Abstract
Aims
Culture media compositions and bioprocess conditions were studied to improve the production of cell biomass and indolic phytohormones by Herbaspirillum seropedicae BR11471, a plant growth promoting bacterium, and different inoculant formulations were also produced and tested for their stability and shelf life.
Methods
Response surface methodology (RSM) based on central composite rotation designs (CCRD) was used to find bioprocess variables that lead to an increase in bacterial biomass and yield of indolic compounds. The major components of DYGS medium were optimized in small-scale shaken cultivations, in two sets of CCRD. High performance liquid chromatography was used to determine nutrient consumption and to correlate it with cell biomass production, and the Salkowski method was used to quantify indoles. Hydrolytic activity in the formulations was quantified with the fluorescein diacetate assay.
Results
Glycerol (5.5 g L−1) and yeast extract (2.8 g L−1), as the main carbon and nitrogen sources, respectively, increased biomass production by 87.5% when compared to original DYGS medium, reaching 3.0 g L−1 of dry cell weight (DCW). In a 2.0 L bioreactor, the optimized medium was used to enhance process conditions for DCW and indole-3-acetic acid (IAA). Biomass production reached 3.4 g L−1 and was restrained at highest air flow levels. The conditions of 34-36 °C, 150 rpm and 4.0 L min−1 of air flow rate resulted in 11.97 mg L−1 of IAA, an increase of 370% over original DYGS at 30 °C. Peat can still be regarded as a good cell carrier for solid state inoculants, whilst the additives tested for liquid formulations are individually more efficient than the mixture.
Conclusions
The production of inoculants containing H. seropedicae strain BR11471 can be efficiently improved with the use of the RSM approach i.e. it maximizes the production of biomass and indolic compounds, and reduces culture media components, both key factors for large-scale industrial production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The inoculation of crops with plant growth promoting bacteria (PGPB) is an emerging technology with increasing diffusion and potential use to increase productivity of agricultural goods. As beneficial interactions of plants with microorganisms become better understood, crops can be safely stimulated by the use of microorganisms as biofertilizers, for crop protection, environmental remediation, biocontrol, and to reduce overall dependence on agricultural chemicals (Adesemoye et al. 2009; Estrada et al. 2013; Hayat et al. 2010). After many years of research, a number of bacterial strains are ready to be introduced into agricultural practice such as some Bacillus, Pseudomonas and Azospirillum species, in addition to the well-known rhizobia group. However, crops inoculated with PGPB still represent only a small fraction of world agriculture. Factors that limit wider utilization of biological products are related to the production, formulation, storage and application of these bacteria (Bashan et al. 2014; O'Callaghan 2016). Inoculation technology with PGPB has had little impact on the productivity of most countries with rural activity. The persistent low confidence of farmers in PGPB-containing inoculants is mainly due to the lack of consistent data on their efficiency and their low quality (Bernabeu et al. 2018; Bashan et al. 2014; Compant et al. 2010; Lucy et al. 2004).
Herbaspirillum seropedicae was first discovered in Brazil by Baldani et al. (1986) and has been isolated from various cereal crops (Monteiro et al. 2012; Balsanelli et al. 2015). It is a Gram-negative diazotrophic proteobacterium known for positive effects on the growth of rice, maize, sugarcane, among others (James et al. 2002; Canellas et al. 2013; Estrada et al. 2013; Rothballer et al. 2008). It has the ability to produce plant hormones and siderophores, promote mineral solubilization, and to perform biological nitrogen fixation (BNF) (Bastián et al. 1998; Richardson et al. 2009; Monteiro et al. 2012; Rosconi et al. 2013; Wagh et al. 2014). Amadeo et al. (2011) also showed that inoculation with H. seropedicae BR11417 increased maize productivity up to 34%. The effectiveness of inoculants containing PGPB depends on a successful colonization of the inoculated plant tissues. Desirable effects could be boosted or anticipated in the field by aggregating substances like auxins, which can be produced during the bioprocesses, and be present in the final product (Bashan et al. 2014; Silva et al. 2012).
The development of a cost-competitive bioprocess is a key point to obtain large scale feasibility of bioproducts (Amadeo et al. 2011; Chebotar et al. 2015; Chen et al. 2012). Response surface methodology (RSM) is a set of statistical techniques based on the design of experiments for building production models, evaluating the effects of multiple factors simultaneously, overcoming the limitations of classical methods of optimization (Hajji et al. 2008; Lotfy et al. 2007; Mutalik et al. 2008; Xie et al. 2012).
The aims of this study were to select the most suitable culture medium for cell biomass production of H. seropedicae BR11417 in shaken cultivations, to apply central composite rotatable designs (CCRDs) of RSM for optimization of medium and conditions in a bioreactor, and finally to evaluate the overall quality of experimental inoculants formulated for agricultural use.
Materials and methods
Microorganism and culture media
Herbaspirillum seropedicae BR11417 (ZAE94) was obtained from the Culture Collection of Johanna Döbereiner Biological Resources Center (CRB-JD, Embrapa Agrobiologia, Seropédica, RJ, Brazil). Slants of potato agar medium were used to prepare stock cultures used thereafter for all experiments, and these were also kept frozen at -80 °C in 30% glycerol as cryo-protectant. Colonies were grown in DYGS medium (in g L−1: glucose, 2.0; malic acid, 2.0; yeast extract, 2.0; peptone, 1.5; glutamic acid, 1.5; K2HPO4, 0.5; MgSO4.7H2O, 0.5) at 35 °C for 24 h. To confirm the identity of the strain, semi-solid JNFb medium was used with bromothymol blue as the pH indicator, as described by Baldani et al. (2014). Cell growth and production of indolic hormones were tested on traditional media used for PGPB growth such as: DYGS, LGI, LGI-P, JNFb, and also LB (Baldani et al. 2014). Two variations of basic DYGS medium were also initially used to select the most favorable carbon source: DYGS-1 contained glucose and DYGS-2 contained glycerol (both at 5.5 g L−1) as the main carbon source. Malic acid, peptone and glutamic acid were all removed for both DYGS-1 and DYGS-2.
Culture conditions
Batch cultures in shaken Erlenmeyer flasks were carried out at 150 rpm, and at 30 or 35 °C in a floor incubator shaker in orbital mode (IS-971-R, Lab Companion). Batch bioreactor experiments were conducted in 2.0 L vessels with control of air flow and temperature, and by monitoring pH and dissolved oxygen (Biostat B-plus twin, Sartorius Stedim Biotech). Operation conditions were set according to each CCRD. In every up-scaling step, from stocks to bioreactor, a ratio of 10% was used for inoculum size.
Inoculant formulations and bacterial survival
Selected culture conditions for H. seropedicae BR11417 were conducted in a 2.0 L batch bioreactor, and were used for inoculant formulation immediately after cells reached late exponential phase. Inoculants were prepared by mixing culture broths with a carrier into four different formulations: (a) peat-based - PI, 3 parts of sterilized ground peat with 2 parts of cell broth; (b) Xanthan gum – XI, at a final concentration of 5 g L−1; (c) PVP (polyvinylpyrrolidone) – PV, at a final concentration of 5 g L−1; (d) Xanthan gum and PVP – XP, 2.5 g L−1 for each component; (e) control - CB, only the culture broth. Liquid formulations (XI, PV and XP) were stabilized in a phosphate buffered saline solution (PBS) containing citric acid and dibutylhydroxytoluene (48 and 1.2 mg L−1, respectively). Inoculants containing 50 g (peat-based) or 50 mL (liquid formulations) were packed into sealed plastic bags or small high-density polyethylene (HDPE) bottles, respectively, previously sterilized in the autoclave at 121 °C. Food grade xanthan gum 200 Mesh (Synth) and polyvinylpirrolidone K-30 (Dinâmica) were obtained from local supplier, and peat of Argentinian origin was used after neutralization with calcium carbonate. All formulations were kept in a dark storage room at 25–30 °C. Shelf life was determined at different times (0, 1, 2, 4 and 6 months) by plate counting after spreading on JNFb medium (Baldani et al. 2014), and the number of viable cells were expressed as colony forming units (CFU) per gram or milliliter, whether the material was solid (peat) or liquid, respectively. Total metabolic activity of cells in the formulations was assayed by the Fluorescein Diacetate method (FDA) according to Green et al. (2006). After 2 h of incubation the production of fluorescein was measured at 490 nm and correlated to a freshly prepared standard curve.
Analytical tests and calculations
Cell growth was estimated by the measure of optical density at 600 nm. Calibration curves were made to correlate optical density (OD600) and dry cell weight (DCW). Samples of 10 mL of culture broth were centrifuged, washed twice with distilled cold water and filtered through 0.22 μm disk filters (Millipore). The filters were dried at 65 °C to constant weight. Cell counts were determined after serial dilutions and spreading of samples on JNFb medium, and it was expressed as CFU g−1 or CFU mL−1.
Glucose and glycerol concentrations were determined in culture supernatants at different times. They were measured in a LC-20A Modular HPLC, with a RI detector (Shimadzu). Samples of 20 μL were eluted at 0.8 mL min−1 with 0.005 M H2SO4 as the mobile phase in a HPX-87H column at 65 °C. A calibration curve was prepared with HPLC-grade reagents (Merck). Yield coefficients (Y) were calculated as the amount of desired product formed (g) in ratio to mass of a compound utilized or formed (g), to express the conversion of the carbon source to cell biomass (YX/S); the production of IAA related to the total biomass (YP/X); and the production of IAA related to consumed substrate (YP/S).
Indolic compounds were quantified using Salkowski reagent according to the microplate assay (Sarwar and Kremer 1995). The highest grade available indole-3-acetic acid (Sigma-Aldrich) was used for the calibration curve, freshly prepared for every set of samples. The concentrations of indoles were expressed as mg L−1 of indole-3-acetic acid (IAA) equivalent. All results of every test are the mean of at least three replicates.
Experimental designs
The factors affecting bacterial biomass production were selected based on the growth behavior of H. seropedicae BR11417 on the media traditionally used for PGPB (Baldani et al. 2014), in the first experiments with shaken flask cultivations. The components, levels and codes used for media optimization are shown in Table 1. To improve the nitrogen source a 22 central composite rotation design (CCRD-1) was centered on four combinations of yeast extract and peptone, the main nitrogenous components of DYGS medium. The optimal concentrations of salts in the DYGS medium were also evaluated in a set of ten experiments of four combinations, in the CCRD-2 (Table 2). Once these components were analyzed, their optimal concentrations were used in the succeeding experiments. In this second experimental design, the carbon source was then tested in terms of conversion into cell biomass under five levels employed during shaking flask cultivations: 2.5, 5.5, 8.25, 11.0 and 13.75 (g L−1). A 23 factorial CCRD-3 was further employed to test the independent variables temperature (°C), aeration (L min−1) and agitation (rpm) in a 2.0 L bioreactor. The process variables, levels and codes used for media optimization are shown in Table S1. To fit the polynomial model, 17 experiments with 8 combinations were required to evaluate cell concentration (DCW) and indole acetic acid production (IAA). Each experiment was performed in triplicate.
Statistical analysis
Statistica 10 (StatSoft) was used to design, calculate and analyze the response models obtained, and to generate surface contour plots. Regression analysis and analysis of variance (ANOVA) were performed for determining significance of the model terms and for fitting the mathematical models to experimental data. The adequacy of the model was determined using F-value, P value, residual standard deviation and coefficient of determination (R2).
Results
Optimization of medium composition
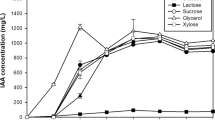
The growth of H. seropedicae BR11417 and the production of indoles were evaluated in shaken cultivations on different media traditionally used for isolation, characterization and even production of PGPB-containing inoculants. After 24 h of cultivation, at 30 °C and 150 rpm, DYGS-1 medium produced 3.3 × 107 CFU mL−1 and 3.15 mg L−1 of total indoles (Fig. 1a). Under the same conditions of temperature and agitation, LB performed better than LGI, LGI-P and JNFb for both parameters. When temperature was raised to 35 °C production of biomass and indoles was similar for DYGS-1 for the same period of cultivation at 30 °C, however the production of indoles was lower for LB, LGI, LGI-P and JNFb (data not shown). Furthermore, when H. seropedicae BR11417 was grown at 35 °C (DYGS-2) the increase in cell counts and production of indolic compounds were more pronounced.
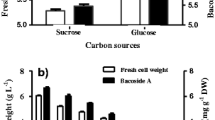
Cell growth and indolics (IAA) production of H. seropedicae BR11417 in (a) shaken cultures for 24 h, at 150 rpm and 30 °C (DYGS-1, LGI, LGI-P, JNFb and LB), and at 35 °C (DYGS-2); b growth conditions on original DYGS (Silva et al. 2012), and on modified DYGS, with glucose or glycerol as the main carbon sources
Despite its utility in the laboratory, DYGS is a complex and costly medium for large scale industrial inoculant production. Therefore, a comparative growth assay was performed at 35 °C, based on the evaluation of the above tests. Accordingly, glucose- and glycerol-based media (both at 5.5 g L−1) were formulated for maximizing economical biomass conversion (Fig. 1b). The original unmodified DYGS medium produced 1.55 g L−1 of cells after 12 h of growth and a yield (YX/S) of 0.24, but the glucose-based medium produced 1.86 g L−1 of cell biomass after 21 h and a yield of 0.30, and the glycerol-based medium resulted in cell concentrations reaching 2.44 g L−1 with an average yield of 0.40 after 30 h of cultivation. Therefore, based on these results, glycerol was chosen as the only carbon source to be added to the so-called modified DYGS for the succeeding optimization experiments.
In order to select a nitrogen source and to adjust the basic saline composition of the modified DYGS medium, two groups of 10 experiments were run and the results are shown in Table 2. In Table 2, on the left side (CCRD-1), the process variables peptone (X1) and yeast extract (X2) are presented with their actual levels, coded levels and mean experimental responses. On the right side of Table 2 (CCRD-2), the process variables potassium phosphate (X3) and magnesium sulphate (X4) are also presented with their levels and mean experimental responses. For both designs, treatment three showed the highest bacterial biomass production, 2.70 g L−1 for the nitrogen source, and 2.83 g L−1 for the salts. These levels of production were obtained at low peptone and phosphate levels. These combinations in treatment 3 produced 74% and 82.6%, respectively, more bacterial biomass than the original DYGS medium.
The analysis of variance (ANOVA) of the regression model was performed to demonstrate the significance according to Fisher’s F-test (Table 3). Also, for both designs, the computed F-values (20.55 for CCRD-1, 18.00 for CCRD-2) were much higher than the tabular value indicating that 96.25% (for CCR-1) and 95.74% (for CCR-2) of the variation among treatments were explained by the model. To verify the significance of each regression coefficient, Student’s t test and P-values were used to understand mutual interactions (Heck et al. 2005). The multiple regression analysis (Electronic Supplementary Material, Table S2) of the experimental data for both designs allowed a second order polynomial fitness, where peptone showed no significance for CCRD-1. To explain the bacterial biomass production (X) according to the models, the statistical insignificant terms must be eliminated and the two equations are:
-
(a)
X = 2.398 + 0.529 X2–0.239 X22, where X2 is yeast extract; and
-
(b)
X = 3.029–1.95X32–0.125 X42, where X3 is potassium phosphate and X4 is magnesium sulphate.
The predicted values for biomass production according to these models are also presented in Table 2, following the recorded experimental values. Peptone did not influence the production of bacterial biomass. According to the model, yeast extract can be used as the sole nitrogen source. Figure 2 shows contour shapes for the factors that affected DCW production. Increasing concentrations of yeast extract resulted in higher production of bacterial biomass. However, the impact of peptone on cell production was not so evident, and the flattened nature of the contour in Fig. 2a depicts a poor interaction between peptone and yeast extract. Furthermore, by increasing the concentration of yeast extract above 4.0 g L−1 the effect was negative on cell production, either with or without peptone added. In contrast, the concentration of major salts showed a precise and strict range when biomass production was increased (Fig. 2b).
The basic optimized medium was used to test the impact of different concentrations of glycerol on the biomass production by H. seropedicae BR11417. Accordingly, five levels were tested (Fig. 3), and the original concentration of 5.5 g L−1 of glycerol in the modified DYGS resulted in 2.98 g L−1 of DCW. For the medium containing 13.75 g L−1 of glycerol, DCW reached only 2.54 g L−1.
Influence of glycerol concentration on biomass production with the modified DYGS medium optimized for nitrogen source and salts composition. Glycerol concentrations are (in g L−1): 2.5 (Test 1), 5.5 (2), 8.25 (3), 11.0 (4) and 13.75(5). All tests were run in triplicates in a shaker incubator at 150 rpm and 35 °C
Optimization of bioreactor operation for bacterial biomass and IAA production
The previously optimized DYGS medium was used to support 2.0 L bioreactor experiments. The treatments with coded combinations of the three factors are shown in Table 4, along with the actual measurements and the predicted values. Treatments five, 10 and 17, with all levels set to flat (coded level = 0), presented DCW above 3.1 g L−1 and IAA levels above 11.4 mg L−1. Maximum biomass concentration of 3.34 g L−1 was obtained at 39.2 °C (level + 1), 194.6 rpm (+1) and 2.2 L min−1 of air flow (level − 1). Maximum IAA production of 11.97 mg L−1 was obtained when H. seropedicae BR11417 was grown at the same settings for agitation and air flow, and at a lower temperature (30.8 °C, level − 1), but not the lowest one (28 °C, level − 1.68). When temperature was set to the highest level (42 °C) a major impact was observed on yield coefficients for biomass and IAA over consumed substrate.
The combination of low temperature of growth (30.8 °C), low agitation (105.4 rpm) and low air flow (2.2 L min−1) resulted in high yield of IAA over consumed substrate and bacterial biomass production. Analysis of variance (ANOVA) demonstrated high significance for biomass production (Table 5), as the computed F-value (4.26) is higher than the tabular F-value (2.72), yet little variations were observed at the axial points of temperature when the experimental values were correlated to the predicted ones. The coefficient of determination (R2) was calculated to be 0.8457 for biomass production, indicating that the model could explain over 84% of the variability. For IAA production, ANOVA indicated that the model is highly significant, and an R2 = 0.92 suggests a satisfactory representation of the process model.
To verify the significance of each coefficient and to understand the patterns of interactions between variables, t-tests and P value were applied (Tables S3 and S4). The regression coefficients to be considered in constructing a model for biomass and indoles production were those whose values were superior to tabular t, and with P-values less than the significance level. Second-order temperature, temperature, agitation and aeration were significant for bacterial biomass production. For the production of indoles, the model revealed that temperature and agitation were significant as well as the interaction between these two factors. The models that explain biomass (X) and IAA (Y) production are:
-
(c)
X = 3.131 + 0.163Y1–0.378Y12 + 0.146Y2–0.199Y3, where Y1 is temperature, Y2 is agitation and Y3 is aeration;
-
(d)
Y = 11.503 + 0.701Y1–1.280Y12–1.970Y3–1.727Y32 + 1.215Y1.Y3, where Y1 is temperature and Y3 is aeration.
The contour shapes for biomass and IAA responses can be seen in Fig. 4, when cells of H. seropedicae BR11417 were grown in the medium optimized for carbon and nitrogen sources. These plots clearly demonstrate that biomass can be increased as agitation is raised (Fig. 4a), as long as the temperature is kept at moderate settings (34–36 °C). The impact of aeration in the culture media went on the opposite way, so less air flow (1–2 L min−1) promoted high biomass production (Fig. 4b). Interestingly, aeration and agitation did not interact in promoting biomass production, and this behavior would be difficult to perceive by using the one-variable-at-a-time approach. These two parameters are reportedly of major importance for bioproducts and cell mass production in aerobic cultures, as reported by Xie et al. (2012). Temperature was identified as a key process variable in the production of indoles by H. seropedicae BR11417, however the interaction of temperature and aeration (Fig. 4e) was the more evident result from the surface plots.
Quality and stability of formulations
Studies on the shelf life of different formulations containing H. seropedicae BR11417 were carried out by plate counting after serial dilutions, and at different times after packaging. Overall microbial activity, expressed as units of formazan produced after hydrolysis of fluorescein diacetate were also measured in the same moment for each sample. The same culture condition was used to prepare all the formulations. Peat formulation showed a superior hydrolytic activity in the early months compared to other inoculants (Fig. 5), and it resulted in good cell stability. After the fourth month cell counts experienced a rapid decline. In the control, where no additive was used, cell counts dropped dramatically, and were accompanied by low levels of metabolic activity. Inoculants with xanthan gum formulation (XI) and polyvinylpirrolidone (PV) additives showed a similar behavior to controls regarding overall hydrolytic activity, but XI presented better results for cell stability after 2 months. The mixture of XI and PV was inefficient in maintaining shelf life with a fast decrease in cell counts after two months of storage, and a continuous decline up to the sixth month of analysis. Total indoles were measured from the onset of formulation production, and after 2 and 4 months of storage. In the formulations XI, PV and XP total indolic compounds were stable and did not vary perceptively (data not shown). For the peat formulation it was not possible to perform the photometric assay described above, and in the control, where no stabilizing agent was added, indoles were poorly detected.
Cell viability of H. seropedicae inoculant formulations. a shows the cell counting along 6 months of storage per unit (mL for liquid or g for solid formulation). b shows overall metabolic activity FDA hydrolysis expressed as formazan units produced per 100 ml of liquid formulations, or per 100 g of peat formulation. Symbols: square, control (CB); circle, xanthan gum (XI); triangle, polyvinylpirrolidone (PI); star, xanthan gum +PVP (XP); diamond, peat (PI) . Mean data shown and standard errors bars are representative of three replicates
Discussion
Herbaspirillum seropedicae BR 11417 and other strains have a great potential for use as PGPB (Alves et al. 2015; Canellas et al. 2013; Trovero et al. 2018). However, apart from symbiotic rhizobia (and their legume hosts), Azospirillum spp. are by far the most studied PGBP, and commercial inoculants based upon them are available since the end of the twentieth century, mainly in developing countries. Few studies have reported inoculant production or studied the factors affecting growth and phytohormone production for these bacteria (Cappuyns et al. 2007; Ona et al. 2005). Bashan et al. (2011) proposed two media for cultivation of Azospirillum strains which were based on modified TYG (tryptone, yeast extract and glucose) medium where the glucose was replaced by Na-gluconate or glycerol, but neither the media composition nor the growth conditions were optimized. Trujillo-Roldán et al. (2013) performed scale-up experiments for inoculant formulation with Azospirillum spp. that were based on oxygen transfer parameters and with the use of an optimized NFb medium.
Many microbial isolates with PGPB capacities are identified annually, yet the majority of strains do not reach the formulation stage, as discussed by Bashan et al. (2014). Bastián et al. (1998) were the first to identify the production of IAA and gibberellins by H. seropedicae on chemically defined NFb medium, and these authors discussed the importance of studying the production of phytohormones in the relations of endophytic microorganisms with host plants.
Most inoculant production plants were originally designed for cultivations of relatively slow-growing rhizobia, so oxygen transfer and aeration are not easily measurable nor adjustable at the factory. Since medium composition is also a major issue in terms of production costs, the present study succeeded in optimizing biomass production for H. seropedicae BR11417 by adjusting the nitrogen and salt composition of the modified DYGS medium, and by substituting all three carbon sources for glycerol alone. Adnan et al. (2014) used a glycerol-based medium at 34.5 g L−1 to optimize ethanol production with recombinant E. coli, however the biomass never exceeded 0.6 g L−1. They affirmed that higher glycerol concentrations are believed to produce osmotic pressure within the bacterial cell, causing cell damage due to the purging of water molecules.
To properly formulate a bacterial suspension is one of the most common barriers to the commercialization of inoculant products to enhance crop yields (Stephens and Rask 2000; Bashan et al. 2014). Every industry develops its proprietary formulations containing a diverse variety of additives. While the culture broth solely without amendments is still used for testing the efficiency of PGPB (Bernabeu et al. 2018), the importance of a proper formulation and its effect on cell viability and ultimately how it limits the efficacy of inoculants in the field is largely recognized (Berninger et al. 2018). In the present study we used two common and well known additives, xanthan gum and PVP for liquid formulations, and traditional peat as the carrier for solid inoculants. Used individually, they all performed and maintained a similar shelf life as compared to unamended culture broth. Novel materials and methods to evaluate cell viability have been the focus of recent studies (Lobo et al. 2018), but little is known concerning the development of bioprocesses for the production of indolic compounds. Indole acetic acid is an intermediate metabolite; many biochemical routes are involved in its synthesis and consumption by PGPB, and, moreover, it can be easily and naturally oxidized (Pedraza et al. 2004; Spaepen et al. 2007). The stabilization of indoles in a commercial formulation does not imply any practical effectiveness when used in the field.
Response Surface Methodology is a set of important statistical designs and numerical techniques used to optimize bioprocesses and predict biological behaviors. RSM is based on the fit of experimental data to polynomial equations, and it not only defines the effect of independent variables, but also their interaction and even quadratic effects (Hallenbeck et al. 2015; Liu et al. 2017). In recent years it has been applied for optimization of experiments in fields like phytochemistry and bioprocessing or food and chemical engineering, always emphasizing practical applications (Pandey et al. 2018; Sharma et al. 2018; Amiri et al. 2019). In rotatable designs the variance of predicted response is constant at all points that are equidistant from the design center. Given the number of experimental factors tested and their levels, CCRDs allow an economic design for the response surface due to the reduced number of combinations for the levels of factors studied when compared to the full factorial (Myers et al. 2016).
During the present study, it was possible to optimize medium and bioprocess conditions for cell growth and indoles production by H. seropedicae BR11417. Equations for modeling indolic compounds and bacterial biomass production were developed based on cultivation experiments with varying process parameters. As far as the authors are aware, this study is the first successful application of the RSM design for inoculant production by any Herbaspirillum strain, a PGPB genus with interesting and desirable features to be used by farmers (Monteiro et al. 2012). The maximal amount of DCW accumulated was 3.34 g L−1 (a 2.15-fold increase as compared to original DYGS medium) and 11.97 mg L−1 of IAA (3.8-fold increase) when the optimized conditions were used. In addition to establishing optimal medium composition, the methodology presented here also makes it possible to predict yields when the variables are altered in some way. The coefficients of determination were 84.6 and 92.4 for DCW and IAA, respectively, confirming the validity of the model. Inoculants produced from these culture conditions have maintained good stability over long periods in spite of the main carrier chosen in the formulation. We expect that the results of this study can support and stimulate inoculant industries in the development of new bioproducts aimed at a more sustainable agriculture.
References
Adesemoye AO, Torbert HA, Kloepper JW (2009) Plant Growth-Promoting Rhizobacteria Allow Reduced Application Rates of Chemical Fertilizers. Microb Ecol 58:921–929. https://doi.org/10.1007/s00248-009-9531-y
Adnan NAA, Suhaimi SN, Abd-Aziz S, Hassan MA, Phang L-Y (2014) Optimization of bioethanol production from glycerol by Escherichia coli SS1. Renew Energy 66:625–633. https://doi.org/10.1016/j.renene.2013.12.032
Alves GC, Videira SS, Urquiaga S, Reis V (2015) Differential plant growth promotion and nitrogen fixation in two genotypes of maize by several Herbaspirillum inoculants. Plant Soil 387:307–321. https://doi.org/10.1007/s11104-014-2295-2
Amadeo I, Mauro LV, Ortí E, Forno G (2011) Determination of robustness and optimal work conditions for a purification process of a therapeutic recombinant protein using response surface methodology. Biotechnol Prog 27:724–732. https://doi.org/10.1002/btpr.588
Amiri S, Shakeri A, Sohrabi MR, Khalajzadeh S, Ghasemi E (2019) Optimization of ultrasonic assisted extraction of fatty acids from Aesculus hippocastanum fruit by response surface methodology. Food Chem 271:762–766. https://doi.org/10.1016/j.foodchem.2018.07.144
Baldani JI, Baldani VLD, Seldin L, Dobereiner J (1986) Characterization of Herbaspirillum seropedicae gen. Nov. sp. nov. a root-associated nitrogen-fixing bacterium. Int J Syst Bacteriol 36:86–93
Baldani JI, Reis VM, Videira SS, Boddey LH, Baldani VLD (2014) The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil 384:413–431. https://doi.org/10.1007/s11104-014-2186-6
Balsanelli E, Tadra-Sfeir MZ, Faoro H, Pankievicz VCS, de Baura VA, Pedrosa FO, de Souza EM, Dixon R, Monteiro RA (2015) Molecular adaptations of Herbaspirillum seropedicae during colonization of the maize rhizosphere. Environ Microbiol 18:2343–2356. https://doi.org/10.1111/1462-2920.12887
Bashan Y, Trejo A, de-Bashan LE (2011) Development of two culture media for mass cultivation of Azospirillum spp. and for production of inoculants to enhance plant growth. Biol Fertil Soils 47:963–969. https://doi.org/10.1007/s00374-011-0555-3
Bashan Y, de-Bashan LE, Prabhu SR, Hernandez J-P (2014) Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013). Plant Soil 378:1–33. https://doi.org/10.1007/s11104-013-1956-x
Bastián F, Cohen A, Piccoli P, Luna V, Baraldi R, Bottini R (1998) Production of indole-3-acetic acid and gibberellins A1 and A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically-defined culture media. Plant Growth Regul 24:7–11
Bernabeu PR, García SS, López AC, Vio SA, Carrasco N, Boiardi JL, Luna MF (2018) Assessment of bacterial inoculant formulated with Paraburkholderia tropica to enhance wheat productivity. W J Microbiol Biotechnol 34:81–91. https://doi.org/10.1007/s11274-018-2461-4
Berninger T, González-López O, Bejarano A, Preininger C, Sessitsch A (2018) Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb Biotechnol 11:277–301. https://doi.org/10.1111/1751-7915.12880
Canellas LP, Balmori DM, Médici LO, Aguiar NO, Campostrini E, Rosa RCC, Façanha AR, Olivares FL (2013) A combination of humic substances and Herbaspirillum seropedicae inoculation enhances the growth of maize (Zea mays L). Plant Soil 366:119–132. https://doi.org/10.1007/s11104-012-1382-5
Cappuyns AM, Bernaerts K, Smets IY, Ona O, Prinsen E, Vanderleyden J, Van Impe JF (2007) Optimal fed batch experiment design for estimation of monod kinetics of Azospirillum brasilense: from theory to practice. Biotechnol Prog 23:1074–1081
Chebotar VK, Malfanova NV, Shcherbakov AV, Ahtemova GA, Borisov AY, Lugtenberg B, Tikhonovich IA (2015) Endophytic Bacteria in microbial preparations that improve plant development (review). Appl Biochem Microbiol 51(3):271–277. https://doi.org/10.1134/S0003683815030059
Chen J, Huang PT, Zhang KY, Ding FR (2012) Isolation of biosurfactant producers, optimization and properties of biosurfactant produced by Acinetobacter sp. from petroleum-contaminated soil. J Appl Microbiol 112:660–671. https://doi.org/10.1111/j.1365-2672.2012.05242.x
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678. https://doi.org/10.1016/j.soilbio.2009.11.024
Estrada GA, Baldani VLD, Oliveira DM, Urquiaga S, Baldani JI (2013) Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant Soil 369:115–129. https://doi.org/10.1007/s11104-012-1550-7
Green VS, Stott DE, Diack M (2006) Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biol Biochem 38:693701. https://doi.org/10.1016/j.soilbio.2005.06.020
Hajji M, Rebai A, Gharsallah N, Nasri M (2008) Optimization of alkaline protease production by Aspergillus clavatus ES1 in Mirabilis jalapa tuber powder using statistical experimental design. Appl Microbiol Biotechnol 79:915–923. https://doi.org/10.1007/s00253-008-1508-0
Hallenbeck PC, Grogger M, Mraz M, Veverka D (2015) The use of design of experiments and response surface methodology to optimize biomass and lipid production by the oleaginous marine green alga, Nannochloropsis gaditana in response to light intensity, inoculum size and CO2. Bioresour Technol 184:161–168. https://doi.org/10.1016/j.biortech.2014.09.022
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60:579–598. https://doi.org/10.1007/s13213-010-0117-1
Heck JX, Soares LHB, Ayub MAZ (2005) Optimization of xylanase and mannanase production by Bacillus circulans strain BL53 on solid-state cultivation. Enz Microb Technol 37:417–423. https://doi.org/10.1016/j.enzmictec.2005.02.015
James EK, Gyaneshwar P, Mathan N, Barraquio QL, Reddy PM, Iannetta PPM, Olivares FL, Ladha JK (2002) Infection and colonization of rice seedlings by the plant growth-promoting bacterium Herbaspirillum seropedicae Z67. Mol Plant Microbe Interact 15:894–906. https://doi.org/10.1094/Mpmi.2002.15.9.894
Liu J, Li G, Sui Y (2017) Optimization of culture medium enhances viable biomass production and biocontrol efficacy of the antagonistic yeast Candida diversa. Front Microbiol 8:1–7. https://doi.org/10.3389/fmicb.2017.02021
Lobo CB, Tomás MSJ, Viruel E, Ferrero MA, Lucca ME (2018) Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol Res 219:12–25. https://doi.org/10.1016/j.micres.2018.10.012
Lotfy WA, Ghanem KM, El-Helow ER (2007) Citric acid production by a novel Aspergillus niger isolate: II. Optimization of process parameters through statistical experimental designs. Bioresour Technol 98:3470–3477. https://doi.org/10.1016/j.biortech.2006.11.032
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 86:1–25. https://doi.org/10.1023/B:ANTO.0000024903.10757.6e
Monteiro RA, Balsanelli E, Wassem R, Marin AM, Brusamarello-Santos LCC, Schmidt MA, Tadra-Sfeir MZ, Pankievicz VCS, Cruz LM, Chubatsu LS, Pedrosa FO, Souza EM (2012) Herbaspirillum-plant interactions: microscopical, histological and molecular aspects. Plant Soil 356:175–196. https://doi.org/10.1007/s11104-012-1125-7
Mutalik SR, Vaidya BK, Joshi RM, Desai KM, Nene SN (2008) Use of response surface optimization for the production of biosurfactant from Rhodococcus spp. MTCC 2574. Bioresour Technol 99:7875–7880. https://doi.org/10.1016/j.biortech.2008.02.027
Myers RH, Montgomery DC, Anderson-Cook CM (2016) Response surface methodology: process and product optimization using designed experiments, 4th Edition. John Wiley & Sons. ISBN: 978-1-118-91601-8
O'Callaghan M (2016) Microbial inoculation of seed for improved crop performance: issues and opportunities. Appl Microbiol Biotechnol 100:5729–5746. https://doi.org/10.1007/s00253-016-7590-9
Ona O, Van IJ, Prinsen E, Vanderleyden J (2005) Growth and indole-3-acetic acid biosynthesis of Azospirillum brasilense Sp245 is environmentally controlled. FEMS Microbiol Lett 246:125–132
Pandey A, Belwal T, Sekar KC, Bhat ID, Rawal RS (2018) Optimization of ultrasonic-assisted extraction (UAE) of phenolics and antioxidant compounds from rhizomes of Rheum moorcroftianum using response surface methodology (RSM). Ind Crop Prod 119:218–225. https://doi.org/10.1016/j.indcrop.2018.04.019
Pedraza RO, Ramirez-Mata A, Xiqui ML, Baca ME (2004) Aromatic amino acid aminotransferase activity and indole-3-acetic acid production by associative nitrogen-fixing bacteria. FEMS Microbiol Lett 233:15–21
Richardson AE, Barea J-M, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339. https://doi.org/10.1007/s11104-009-9895-2
Rosconi F, Davyt D, Martínez V, Martínez M, Abin-Carriquiry JA, Zane H, Butler A, de Souza EM, Fabiano E (2013) Identification and structural characterization of serobactins, a suite of lipopeptide siderophores produced by the grass endophyte Herbaspirillum seropedicae. Environ Microbiol 15:916–927. https://doi.org/10.1111/1462-2920.12075
Rothballer M, Eckert B, Schmid M, Fekete A, Schloter M, Lehner A, Pollmann S, Hartmann A (2008) Endophytic root colonization of gramineous plants by Herbaspirillum frisingense. FEMS Microbiol Ecol 66:85–95. https://doi.org/10.1111/j.1574-6941.2008.00582.x
Sarwar M, Kremer RJ (1995) Determination of bacterially derived auxins using a microplate method. Lett Appl Microbiol 20:282–285
Sharma D, Yadav KD, Kumar S (2018) Biotransformation of flower waste composting: optimization of waste combinations using response surface methodology. Bioresour Technol 270:198–2017. https://doi.org/10.1016/j.biortech.2018.09.036
Silva MF, Antônio CS, Oliveira PJ, Xavier GR, Rumjanek NG, Soares LHB, Reis VM (2012) Survival of endophytic bacteria in polymer-based inoculants and efficiency of their application to sugarcane. Plant Soil 356:231–243. https://doi.org/10.1007/s11104-012-1242-3
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Stephens JHG, Rask HM (2000) Inoculant production and formulation. Field Crop Res 65:249–258. https://doi.org/10.1016/S0378-4290(99)00090-8
Trovero MF, Scavone P, Platero R, Souza EM, Fabiano E, Rosconi F (2018) Herbaspirillum seropedicae differentially expressed genes in response to iron availability. Front Microbiol 9:1–12. https://doi.org/10.3389/fmicb.2018.01430
Trujillo-Roldán MA, Valdez-Cruz NA, Gonzalez-Monterrubio CF, Acevedo-Sánchez EV, Martínez-Salinas C, García-Cabrera RI, Gamboa-Suasnavart RA, Marín-Palacio LD, Villegas J, Blancas-Cabrera A (2013) Scale-up from shake flasks to pilot-scale production of the plant growth-promoting bacterium Azospirillum brasilense for preparing a liquid inoculant formulation. Appl Microbiol Biotechnol 97:9665–9677. https://doi.org/10.1007/s00253-013-5199-9
Wagh J, Bhandari P, Shah S, Archana G, Kumar GN (2014) Overexpression of citrate operon in Herbaspirillum seropedicae Z67 enhances organic acid secretion, mineral phosphate solubilization and growth promotion of Oryza sativa. Plant Soil 383:73–86. https://doi.org/10.1007/s11104-014-2161-2
Xie T, Sun Y, Du K, Liang B, Cheng R, Zhang Y (2012) Optimization of heterotrophic cultivation of Chlorella sp. for oil production. Bioresour Technol 118:235–242. https://doi.org/10.1016/j.biortech.2012.05.004
Acknowledgements
The authors would like to thank the financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – Grant number 001), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Euan K. James.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Scheidt, W., dos Santos Pedroza, I.C.P., Fontana, J. et al. Optimization of culture medium and growth conditions of the plant growth-promoting bacterium Herbaspirillum seropedicae BR11417 for its use as an agricultural inoculant using response surface methodology (RSM). Plant Soil 451, 75–87 (2020). https://doi.org/10.1007/s11104-019-04172-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04172-0