performance of microbial cells [23, 24]. The limitation of R. oryzae for lactic acid production is the side-product ethanol which is mainly synthesized under oxygen-limiting condition [25]. Excess aeration rate affects the fungal morphology, and increasing aeration rate [26] stimulates the formation of large pellets. Our results demonstrated that k La of 3.9 h−1 was beneficial to both formation of middle-size pellets and lactic acid production. The smaller pellets were formed at low k La value with lower lactic acid titer. This indicated that oxygen-limiting condition inhibits fungal growth and lactic acid formation.
Fungal pellets as natural immobilized cells were reused in repeated batch cultivation. The operation with cell recycling was promoted cell adaptation to the medium. This extended the lactic acid production phase of the culture [27]. During repeated batch cultivation, OUR was lower than that in normal batch fermentation. In addition, OUR decreased slightly with cycles in the earlier three cycles but approached a constant in the following cycles. The lactic acid concentration and productivity were improved, and it might be concluded that fermentation using pellet recycling is an efficient process.
References
Yin, P., Naoki, N., Yuuko, K., Kazutoyo, Y., Yongsoo, P., & Mitsuyasu, O. (1997). Enhanced production of l(+)-lactic acid from corn starch in a culture of Rhizopus oryzae using an air-lift bioreactor. Journal of Fermentation and Bioengineering, 3, 249–253.
Koutinas, A. A., Fabien, M., Ruohang, W., Grant, M. C., & Colin, W. (2007). Development of an oat-based biorefinery for the production of l(+)-lactic acid by Rhizopus oryzae and various value-added coproducts. Journal of Agricultural and Food Chemistry, 55, 1755–1761.
Akerberg, C., & Zacchi, G. (2000). An economic evaluation of the fermentative production of lactic acid from wheat flour. Bioresource Technology, 75, 119–126.
Zhang, Z. Y., Bo, J., & Joan, M. K. (2008). Production of l(+)-lactic acid using acid-adapted precultures of Rhizopus arrhizus in stirred tank reactor. Applied Biochemistry and Biotechnology, 149, 265–276.
Yin, P., Yahiro, K., Ishigaki, T., Park, Y., & Okabe, M. (1998). l(+)-Lactic acid production by repeated batch culture of Rhizopus oryzae in air-lift bioreactor. Journal of Fermentation and Bioengineering, 85, 96–100.
Huang, L. P., Jin, B., Lant, P., Qiao, X., Chen, J., & Sun, W. (2004). Direct fermentation of potato starch in wastewater to lactic acid by Rhizopus oryzae. Biotechnology and Bioprocess Engineering, 9, 245–251.
Dong, X. Y., Bai, S., & Sun, Y. (1996). Production of l(+)-lactic acid with Rhizopus oryzae immobilized in polyurethane foam cubes. Biotechnological Letters, 18, 225–228.
Efremenko, E., Spiricheva, O., Varfolomeyev, S., & Lozinsky, V. (2006). Rhizopus oryzae fungus cells producing l(+)-lactic acid: kinetic and metabolic parameters of free and PVA-cryogel-entrapped mycelium. Applied Microbiology and Biotechnology, 72, 480–485.
Znidarsic, P., Komel, R., & Pavko, A. (1998). Studies of a pelleted growth form of Rhizopus nigricans as a biocatalyst for progesterone 11 α-hydroxylation. Journal of Biotechnology, 60, 207–216.
Myers, R. H., & Montgomery, D. C. (2002). Response surface methodology. New York: Wiley.
Chang, N. Y., Huang, J. C., Lee, C. C., Shih, I. L., & Tzeng, Y. M. (2002). Use of response surface methodology to optimize culture medium for production of lovastatin by Monascus ruber. Enzyme and Microbial Technology, 30, 889–894.
Mitchell, J. K. (2003). Development of a submerged-liquid sporulation medium for the potential smartweed bioherbicide Septoria polygonorum. Biological Control, 27, 293–299.
Boyaci, I. H. (2005). A new approach for determination of enzyme kinetic constants using response surface methodology. Biochemical Engineering Journal, 25, 55–62.
Praneetratananon, S., Wakisaka, M., Shirai, Y., & Kitpreechavanich, V. (2005). Kitchen refuse: a novel substrate for l(+)-lactic acid production by Rhizopus oryzae in submerged fermentation. Japan Journal of Food Engineering, 6, 45–51.
Praneetratananon, S., Wakisaka, M., Shirai, Y., & Kitpreechavanich, V. (2005). Enhancement of lactic acid production from kitchen refuse by Rhizopus oryzae KPS106 immobilized on loofa sponge. Japan Journal of Food Engineering, 6, 45–51.
Miura, S., Arimura, T., Hoshino, M., Kojima, M., Dwiarti, L. A., & Okabe, M. (2003). Optimization and scale-up of l-lactic acid fermentation by mutant strain Rhizopus sp. MK-96–1196 in airlift bioreactor. Journal of Bioscience and Bioengineering, 96, 65–69.
Taguchi, H., & Humphrey, A. E. (1966). Dynamic measurement of the volumetric oxygen transfer coefficient in fermentation systems. Journal of Fermentation Technology, 44, 881–889.
Tay, A., & Yang, S. T. (2002). Production of l(+)-lactic acid from glucose and starch by immobilized cells of Rhizopus oryzae in a rotating fibrous bed bioreactor. Biotechnology and Bioengineering, 80, 1–12.
Bai, D. M., Li, S. Z., Liu, Z. L., & Cui, Z. F. (2008). Enhanced l(+)-lactic acid production by an adapted strain of Rhizopus oryzae using corncob hydrolysis. Applied Biochemistry and Biotechnology, 144, 79–85.
Zhang, Z. Y., Jin, B., & Kelly, J. M. (2007). Production of lactic acid from renewable materials by Rhizopus fungi. Biochemical Engineering, 35, 251–263.
Liao, W., Liu, Y., & Chen, S. (2007). Studying pellet formation of a filamentous fungus Rhizopus oryzae to enhance organic acid production. Applied Biochemistry and Biotechnology, 136–140, 689–701.
Zhou, Y., Du, J., & Tsao, G. T. (2000). Mycelial pellet formation by Rhizopus oryzae ATCC 20344. Applied Biochemistry and Biotechnology, 84–86, 779–789.
Abd-Aziz, S., Fernandez, C. C., Md. Salleh, M., Md. Illias, R., & Hassan, M. A. (2007). Effect of agitation and aeration rates on chitinase production using Trichoderma virens UKM1 in 2-l stirred tank reactor. Applied Biochemistry and Biotechnology, 150, 193–204.
Liu, B. L., Rou, T. M., Rao, Y. K., & Tzeng, Y. M. (2007). Effect of pH and aeration rate on the production of destruxins A and B from Metarhizium anisopliae. International Journal of Applied Science and Engineering, 5, 17–26.
Skory, C. D., Freer, S. N., & Bothast, R. J. (1998). Production of l-lactic acid by Rhizopus oryzae under oxygen limiting conditions. Biotechnological Letters, 20, 191–194.
Bai, D. M., Jia, M. Z., Zhao, X. M., Ban, R., Shen, F., Li, X. G., et al. (2003). l(+)-lactic acid production by pellet-form Rhizopus oryzae R1021 in a stirred tank fermenter. Chemical Engineering Science, 58, 785–791.
Cunha, M. A. A., Rodrigues, R. C. B., Santos, J. C., Converti, A., & Silva, S. S. (2007). Repeated-batch xylitol bioproduction using yeast cells entrapped in polyvinyl alcohol–hydrogel. Current Microbiology, 54, 91–96.
Acknowledgment
This work has been financially supported by the National Metal and Materials Technology Center, National Science and Technology Development Agency (NSTDA), Thailand through project, “Development of Artificial Intelligent Control for Fermenting Reactor and Fermentation Process”, no. MT-B-49-POL-07-345-I.
We thank Dr. Jean-Jacques Sanglier for discussion and critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maneeboon, T., Vanichsriratana, W., Pomchaitaward, C. et al. Optimization of Lactic Acid Production by Pellet-Form Rhizopus oryzae in 3-L Airlift Bioreactor Using Response Surface Methodology. Appl Biochem Biotechnol 161, 137–146 (2010). https://doi.org/10.1007/s12010-009-8860-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8860-0