Abstract
Delignified sugarcane bagasse from the sugar industry was used as a carrier for Saccharomyces cerevisiae SC90 immobilization and ethanol production. The proficiency of cell immobilization of S. cerevisiae SC90 on delignified sugarcane bagasse was determined by the amount of cell retention on the carrier and by scanning electron microscopy (SEM). S. cerevisiae SC90 showed the highest cell immobilization on day 1 when diluted molasses (231 g/L of total sugar) was used as a substrate. The efficiency of ethanol production by the immobilized cells was compared with cells grown in suspension in the repeated batch process. Immobilized cells exhibited a higher ethanol production than the suspended system for all five consecutive batches without any requirement for cell adaptation. The maximum ethanol yield (YP/S) of the immobilized cells was 0.42 ± 0.02 g/g (82.35% theoretical yield) in a 3 L packed bed bioreactor when the production could be prolonged up to five consecutive batches. As an additional bonus, the high protein spent yeast cells mixed with delignified sugarcane bagasse can be explored as an animal feed in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The novelty and significance of this work lies in the usage of delignified sugarcane bagasse as immobilized yeast supporter to serve bioethanol production under the repeated batches process using molasses as a substrate. The efficiency of ethanol production by the immobilized cells was significantly higher than the suspended culture under the five cycles of repeated batches without any requirement for the cell adaptation. The spent immobilized yeast on sugarcane bagasse represented its potential to be used as animal feed supplement based on composition analysis.
Introduction
Concerns about the economic and environmental effects of depletion of nonrenewable fossil fuel sources have led to exploration of bioethanol as a potential energy source to replace petroleum. As the demand for ethanol increases, there is a need to search for less expensive technology with the high process competitiveness in order to reduce the cost of production. In Thailand, bioethanol production from molasses-based ethanol provides high net energy for vehicles, since sugarcane is a prime economic crop [1]. Bioethanol has been widely produced under batch or fed batch processes using free, suspended cells. Others have used cell immobilization based on cell entrapment in gels such as carrageen and calcium alginate [2,3,4]. However, there are drawbacks among these types of cell immobilization processes such as instability caused by gel degradation and lack of metabolite mass transfer.
Recently, natural cell immobilization has emerged as an effective biocatalyst, as it overcomes these limitations that usually appear through the other chemical immobilization techniques. The system not only increases the production and yield of ethanol, but also provides a method for cell recycling and facilitates product recovery, thereby minimizing bioethanol production cost in various bioprocesses [5,6,7]. Natural cell immobilization occurs by direct adsorption of microorganisms onto the biotic or the abiotic carriers. Carriers support growth of microorganisms and protect cells from toxic inhibitors and high sugar and ethanol concentrations, which can be present in the batch culture [8,9,10]. This process provides higher biomass concentration and enhanced biological stability, which could improve ethanol production rates by reducing the fermentation time. The presence of high biomass concentrations also minimizes the risk of contamination in the process [11]. Natural or biotic carriers have been studied in the past, including wood blocks, guava pieces, porous cellulose, apple cuttings, loofa sponges, sorghum bagasse, saw dust, watermelon rinds, silk cocoons and delignified agricultural materials [11,12,13,14,15,16,17]. Since sugarcane is a prime economic crop in Thailand, while Thailand left over bagasse around 30.68 million tons per year [18]. Sugarcane bagasse, the fibrous matter that remains after sugarcane is crushed and squeezed to extract the sugar, is a remarkable by-product that could be developed as a natural carrier. Additionally, the spent cell/carrier mix could be further modified to serve as an animal feed supplement at the end of the fermentation process.
Saccharomyces cerevisiae has been widely used for bioethanol production because it can use various types of substrates and is tolerant to a wide range of pH and ethanol concentrations [13]. It has been found to immobilize on different types of natural carriers including sorghum bagasse, sugarcane pieces, wood blocks, silk cocoons, and loofa sponges [11,12,13,14, 19]. Yeast cells immobilization on agricultural materials have been found to produce almost ten times more ethanol production than cultures grown in suspension with the faster fermentation per unit volume [19]. In addition, the cost of product recovery can be reduced with the immobilized cells. Previously, yeast immobilization on sugarcane bagasse and its used in bioethanol fermentation was limited especially for the scale up the process. The spent agricultural carrier, such as delignified lignocellulose, can be used as protein enriched animal feed [20, 21]. Pretreatment processes can be used to increase digestibility of lignocellulose by removing lignin. Thus, ruminal microorganisms can easily access the nutritional part of the delignified lignocellulose [22]. Hence, the immobilization of bioethanol producing yeast on various sources of glucose rich substrates represents a promising technology for industrial scale ethanol production with high economic benefits.
The aim of this work was to study the potential of using immobilized S. cerevisiae on sugarcane bagasse for ethanol production using molasses as a substrate. Sugarcane bagasse and molasses are natural and abundantly available from the Thai sugar industry. Delignified sugarcane bagasse provides various benefits in terms of mechanical strength, light weight, high surface area and eventually as animal feed. S. cerevisiae SC90 was immobilized on delignified sugarcane bagasse using molasses as a substrate to optimize the immobilization and fermentation processes. Immobilization efficiency of yeast was analyzed by assessing the cell retention of the immobilized cells and visualized with scanning electron microscopy (SEM). Ethanol production by the immobilized yeast was determined under batch and repeated batch processes and compared with that of suspended cultures using ethanol yield (YP/S) and percent theoretical yield as the kinetic parameters. The spent immobilized yeast absorbed on delignified bagasse was determined on its nutritional value to be a valuable commodity as an animal feed supplement.
Materials and Methods
Yeast Strain and Culture Preparation
Saccharomyces cerevisiae SC90 (TISTR 5606) was obtained from Thailand Institute of Scientific and Technological Research (TISTR). The culture was grown in Yeast Extract–Peptone–Dextrose medium (YPD) medium containing 20 g/L glucose, 10 g/L yeast extract, and 20 g/L peptone, pH 4.8. Starter cultures were prepared by transferring a single colony to 5 mL YPD broth, pH 4.8 and incubating at 30 °C with shaking at 120 rpm for 12 h until the OD600 was approximately 1.0. The seed culture of 50 mL YPD broth was grown under the same conditions.
Carrier and Medium Preparations
Sugarcane bagasse and molasses were obtained from Khon Kaen Sugar Industry (KSL) Green Innovation Public Company Limited, Thailand. Sugarcane pieces were obtained and cut into small pieces of 1 cm length. Sugarcane bagasse was delignified using 1:10 (w/v) ratio of potassium hydroxide in 0.01% (v/v) acetic acid at 70 °C for 1 h. The process was repeated 3 times. Then the bagasse was neutralized by rinsing with water and dried in an oven at 60 °C until the dry weight was constant [23]. The concentration of total sugar in molasses was measured by using Fehling’s titrimetric method and the molasses was diluted to a specific sugar concentration (as described below) with distilled water before use [24]. The subsequently diluted molasses was supplemented with 1 g/L (NH4)2SO4 and 1 g/L KH2PO4, pH 4.8. Molasses and delignified bagasse were sterilized at 121 °C, 15 min.
Determination of Optimum Conditions for Cell Immobilization
To begin the batch process, 10% (v/v) of the starter culture (OD600 approximately 1.0) was inoculated into a flask containing 10% (w/v) delignified sugarcane bagasse in a total volume of 50 mL culture medium. YPD and molasses (with total sugar concentration of 40, 94, 140, 185 and 231 g/L) were used as the culture media. These specific growth conditions for immobilized cells were cultured at 30 °C and 80 rpm for 1–3 days. The optimum immobilization time and condition were evaluated by quantitative and qualitative analysis.
Quantitative analysis was determined by measuring the increase in dried mass of immobilized cells adsorbed on sugarcane bagasse to the dry mass of carrier (w/w) or cell retention of the carrier. Specifically, suspended cells were rinsed out of the batch and the remaining immobilized cells on the sugarcane bagasse were washed twice using sterile distilled water and subsequently oven dried at 70 °C until the weight was stable. The immobilization efficiency based on the cell retention in each specific medium condition and time was calculated as dried mass of immobilized cell on sugarcane bagasse (g) to the dry mass of sugarcane bagasse starting material (g).
Qualitative analysis of cells immobilized on sugarcane bagasse and grown under each test condition was performed using a scanning electron microscope (SEM) (JCM-6000, Japan). The immobilized cells on the sugarcane bagasse on day 1 were oven dried at 70 °C and followed by freeze drying at − 35 °C for 3.5 h, − 5 °C for 8 h, 15 °C for 8 h, and 35 °C for 3 h (FD8-Economic Series, Thailand). The sample was prepared for SEM by fixing the specimen with tape and then sputtered with gold under high vacuum conditions. Each sample was examined at 2000-fold magnification using SEM.
Batch and Repeated Batch Fermentation
Saccharomyces cerevisiae SC90 suspended and immobilized cultures were fermented for five consecutive batches using molasses as a substrate for ethanol production. Suspended and immobilized cultures of S. cerevisiae SC90 were compared for fermentation efficiencies under shaking conditions in flasks containing 50 mL molasses supplemented with (NH4)2SO4 and KH2PO4 (molasses 231 g/L of total sugar and supplemented with 1 g/L (NH4)2SO4 and 1 g/L KH2PO4), pH 4.8 in 125 mL Erlenmeyer flask. The flask of immobilized cells additionally contained 10% (w/v) delignified bagasse inoculated with 10% (v/v) of the starter culture (OD600 approximately 1.0). The flask of suspended culture contained only fermentation medium with 10% (v/v) of the starter culture (OD600 approximately 1.0). Flasks were shaken at 80 rpm at 30 °C for 24 h and then further incubated without shaking for another 24 h (JSSI-100C, Korea) only for the first batch. The following four batches were grown under anaerobic conditions for 48 h. The experiment was monitored by removing a 5 mL sample at the end of 48 h in order to analyze the concentration of total sugar (g/L) and ethanol (g/L). Repeated batches of immobilized cells were grown by discarding the supernatant and replacing it with fresh molasses to continue the fermentation in the following batch. The suspended cultures were propagated by centrifuging the cell cultures at 5000 rpm (PLC-012, Taiwan), resuspending in YPD molasses growth media under sterile conditions, and fermenting in the following batch. The repeated batch was operated for a total of five consecutive cycles. The yield (YP/S, grams of ethanol produced per grams of total sugar utilized) and percentage theoretical yield of ethanol (%) (percent of grams of product obtained per theoretical yield in grams) was based on ethanol production from the total sugar consumed. These parameters were compared between suspended and immobilized cultures.
Scale up experiments were performed in a 3 L stirred tank bioreactor (GBJX-5C, Zhenjiang, China) with a packed bed containing the immobilized cell bed. The temperature and pH were controlled at 30 °C and pH 4.8 respectively. Two liters of molasses with an initial concentration of 231 g/L of total sugar was supplemented with 1 g/L (NH4)2SO4 and 1 g/L KH2PO4, was then packed with 10% (w/v) delignified bagasse and inoculated with 10% (v/v) of the seed culture. The fermentation was conducted for a duration of 48 h; the first 24 h was operated with a controlled agitation speed of 80 rpm and then without agitation for the last 24 h in the first batch and under anaerobic conditions for an additional four batches with 48 h in each batch. Ten milliliter samples were collected at the end of 48 h for ethanol and sugar analysis. The fermentation was performed for a total of five consecutive batches by aspirating out the supernatant and refilling the fermenter with fresh molasses. Ethanol yields (YP/S) and percentage theoretical yield (%) were observed for each batch.
The ethanol produced was analyzed by gas chromatography (GC) (HP Innowax Agilent 6890N), using an Innowax column (29.8 m × 0.25 mm × 0.25 mm) with a flame ionization detector (FID). The column temperature was 150 °C, the program run time was 5.5 min, the ethanol retention time was about 1.9 min, the carrier gas was nitrogen (16 kPa), the injector temperature was 175 °C, the detector temperature was 250 °C, the flow rate was 40 mL/min, the split ratio was 1:50, and the velocity of H2 flow was 60 mL/min, with a sample quantity of 1 mL. One part of the supernatant was filtered through a 0.22 mm cellulose acetate filter prior to GC analysis. Standard ethanol solutions were prepared at 0.1%, 0.3%, 1%, 5% and 10% (v/v), using 95% absolute ethanol. The concentration of non-fermentable sugar was determined based on total sugar using Fehling’s titrimetric method [24].
Analysis of Delignified Sugarcane Bagasse for Potential Use as Animal Feed
The spent immobilized yeast adsorbed on sugarcane bagasse was collected after completion of all five cycles of repeated batches by discarding the supernatant. The material was then oven dried at 60 °C for 24 h. Chemical composition of immobilized yeast cells on the delignified sugarcane bagasse was analyzed by Betagro Science Center Co., Ltd using the standard methods and testing for the following components; ash (AOAC (2012) 942.05), energy (Compendium of Methods for Food Analysis) (2003, Food Composition and Nutrition Labeling Chapter, p 2–9), fiber (Inhouse Method: TI-C00-040 based on AOAC (2012) 978.10), fat (Inhouse Method: TI-C00-015 based on AOAC (2012) 920.39), moisture (ISO 6496 : 1999) and protein (Inhouse Method: TI-C00-016 based on ISO 5983-2: 2005). The chemical composition of spent immobilized yeast on delignified sugarcane bagasse was compared with the delignified sugarcane bagasse as a control.
Statistical Analysis
All experiments were performed in triplicate, and the results were statistically analyzed by ANOVA with Duncan’s multiple range test. The null hypothesis was accepted or rejected with 95% confidence interval (p < 0.05).
Results and Discussion
Sugarcane bagasse was chosen to be a carrier due to its surface characteristics, high water retention, high water content, high water absorption index, and low lignin content [25]. These properties could facilitate adhesion and growth of microorganisms which require a water content of 30–80% for solid-state fermentation [25, 26]. Sugarcane bagasse has an initial water content of 7.77% (w/w); however, after being submerged in water, its content can reach 84.27% (w/w) [25, 26]. Sugarcane bagasse exhibited a higher water absorption (8.58 g/g) index than other materials such as corn cobs (3.77 g/g), sugar beet pulp (6.59 g/g), loofa sponge (7.76 g/g), and coffee husks (8.30 g/g) [27]. All of these materials, including sugarcane bagasse, have in common a large amount of lignocellulosic in their structure, and this is the main composition of agricultural waste. Lignocellulosic materials are composed of cellulose, hemicellulose, and lignin with lignin covering the structure of cellulose and hemicellulose to create a smooth surface that impedes the adhesion of cells to the material [28]. Removing lignin is a promising technique to increase the microbial adhesion or immobilization to the agricultural waste.
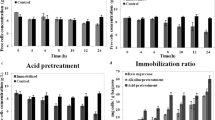
To explore the capacity of immobilization efficiency, S. cerevisiae SC90 was cultured in rich medium (YPD) and molasses at different sugar concentrations in the presence of delignified sugarcane bagasse. After 3 days, the dry weight of immobilized cells adsorbed on sugarcane bagasse per dried weight of sugarcane bagasse (g/g) (Fig. 1) was recorded and compared. According to the dry weight basis, molasses was an optimal substrate for cell growth in the presence of delignified sugarcane bagasse. Immobilized yeast grown in the presence of molasses at a concentration of 231 g/L grew better (0.31 ± 0.06 g/g) when compared to other concentrations of molasses, as well as to YPD alone. Additionally, immobilization was optimized in a short period of time (within 1 day). The efficiency of yeast immobilization was related to the concentration of total sugars in molasses; however, excess amounts of reducing sugars could inhibit the growth of microorganisms and product formation [29]. According to our study, molasses was the optimal culture medium for cell immobilization; there was a shorter lag phase for medium adaptation when immobilization and fermentation media were the same. Therefore, diluted molasses containing 231 g/L of total sugar was used as both the immobilization medium and fermentation medium in subsequent experiments.
Immobilization efficiency of S. cerevisiae SC90 is expressed as the ratio of dried weight of immobilized yeast cells on delignified sugarcane bagasse per dried weight of sugarcane bagasse (g/g) grown on Yeast peptone dextrose medium (YPD) and molasses containing 40–231 g/L of total sugar as substrates. The data were analyzed over 3 days of cultivation. Error bars indicate the standard deviation (n = 3)
The adhesion of yeast on delignified agricultural wastes was reported to depend on electrostatic interactions between the support and the negatively charged cell surface through physical adsorption [19]. The removal of lignin makes cellulose accessible to the cells, thus exposing a large number of hydrophilic groups with a positive charge that can absorb negatively charged cells [30]. The delignified process of lignocellulosic material using potassium hydroxide was proven to increase the bacterial adhesion [23]. In our work, the lignin content of the delignified bagasse was reduced approximately 1.7 times of the non delignified bagasse after the pretreatment. We therefore used scanning electron microscopy (SEM) to compare the surface structure of non delignified sugarcane bagasse with delignified sugarcane bagasse (Fig. 2). As our result, the removal of lignin through chemical processes tends to deform the rigid structure of bagasse and enhances opportunities for bacterial cell immobilization on the structure of cellulose and hemicelluloses by increasing the porosity and the roughness of the supporting material (Fig. 2a, b). This is in agreement with reports which showed yeast cells favor cell adhesion to lignocellulosic supports treated by delignification [31]. We also compared yeast immobilization on delignified bagasse on day 1 when cultured in YPD and molasses (Fig. 2 c and d). Yeast cells homogeneously adhered to the surface of delignified bagasse in a similar pattern when cultured in YPD and molasses. Other studies have reported that a low level of continuous shaking during immobilization could also lead to better immobilization performance [32]. We conclude that the optimum immobilization condition was on day 1 at 30 °C, with shaking at 80 rpm, in rich media containing molasses with 231 g/L of total sugar.
Scanning electron microscopy (SEM) shows a the surface structure of non delignified sugarcane bagasse, b the surface structure of delignified sugarcane bagasse, c yeast immobilization on the delignified sugarcane bagasse when cultured in Yeast Peptone Dextrose (YPD) and d yeast immobilization on delignified sugarcane bagasse when cultured in molasses containing 231 g/L total sugar. The pictures were taken with ×2000 magnification on day 1
The fermentation efficiency of yeast cells immobilized on delignified sugarcane bagasse (IM) versus those suspended in culture [free cell (FC)] were compared. A five-cycle repeated batch process using shaking flasks with molasses containing 231 g/L of total sugar and supplemented with (NH4)2SO4 and KH2PO4 as a substrate were used. Ethanol yield (YP/S) and percent theoretical yield were calculated at the end of the 48-h fermentation period (Table 1). The ethanol yield (YP/S) for suspended cultures remained static for the first three batches and then rose for the fourth and fifth batch, suggesting a significant period was needed for adaptation to the fermentation process. In contrast, immobilized cells exhibited a significantly higher ethanol yield from the first batch, suggesting the cells are already primed for fermentation when immobilized as biocatalyst. The ethanol yield from immobilized yeast reached a maximal level in the fourth batch [0.43 ± 0.05 g/g (84.31% theoretical yield)]. Additionally, the ethanol yield of immobilized yeast from each of the batches was maintained at a level that was significantly higher than the suspended cultures. The higher fermentation rate of immobilized yeast compared to suspended cells could be a result of higher cell density and viability of cells on the carrier. For example, the cell density of immobilized yeast could be maintained in the system during media changes while some cell mass could be lost during media replacement in the suspended cell system [25]. The carrier may also act as a protective barrier against extreme environmental conditions caused by pH, temperature, accumulated toxic chemicals in the medium, and high concentrations of substrate, whereas the suspended cells might utilize some sugar for survival instead of producing ethanol [33]. Additionally, the proteins and minerals released from the carrier into the media may favor yeast growth and enhance ethanol productivity [28]. The immobilized yeast produced 1.1 times higher in ethanol production than the free cells and ethanol yields were maintained in all five consecutive batches. The maximum bioethanol yield obtained from immobilized yeast on sugarcane bagasse was three times higher than that of free cells, while the concentration of total cells in immobilized cells system was only about 1.3 times higher than the concentration of cells in a suspended system [25]. Other groups have seen similar results. For example, using silk cocoons as a carrier and blackstrap molasses as a substrate resulted in higher ethanol concentrations in five-cycle batches for immobilized cells (91% theoretical yield) over suspended culture in all batches [14]. It has also been found that yeast immobilized on corn stems increased the ethanol yield and decreased the amount of residual sugar in ethanol fermentation compared to a suspended cell system [34].
We also carried out five-repeated cycles of fermentation in a 3 L stirred tank bioreactor (GBJX-5C, Zhenjiang) using molasses with 231 g/L of total sugar concentration as a substrate. The result from Table 2 shows fermentation efficiency of immobilized S. cerevisiae SC90, which could be maintained for at least three consecutive batches with ethanol yields (YP/S) of 0.41–0.42 g/g and 80–82% theoretical yield. The ethanol yield significantly dropped in the fourth batch to 0.34 g/g (66.67% theoretical yield), perhaps as a result of cell detachment from the support. However, the ethanol yield rose again in the fifth batch to 0.38 g/g (74.51% theoretical yield), suggesting that resumption of cell growth and colonization of the delignified sugarcane bagasse occurred. The ethanol yield (YP/S) from our fermenter experiments was higher than that obtained from the shaking flask conditions, suggesting there are benefits for scale-up production in the well-controlled fermenter system. The stirred tank bioreactor gave an advantage in that good homogenization between substrate and carrier occurred. Larger scale fermentation and high compaction of delignified sugarcane bagasse in molasses might require high speed agitation to obtain a well-mixed condition, which could result in cell damage due to high shear force of the impeller. However, there is precedence for this method, as a stirred tank bioreactor was successfully modified into a packed bed bioreactor for glycerol production by immobilized S. cerevisiae using sintered glass Raschig rings as a carrier packed in a stainless steel basket [35]. This could prevent direct contact between immobilized cells and the impeller. In addition, sieve plate baffles in bioreactors designed by Ramakrishna et al. reduce the compaction problem of the carrier and increase the cross-sectional area of immobilized cells, allowing more area contact for cells with the substrate [36].
In this study, we also analyzed the potential of using spent immobilized yeast cells on delignified sugarcane bagasse as an animal feed supplement. Ruminants can digest only 25–35% (w/w) of lignocellulosic feed because of its complex structure [20]. Therefore, various methods have been tested to increase digestibility of sugarcane bagasse [37]. By using NaOH and lime as an alkaline treatment, the amount of lignin in sugarcane bagasse can be decreased by 41.2%, resulting in an 8.9 fold increase in the accessibility of the cellulose component in delignified sugarcane. This enhanced the digestibility of sugarcane bagasse for ruminants, as ruminal enzymes could easily access the polysaccharide portions of the bagasse [20]. The chemical composition of delignified sugarcane bagasse covered by immobilized yeast and delignified sugarcane bagasse alone was compared (Table 3). The results indicate spent immobilized yeast on delignified sugarcane bagasse has a significantly higher protein content than delignified sugarcane bagasse alone due to the presence of immobilized yeast cells. Therefore, the crude protein in the spent immobilized yeast cells, along with the delignified sugarcane bagasse, can be further used as protein enriched (SCP production) animal feed. Molasses contains minerals and trace elements, including B complex vitamins, which have been shown to increase milk production in cows and improve wool quality in sheep. Residual molasses that remains on the spent delignified fiber could be utilized by ruminal microorganisms to enhance the digestive activity [38]. Further investigation will be necessary to analyze digestibility and toxicity in the animals.
Conclusions
The results of our study suggest that sugarcane bagasse may be a valuable commodity for ethanol production. Batch and repeated batch fermentations of immobilized S. cerevisiae SC90 on delignified sugarcane bagasse showed high ethanol production with molasses as the sugar source. The immobilized biocatalysts had high fermentation ability and high stability during five repeated batches. Immobilization of cells on the bagasse eliminated the need for cell separation to inoculate the following batches, thus reducing labor and costs for continuous batch fermentations. Additionally, we evaluated the nutritional content of the spent yeast/delignified sugarcane bagasse and found that it had a relatively high protein content and a composition that could serve as animal feed. The immobilization and fermentation technologies described in this study could be applied to other fibrous agricultural bio-waste and other industrial fermentations to reduce the cost of production and maximize productivity of ethanol.
References
Bloyd, C.N.: An Update on Ethanol Production and Utilization in Thailand. Pacific Northwest National Laboratory (PNNL), Richland (2009)
Hamamci, H., Ryu, D.D.Y.: Performance of tapered column packed-bed bioreactor for ethanol production. Biotechnol. Bioeng. 29(8), 994–1002 (1987)
Luong, J.H.T.: Cell immobilization in K-carrageenan for ethanol production. Biotechnol. Bioeng. 27(12), 1652–1661 (1985)
Shindo, S., Takata, S., Taguchi, H., Yoshimura, N.: Development of novel carrier using natural zeolite and continuous ethanol fermentation with immobilized Saccharomyces cerevisiae in a bioreactor. Biotechnol. Lett. 23, 24 (2001)
Gross, R., Hauer, B., Otto, K., Schmid, A.: Microbial biofilms: new catalysts for maximizing productivity of long term biotransformations. Biotechnol. Bioeng. 98(6), 1123–1134 (2007)
Rosche, B., Li, X.Z., Hauer, B., Schmid, A., Buehler, K.: Microbial biofilms: a concept for industrial catalysis? Trends Biotechnol. 27(11), 636–643 (2009)
Winn, M., Foulkes, J.M., Perni, S., Simmons, M.J.H., Overton, T.W., Goss, R.J.M.: Biofilms and their engineered counterparts: a new generation of immobilised biocatalysts. Catal. Sci. Technol. 2(8), 1544–1547 (2012)
Li, X.Z., Webb, J.S., Kjelleberg, S., Rosche, B.: Enhanced benzaldehyde tolerance in Zymomonas mobilis biofilms and the potential of biofilm applications in fine-chemical production. Appl. Environ. Microbiol. 72(2), 1639–1644 (2006)
Todhanakasem, T., Sangsutthiseree, A., Areerat, K., Young, G.M., Thanonkeo, P.: Biofilm production by Zymomonas mobilis enhances ethanol production and tolerance to toxic inhibitors from rice bran hydrolysate. New Biotechnol. 31(5), 451–459 (2014)
Yu, J., Yue, G., Zhong, J., Zhang, X., Tan, T.: Immobilization of Saccharomyces cerevisiae to modified bagasse for ethanol production. Renew. Energy 35(6), 1130–1134 (2010)
Yu, J., Zhang, X., Tan, T.: A novel immobilization method of Saccharomyces cerevisiae to sorghum bagasse for ethanol production. J. Biotechnol. 129(3), 415–420 (2007)
Guénette, M., Duvnjak, Z.: Wood blocks as a carrier for Saccharomyces cerevisiae used in the production of ethanol and fructose. Chem. Eng. J. Biochem. Eng. J. 61(3), 233–240 (1996)
Ogbonna, J.C., Mashima, H., Tanaka, H.: Scale up of fuel ethanol production from sugar beet juice using loofa sponge immobilized bioreactor. Bioresour. Technol. 76(1), 1–8 (2001)
Rattanapan, A., Limtong, S., Phisalaphong, M.: Ethanol production by repeated batch and continuous fermentations of blackstrap molasses using immobilized yeast cells on thin-shell silk cocoons. Appl. Energy 88(12), 4400–4404 (2011)
Kourkoutas, Y., Koutinas, A.A., Kanellaki, M., Banat, I.M., Marchant, R.: Continuous wine fermentation using a psychrophilic yeast immobilized on apple cuts at different temperatures. Food Microbiol. 19(2–3), 127–134 (2002)
Iconomou, L., Psarianos, C., Koutinas, A.: Ethanol fermentation promoted by delignified cellulosic material. J. Ferment. Bioeng. 79(3), 294–296 (1995)
Sakurai, A., Nishida, Y., Saito, H., Sakakibara, M.: Ethanol production by repeated batch culture using yeast cells immobilized within porous cellulose carriers. J. Biosci. Bioeng. 90(5), 526–529 (2000)
Liang, L., Zhang, Y., Zhang, L., Zhu, M., Liang, S., Huang, Y.: Study of sugarcane pieces as yeast supports for ethanol production from sugarcane juice and molasses. J. Ind. Microbiol. Biotechnol. 35(12), 1605–1613 (2008)
Ahmadi, F., Zamiri, M.J., Khorvash, M., Ziaee, E., Polikarpov, I.: Pre-treatment of sugarcane bagasse with a combination of sodium hydroxide and lime for improving the ruminal degradability: optimization of process parameters using response surface methodology. J. Appl. Anim. Res. 44(1), 287–296 (2016)
Bruno, R.G.S., Rutigliano, H.M., Cerri, R.L., Robinson, P.H., Santos, J.E.P.: Effect of feeding Saccharomyces cerevisiae on performance of dairy cows during summer heat stress. Anim. Feed Sci. Technol. 150(3–4), 175–186 (2009)
Fontana, J.D., Ramos, L.P., Deschamps, F.C.: Pretreated sugar cane bagasse as a model for cattle feeding. Appl. Biochem. Biotechnol. 51(1), 105–116 (1995)
Todhanakasem, T., Tiwari, R., Thanonkeo, P.: Development of corn silk as a biocarrier for Zymomonas mobilis biofilms in ethanol production from rice straw. J. Gen. Appl. Microbiol. 62(2), 68–74 (2016)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3), 426–428 (1959)
Sugarcane Bagasse.: Feedipedia, a programme by INRA, CIRAD, AFZ and FAO (2015). https://www.feedipedia.org/node/559
Anita, S.H., Mangunwardoyo, W.: Sugarcane Bagasse as a carrier for the immobilization of Saccharomyces cerevisiae in bioethanol production. Makara J. Technol. 20(2), 73–81 (2016)
Razmovski, R., Vučurović, V.: Bioethanol production from sugar beet molasses and thick juice using Saccharomyces cerevisiae immobilized on maize stem ground tissue. Fuel 92(1), 1–8 (2012)
Mussatto, S.I., Aguilar, C.N., Rodrigues, L.R., Teixeira, J.A.: Fructooligosaccharides and β-fructofuranosidase production by Aspergillus japonicus immobilized on lignocellulosic materials. J. Mol. Catal. B 59(1–3), 76–81 (2009)
Genisheva, Z., Mussatto, S.I., Oliveira, J.M., Teixeira, J.A.: Evaluating the potential of wine-making residues and corn cobs as support materials for cell immobilization for ethanol production. Ind. Crops Prod. 34(1), 979–985 (2011)
Periyasamy, S., Venkatachalam, S., Ramasamy, S., Srinivasan, V.: Production of bio-ethanol from sugar molasses using Saccharomyces cerevisiae. Mod. Appl. Sci. 3(8), 32 (2009)
Peinado, R.A., Moreno, J.J., Villalba, J.M., Gonzlez-Reyes, J.A., Ortega, J.M., Mauricio, J.C.: Yeast biocapsules: a new immobilization method and their applications. Enzym. Microb. Technol. 40(1), 79–84 (2006)
Sahin, H.T., Arslan, M.B.: A study on physical and chemical properties of cellulose paper immersed in various solvent mixtures. Int. J. Mol. Sci. 9(1), 78–88 (2008)
Kourkoutas, Y., Bekatorou, A., Banat, I.M., Marchant, R., Koutinas, A.A.: Immobilization technologies and support materials suitable in alcohol beverages production: a review. Food Microbiol. 21(4), 377–397 (2004)
Vučurović, V.M., Razmovski, R.N., Popov, S.D.: (2009) Ethanol production using Saccharomyces cerevisiae cells immobilised on corn stem ground tissue. Zbornik Matice srpske za prirodne nauke 116:315–322
Nuanpeng, S., Laopaiboon, L., Srinophakun, P., Klanrit, P., Jaisil, P., Laopaiboon, P.: Ethanol production from sweet sorghum juice under very high gravity conditions: batch, repeated-batch and scale up fermentation. Electron. J. Biotechnol. 14(1), 4–5 (2011)
Bisping, B., Rehm, H.J.: Glycerol production by cells of Saccharomyces cerevisiae immobilized in sintered glass. Appl. Microbiol. Biotechnol. 23(3–4), 174–179 (1986)
Ramakrishna, S.V., Sreedharan, V.P., Prema, P.: Continuous ethanol production with immobilized yeast cells in a packed bed reactor. In: Bioreactor Immobilized Enzymes and Cells: Fundamentals and Applications-I. Elsevier, Amsterdam (1988)
Karp, S.G., Woiciechowski, A.L., Soccol, V.T., Soccol, C.R.: Pretreatment strategies for delignification of sugarcane bagasse: a review. Braz. Arch. Biol. Technol. 56(4), 679–689 (2013)
Cleasby, T.G.: The feeding value of molasses. South Afr. Sugar J. 1, 47–360 (1963)
Acknowledgements
Briana M. Young is gratefully acknowledged for her proofreading of the manuscript. This research was financially supported by The Thailand Research Fund and KSL Green Innovation Public Company Limited (TRF 60I0004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sowatad, A., Todhanakasem, T. Bioethanol Production by Repeated Batch Using Immobilized Yeast Cells on Sugarcane Bagasse. Waste Biomass Valor 11, 2009–2016 (2020). https://doi.org/10.1007/s12649-018-0534-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0534-0