Abstract
In this work, cashew apple bagasse (CAB) was used for Saccharomyces cerevisiae immobilization. The support was prepared through a treatment with a solution of 3% HCl, and delignification with 2% NaOH was also conducted. Optical micrographs showed that high populations of yeast cells adhered to pre-treated CAB surface. Ten consecutive fermentations of cashew apple juice for ethanol production were carried out using immobilized yeasts. High ethanol productivity was observed from the third fermentation assay until the tenth fermentation. Ethanol concentrations (about 19.82–37.83 g L−1 in average value) and ethanol productivities (about 3.30–6.31 g L−1 h−1) were high and stable, and residual sugar concentrations were low in almost all fermentations (around 3.00 g L−1) with conversions ranging from 44.80% to 96.50%, showing efficiency (85.30–98.52%) and operational stability of the biocatalyst for ethanol fermentation. Results showed that cashew apple bagasse is an efficient support for cell immobilization aiming at ethanol production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Immobilized cell system has been suggested as an effective means for improving ethanol fermentation, since it is possible to increase process productivity and minimize production costs [1, 2]. The immobilization of cells leads to higher cell densities with consequent increases in reaction rates and productivity. As a result, shorter residence time and smaller reactor size can be employed.
Ethanol production by immobilized yeast cells has been extensively investigated [2–7]. For industrial purposes, an important choice criterion is the carrier cost which, combined with the interest in by-products recycling, has been leading to an increasing search for cheap and available potential cell carriers [1]. Therefore, in this work, cashew apple bagasse, a lignocellulosic residue from the juice industry, is used as cell support in alcoholic fermentation of cashew apple juice.
Cashew apple is a pseudofruit native of Brazil, especially of the North and Northeast regions, where the cashew agroindustry has an outstanding role in the local economy. Cashew apple is a hard, pear-shaped, small and non-climacteric fruit, and is found in three colors: yellow, orange, and red. Its main product is the cashew nut, which is well known around the world. The edible portion, representing 90% of the fruit, is a pseudofruit rich in vitamin C, flavor, and aroma. The industrially processed products are basically consumed by the local market and they do not play an important role in the state or Brazil economy [8–10]. When the pseudofruit is industrially processed for the production of juice, 40% (w/w) of bagasse is produced, which is not used for human consumption and is usually discarded by the local industry [11].
In this work, the ability of cashew apple bagasse as an alternative low-cost support for the immobilization of Saccharomyces cerevisiae cells has been evaluated, aiming to evaluate the efficiency and suitability of immobilized yeast cells for ethanol production from cashew apple juice.
Materials and Methods
Raw Material
Cashew apple (Anacardium occidentalis L.) bagasse was kindly donated by Jandaia Industry of Juice (Ceará, Brazil). It was washed five times with water, dried at 60 °C for 24 h, and milled. The milled CAB was stored at room temperature. Cashew apple juice was extracted by compressing the cashew apple. After compressing, the juice was centrifuged at 3,500 rpm for 20 min (BIO ENG, BE-6000).
Microorganism
The microorganism used was a commercial yeast S. cerevisiae. A pure culture was isolated from baker’s yeast Saf-momento (SAF Argentina, Buenos Aires), inoculated on agar Sabouraud Biolife (peptone 5.0 g L−1, glucose 15.0 g L−1, and agar 15.0 g L−1), and incubated at 30 °C for 48 h.
Inoculum Preparation
Inoculum was obtained in 500-mL Erlenmeyer flasks with a medium volume of 200 mL, consisting of (g L−1): glucose 30; yeast extract 5; (NH4)2SO4 10; KH2PO4 4.5; MgSO4·7H2O 1; ZnSO4 0.65. The medium was sterilized at 110 °C for 10 min. The pH and temperature were maintained at 5.0 and 30 °C, respectively, during 24 h. After that, cells were centrifuged at 10,000×g for 10 min to obtain the initial biomass used in fermentation assays [12] or for cell immobilization.
Support Preparation and Microorganism Immobilization
The support for yeast immobilization was prepared using cashew apple bagasse (CAB) according to the methodology proposed by Branyik et al. [13], with slight modifications. CAB was washed with water and dried at 50 °C. After that, it was treated with HCl 3% for 2.5 h at constant agitation in a thermostatic bath at 60 °C. Then, it was washed with water, dried at 50 °C, and treated with NaOH 2% for 24 h. After that, the resulting solid was washed, dried, grinded, and sieved. In this work, only particles between 0.35 and 0.84 mm were used as support for yeast immobilization.
Pre-treated CAB (12.5 g) was sterilized at 110 °C for 10 min and then mixed with 125 mL of synthetic medium, consisting of (g L−1): glucose 30; yeast extract 5; (NH4)2SO4 10; KH2PO4 4.5; MgSO4·7H2O 1; ZnSO4 0.65. One percent (w/v) of cells was inoculated into the medium containing the support and the mixture was allowed to ferment for 24 h at 30 °C and 150 rpm. The liquid was then decanted and the support containing cells was washed with sterile water and used for the fermentation runs.
Media and Fermentation Assays
The medium for fermentation consisted of cashew apple juice supplemented with the following components: 2.50 g L−1 NH4SO4, 0.50 g L−1 KH2PO4, 0.65 g L−1 MgSO4·7H2O, and 0.65 g L−1 ZnSO4. It was sterilized in an autoclave (Phoenix, Araraquara, SP, Brazil) at 110 °C for 10 min [14] and its initial pH was adjusted to 4.50 by using 1 N HCl. Batch fermentation was carried out in 500-mL Erlenmeyer flasks with 250 or 125 mL of medium, for free cell or immobilized cell system, respectively, in a TE240 rotary shaker (Tecnal, São Paulo, Brazil) at 30 °C and 150 rpm. Samples were collected at time-defined intervals and submitted to analysis. When free cells were used to produce ethanol, initial yeast concentration inoculated into the fermentation medium was 10 g L−1. When fermentation by immobilized cells was conducted, 12.5 g of support containing cells were added to the medium. At the end of fermentation, the support was separated from the broth by filtration and stored under refrigeration.
Repeated Batch Fermentation
Repeated batch fermentation was conducted using yeast cells immobilized in cashew apple bagasse. The fermentation time for each batch was 6 h. At the end of each fermentation run, the support was collected from fermentation broth, washed with sterile water, and transferred to a fresh medium. This procedure was continued for ten runs [15].
Fermentation Parameters
The fermentation parameters and yields were calculated at the end of the fermentation (6 h). The ethanol volumetric productivity (Q P, g L−1 h−1) was calculated as the ratio of ethanol concentration at the end of the run (P f, g L−1) to the fermentation time (t, h)
The yield of ethanol on consumed sugar (Y P/S, g g−1) was defined as
where S 0 (g L−1) and S f (g L−1) are the starting and the final sugar concentrations, respectively.
Sugar conversion was calculated using Eq. 3:
The efficiency of sugar conversion to ethanol (g, %) has been estimated by the relationship:
where Y th is the theoretical value of Y P/S (0.51 g g−1).
The yield of biomass on consumed sugar (Y X/S, g g−1) was defined as:
Analytical Methods
Biomass Content
Optical density (OD = 660 nm) was used to identify free cells growth. A previously constructed calibration curve was used to relate the OD measurements to dry cell concentration in the samples.
The mass of cells adsorbed onto the support particles was quantified by counted using a Neubauer camera. Before counting, 0.5 g of support containing immobilized cells were added to 50 mL of 0.85% NaCl solution, and the mixture was allowed maintained for 24 h at 150 rpm. Afterwards, 0.1 mL of solution (containing freed yeast cells) was mixed to 0.2 mL of a methylene blue solution and placed in the Neubauer camera.
Glucose, Fructose, Ethanol, and Glycerol Concentrations
Total reducing sugars (glucose + fructose), ethanol, and glycerol were analyzed by high-performance liquid chromatography (HPLC) using a Waters HPLC system (Waters, Milford, MA, USA) equipped with a refractive index Waters 2414 detector using an Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA). The eluent was 5 mmol L−1 H2SO4 in Water MilliQ (simplicity 185, Millipore, Billerica, MA, USA) at a flow rate of 0.5 mL min−1 and 65 °C. Samples were identified by comparing the retention times with those of carbohydrates, ethanol, and glycerol standards.
Optical Microphotographs
Optical micrographs were performed using a biological microscope (OPTON, TNB-04B-PL).
Results and Discussion
Immobilization of Yeast Cell to CAB
In order to confirm cell immobilization onto pre-treated CAB as well as the potential of this raw material as support for S. cerevisiae, optical micrographs were taken before immobilization and at the end of fermentation assays (see Fig. 1). The carrier from pre-treated CAB is irregular in shape (Fig.1a), but it can be observed that high populations of yeast cell adhered to its surface (Fig. 1b). This result suggests the use of the biocatalyst in batch fermentation, for instance, allowing recycling cells in repeated batch runs. Figure 1b is representative of all the bagasse observed under the microscope, 20 samples (ten before immobilization and ten after). Most of all observed samples have similar distribution of immobilized cells, as pictured in Fig. 1b.
When lignocellulosic materials act as supports, most of the yeast cells were adsorbed to the surface of the supports and just a few of them are embedded in the inner side of the supports tightly [2]. Cellulosic materials, such as cashew apple bagasse, are solid carriers used for cell immobilization by physical adsorption due to electrostatic forces or by covalent binding between the cell membrane and the carrier [1]. Furthermore, as there are no barriers between the cells and the solution, cell detachment and relocation is possible with possible occurrence of equilibrium between adsorbed and freely suspended cells.
Batch Fermentation in Free and Immobilized Yeast Cells Systems
In a previous work [13], cashew apple juice was used for ethanol production. The effect of initial sugar concentrations (glucose + fructose—S 0) was investigated and maximum ethanol concentration was obtained when 87.7 and 103.1 g L−1 of initial sugar concentration was used in the fermentation medium. Immobilized cell technology has many advantages over conventional systems. One of them is cell immobilization that increases productivity to two or three times [4] by substantially increasing the population density. Therefore, in this work, we compare the performance of a free cell system with an immobilized cell system in the alcoholic fermentation of cashew apple juice. Figure 2 shows the experimental results obtained for substrate consumption, ethanol and glycerol production. Table 1 presents the fermentation parameters obtained in batch fermentation with S. cerevisiae free and immobilized on CAB.
As it can be seen in Fig. 2, in the free cell system it took 9 h to consume 95% of the total sugar and ethanol productivity was 4.29 g L−1 h−1 (see Table 1). In contrast, 94% of the total sugar was consumed after 6 h in the immobilized system, with an ethanol productivity of 6.15 g L−1 h−1, which is 1.4 higher than that of the free cell system. Ethanol concentration and yield were also higher for the immobilized system (Table 1). According to the literature [2], this ability of immobilized cells to produce ethanol for a long time yet remains to be explained but it might be due to the fact that immobilized cells contain significantly higher percentages of saturated fatty acids compared to free cells which leads to greater ethanol tolerance in the immobilized cells, and hence greater survival and productivity in subsequent cycles compared to free cells can be observed. Other authors [16] reported that immobilized cells can retain enzyme activities for a long time due to the different composition of cells (proteins, lipids, RNA, DNA, and inorganic substances) compared to free cells.
Repeated Batch Fermentation with Immobilized Yeast Cells
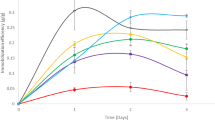
Ethanol production using yeast immobilized in CAB as a carrier was examined by a ten-cycle repeated batch fermentation using cashew apple juice as fermentation media. Results are presented in Fig. 3. Ethanol concentration in each cycle varied in each repeated run, ranging from 19.82 to 37.83 g L−1. A delay in batch fermentations 1–3 was observed in relation to the rest, probably as a consequence of the adaptation of the immobilized yeasts to the fermentation media. Ethanol concentrations were high and quite stable from the fourth batch on. Those results shows that, at the fourth batch, high density of immobilized cells were achieved, which retained their viability at stable levels during the successive batches. Other authors [5] observed that the immobilization of cells is a time-dependent process and they attribute this dependence to two main factors: cell multiplication and the formation of a strong and irreversible adhesion.
The fermentation parameters obtained after repeated batch fermentations of cashew apple juice for ten cycles using yeast immobilized in CAB are summarized in Table 2. Ethanol productivities (from 3.30 to 6.31 g L−1 h−1) were high when compared to other authors’ productivities (see Table 3). Residual sugar concentrations were low after the fourth batch with conversions ranging from 95% to 96.5%, showing operational stability of the biocatalyst for alcoholic fermentation.
From the Gay–Lussac equation for ethanol production under anaerobic conditions, for each kilogram of glucose consumed, 0.51 kg of ethanol can be produced. However, as some of the carbon source is used for biomass generation, the actual ethanol yield is about 90–95% of the theoretical one. In addition to ethanol, carbon dioxide, and biomass, 2 mol of adenosine triphosphate are produced per mole of glucose [17]. In this work, fermentation of cashew apple juice using yeast immobilized in CAB, the efficiency of sugar conversion to ethanol (η) varied from 85.3% to 98.5%, which is considered acceptable when compared to other authors’ values using different substrates and biocatalysts [5].
Conclusions
Optical microphotographs showed that cells colonized the cashew apple bagasse uniformly and abundantly, thus demonstrating the promising ability of this raw material to immobilize them. The immobilized yeast biocatalyst showed high fermentation activity. In repeated batch fermentation, after the third batch, more than 95% total sugar was consumed after 6 h of fermentation with the ethanol yield of 0.46 on average, with an average ethanol productivity of 5.49 g L−1 h−1. The immobilized cells could be reused for at least for ten cycles retaining its original activity. Thus, biocatalysts produced by cell immobilization on CAB were suitable for alcoholic fermentation, with some advantages: ease of handling, good operational stability, and low raw materials and process costs.
References
Santos, D. T., Sarrouh, B. F., Rivaldi, J. D., Converti, A., & Silva, S. S. (2008). Journal of Food Engineering, 86, 542–548.
Yu, J., Zhang, X., & Tan, T. (2007). Journal of biotechnology, 129, 415–420.
Giordano, R. L. C., Hirano, P. C., Gonçalves, L. R. B., & Schmidell Netto, W. (2000). Applied Biochemistry and Biotechnology, 84–86, 643–654.
Najafpour, G., Younesi, H., Ku, S., & Ku, I. (2004). Bioresource Technology, 92, 251–260.
Liang, L., Zhang, Y., Zhang, L., Zhu, M. J., Liang, S., & Huang, Y. (2008). Journal of Industrial Microbiology Biotechnology, 35, 1605–1613.
Plessas, S., & Bekatorou, A. (2007). Bioresource Technology, 98, 860–865.
Kopsahelis, N. (2007). Bioresource Technology. doi:10.1016/j.biortech.2006.03.030.
Assunção, R. B., & Mercadante, A. Z. (2003). Journal of Food Composition and Analysis, 16, 647–657.
Azevedo, D. C. S., & Rodrigues, A. (2000). Journal of Separation Technology, 35, 2561–2581.
Campos, D. C. P., Santos, A. S., Wolkoff, D. B., Matta, V. M., Cabral, L. M. C., & Couri, S. (2002). Desalination, 148, 61–65.
Rodrigues, T. H. S., Pinto, G. A. S., & Gonçalves, L. R. B. (2008). Biotechnology and Bioprocess Engineering, 13, 571–576.
Rocha, M. V. P., Rodrigues, T. H. S., Macedo, G. R., & Gonçalves, L. R. R. (2009). Applied Biochemistry and Biotechnology, 155, 407–417. doi:10.1007/s12010-008-8432-8.
Branyik, T., Vicente, A. A., Machado Cruz, J. M., & Teixeira, J. A. (2001). Biotechnology Letters, 23, 1073–1078.
Pinheiro, A. D. T., Rocha, M. V. P., Macedo, G. R., & Gonçalves, L. R. B. (2008). Applied Biochemistry and Biotechnology, 148, 227–234.
Yamashita, Y., Kurosumi, A., Sasaki, C., & Nakamura, Y. (2008). Biochemical Engineering Journal, 42, 314–319.
Rychtera, M., Basarova, G., & Ivanova, V. (1987). In O. M. Neijssel, R. R. Van der Meer & K. Luvben (Eds.), Fourth European Congress on Biotechnology. 2 (pp. 107–109). Amsterdam: Elsevier.
Soboncan, G., & Glavic, P. (2000). Applied Thermal Engineering, 20(6), 529–543. doi:10.1016/S1359-4311(99)00042-3.
Kourkoutas, Y., Komaitis, M., Koutinas, A., & Kanellaki, M. (2001). Journal of Agricultural and Food Chemistry, 49(3), 1417–1425. doi:10.1021/jf000942n.
Bekatorou, A., Sarellas, A., Ternan, N., & Mallouchos, A. (2002). Journal of Agricultural and Food Chemistry, 50, 7249–7257. doi:10.1021/jf020291q.
Acknowledgments
The authors acknowledge BNB, CNPq, CAPES, and ANP (from Brazil) for the financial support that made this work possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pacheco, A.M., Gondim, D.R. & Gonçalves, L.R.B. Ethanol Production by Fermentation Using Immobilized Cells of Saccharomyces cerevisiae in Cashew Apple Bagasse. Appl Biochem Biotechnol 161, 209–217 (2010). https://doi.org/10.1007/s12010-009-8781-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8781-y