Abstract
The aim of this work was to optimize the enzymatic hydrolysis of the cellulose fraction of cashew apple bagasse (CAB) after diluted acid (CAB-H) and alkali pretreatment (CAB-OH), and to evaluate its fermentation to ethanol using Saccharomyces cerevisiae. Glucose conversion of 82 ± 2 mg/g CAB-H and 730 ± 20 mg/g CAB-OH was obtained when 2% (w/v) of solid and 30 FPU/g bagasse was used during hydrolysis at 45 °C, 2-fold higher than when using 15 FPU/g bagasse, 44 ± 2 mg/g CAB-H, and 450 ± 50 mg/g CAB-OH, respectively. Ethanol concentration and productivity, achieved after 6 h of fermentation, were 20.0 ± 0.2 g L−1 and 3.33 g L−1 h−1, respectively, when using CAB-OH hydrolyzate (initial glucose concentration of 52.4 g L−1). For CAB-H hydrolyzate (initial glucose concentration of 17.4 g L−1), ethanol concentration and productivity were 8.2 ± 0.1 g L−1 and 2.7 g L−1 h−1 in 3 h, respectively. Hydrolyzates fermentation resulted in an ethanol yield of 0.38 and 0.47 g/g glucose with pretreated CAB-OH and CAB-H, respectively. Ethanol concentration and productivity, obtained using CAB-OH hydrolyzate, were close to the values obtained in the conventional ethanol fermentation of cashew apple juice or sugar cane juice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There has been an increase in the worldwide interest in alternative, non-petroleum-based sources of energy as a means to enhance security of the oil supply and due to the negative impact of fossil fuels on the environment. The most common renewable fuel today is ethanol, produced by fermentation of sucrose in Brazil or corn glucose in the USA; however, these raw material bases will not be sufficient to satisfy the international demand. Consequently, future large-scale use of ethanol will most certainly have to be based on production from lignocellulosic materials [1].
In the state of Ceará (northeast of Brazil), the cashew agroindustry has an outstanding role in the local economy, and the cashew apple bagasse (CAB), a lignocellulosic raw material, appears as an alternative for ethanol production. CAB, by-product of the cashew apple juice industry, represents approximately 20% of the total peduncle weight. However, its exploitation is restricted to its use as nutritional complement for animal ration [2]. The official estimate for the Brazilian cashew nut crop for 2008/2009 is around 300,000 tons [3], which accounts for 11% of the world production and corresponds to more than 6 million tons of cashew apple.
Lignocellulosic materials typically contain 55–57% of dry weight (DW) of carbohydrates, which are polymers containing sugar units of five and six carbon atoms [4]. Cashew apple bagasse contains in percent DW, 24.3% cellulose, 12.5% hemicelluloses, 22.5% lignin, 14.2% crude proteins, and 11.3% nonfiber carbohydrates [5]. Cellulose is a biopolymer of β-1,4-linked glucose dimers, and this abundant biopolymer is made up of crystalline and amorphous regions. The amorphous component is attacked/digested more easily by enzymes than crystalline components [6].
The lignocellulosic biomass must be pretreated to make the cellulose fraction more accessible to enzymatic attack. Diverse pretreatment processes have been evaluated technically and economically, aiming at improving enzymatic hydrolysis; these include acid or alkali treatment, steam-explosion, and organic solvents [7–9]. Pretreatment is also necessary to use cashew apple bagasse in bioconversion for the production of ethanol, which represents a promising alternative fuel to reduce environmental problems.
In the bioconversion of solid residues to a readily fermentable stream of glucose monomers, an enzymatic complex should be used [4]. The hydrolysis of cellulose by cellulolytic enzymes has been investigated intensively since the early 1970s, with the objective of developing a process for the production of ethanol [10].
Alternatives for compensating ethanol production costs should be analyzed, for instance by the generation of valuable co-products or the possible use of the obtained hydrolysate after the dilute acid hydrolysis [11, 12] and also the lignin residue after the alkaline hydrolysis [13, 14]. Some authors studied the use of lignin as raw material for production of adsorptive materials by chemical modification [13]. Other authors studied the thermal conversion of non-fermentable lignin, since it can provide the energy required by the entire process, remaining a surplus that can be commercialized in form of electricity. According to them, this is possible due to the high energy value of the lignin (29.54 MJ/kg) that is released during its combustion [14]. Furthermore, arabinose and xylose solutions obtained during pretreatment of lignocellulosic biomass can be used by bacteria as a precursor of synthetic polymers and resins, such as 2,3-butanediol [11]. Xylose solutions can also be converted into xylitol by chemical or biotechnological means, using co-culture of Saccharomyces cerevisiae and Candida tropicalis [12].
The aim of this work was to study the different factors that play an important role in the enzymatic hydrolysis of the cellulose fraction of CAB after dilute acid and combination of dilute acid and alkali pretreatment. The influence of the pretreatments in ethanol production by S. cerevisiae will also be discussed.
Materials and Methods
Raw Material
Cashew apple (Anacardium occidentalis L.) bagasse was kindly donated by Jandaia Industry of Juice (Ceará, Brazil). It was washed five times with water, dried at 60 °C for 24 h, and milled. The milled CAB was stored at room temperature.
Pretreatment of Cashew Apple Bagasse
CAB, solid concentration of 30% (w/v), was slurried in dilute H2SO4 (0.8 mol L−1) and pretreated in autoclave at 121 °C for 15 min; the autoclave vented within 10 min following the end of the cycle. Afterwards, the liquid was collected by filtration, the solid residue (CAB-H) was washed with 100 mM citrate buffer until pH 4.5, and it was dried at 50 °C for 24 h. CAB-H was pretreated to increase cellulose accessibility by partially removing lignin. This pretreatment was conduced as follows: CAB-H was mixed with a 4% NaOH (w/v) solution, which was submitted to thermal treatment at 121 °C for 30 min [4]. The resulting pretreated solid was washed with water until pH 6.0 and dried at 50 °C for 24 h; this solid was named CAB-OH.
Enzymatic Hydrolysis
The saccharification of pretreated CAB-H and CAB-OH with a commercial enzyme extract, Celluclast 1.5 L (Novozyme, Bagsvaerd, Denmark), was performed in triplicate under mild agitation (150 rpm) in 250 mL flasks. The influence of solid content (2 and 16% w/v), temperature (30, 37, and 45 °C), and enzyme loading (15 and 30 FPU/g bagasse) was investigated. Enzyme was added to the flasks in diluted form using 100 mM citrate buffer and pH 5.0, which was selected based on several works presented in literature [4, 7–9, 15] that relate simultaneous saccharification, and fermentation can be conducted at a slightly acid pH. Furthermore, this pH is within the stability range of commercial cellulases (i.e., Celluclast 1.5 L).
Enzyme Activity
Filter paper activity was determined as recommend by Ghose [16], and it was expressed as filter paper units (FPU) per milliliter of mixture. The activity of enzyme used, Celluclast 1.5 L, was 110 FPU/mL of mixture.
Glucose Conversion
In this work, glucose conversion was defined as the amount of glucose released, expressed in milligram per gram of pretreated CAB.
Microorganism and Inoculum Preparation
A pure culture of S. cerevisiae was isolated from baker’s yeast Saf-momento (SAF Argentina, Buenos Aires) at the Bioengineering Laboratory at Federal University of Ceará, Brazil. The culture was inoculated on agar Sabouraud Biolife (peptone 5.0 g L−1, glucose 15.0 g L−1, and agar 15.0 g L−1) and incubated at 30 °C for 48 h. Inoculum was obtained in 500 mL erlenmeyer flasks with a medium volume of 200 mL, consisting of (g L−1): glucose 30; yeast extract 5; (NH4)2SO4 10; KH2PO4 4.5; MgSO4·7H2O 1; ZnSO4 0.65. The medium was sterilized at 110 °C for 10 min. The pH and temperature were maintained at 5.0 and 30 °C, respectively, during 24 h. After that, cells were centrifuged at 10,000×g for 10 min to obtain the initial biomass used in fermentation assays.
Fermentation Assays
Fermentation was conducted using the liquid fraction (CAB-H or CAB-OH hydrolyzate), without any nutritional supplements, obtained after enzymatic saccharification at 45 °C using an enzyme load of 30 FPU/g bagasse and solid percentage of 16% (w/v). An inoculum of 10 g L−1 of S. cerevisiae was used for ethanol production. Batch fermentation experiments were carried in 250 mL flask at 30 °C, 150 rpm, and pH 5.0 using 100 mL of medium.
Analytical Methods
Biomass
Cell concentration was determined by dry weight [17]. Samples were taken from the fermentation media at certain time intervals and centrifuged at 6,000 rpm for 15 min in a BE-6000 centrifuge (BIO ENG, Piracicaba, SP, Brazil). The pellet was dried at 60 °C on a Tecnal TE-397/4 stove (Tecnal, Piracicaba, SP, Brazil) until constant weight.
Glucose and Ethanol Concentrations
Glucose and ethanol were analyzed by high-performance liquid chromatography (HPLC) using a Waters HPLC system (Waters, Milford, MA, USA) equipped with a refractive index Waters 2414 detector using an Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA). The eluent was 5 mmoL L−1 H2SO4 in Water MiliQ (simplicity 185, Millipore, Billerica, MA, USA) at a flow rate of 0.5 mL min−1 and 65 °C. Samples were identified by comparing the retention times with those of carbohydrate and ethanol standards.
Statistical Analysis
Significant effect of temperature, enzyme loading, and solid concentration on glucose conversion was determined by statistical analysis method, the analysis of variance (ANOVA), calculated using Microcal Origin 6.0 (Microcal Software Inc., Northampton, MA, USA).
Results and Discussion
Enzymatic Saccharification
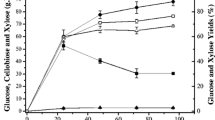
The time course of enzymatic hydrolysis of CAB-H and CAB-OH, using 2% w/v of pretreated solid, is presented in Fig. 1. Soluble components formed during pretreatment can influence cellulose conversion negatively [18]. To minimize the effects of these components when evaluating different substrate concentrations, the pretreated material used was washed with water. It can be observed that glucose was released within 24 h for all investigated conditions. Furthermore, larger amounts of glucose are released when CAB-OH was used instead of CAB-H. The enzymatic saccharification (45 °C, pH 5.0, 72 h) of CAB, pretreated only with dilute acid without NaOH treatment, generated 47 ± 5 mg of glucose per gram of CAB-H. However, saccharification of CAB-OH generated 466 ± 81 mg of glucose per gram of CAB-OH, in the same conditions, 10-fold more than CAB-H. This result can be explained by the high content of lignin in lignocellulosic materials, such as CAB-H [5]. The hydrolysis of sugars from these materials would probably require more enzyme or more extreme pretreatment conditions to achieve higher conversions. Materials with lower lignin contents, such as CAB-OH, would be expected to show higher conversions with shorter hydrolysis times (or digestion), as observed in Fig. 1 [19].
Some authors [4, 18] obtained positive effects on glucose conversion by enhancing temperature and enzyme loading. Therefore, in this work, the effects of temperature (30, 37, and 45 °C) on enzymatic saccharification (15 FPU, pH 5.0, and 2% w/v solid percentage) were evaluated using the two pretreated (CAB-H and CAB-OH) materials (Fig. 1). Glucose conversion increase with temperature, and the highest values were obtained when the enzymatic reaction was carried on at 45 °C (466 ± 81 mg glucose per gram CAB-OH). The analysis of variance showed that temperature promoted a positive effect on glucose conversion. At the 0.05 level, the means are significantly different (for every reaction time investigated, see Table 1. Conversions were higher at 45 °C (more than 2-fold) than at 37 °C, for all conditions evaluated. Other authors [4, 7, 20] studied enzymatic hydrolysis of different lignocellulosic materials using Celluclast 1.5 L and obtained similar results; best reactional conditions were 45 °C, pH 5.0, and 72 h. The optimal temperature of the enzymatic hydrolysis step has often been found to be around 45 °C [4, 7, 18], which is in accordance with the results obtained at short reaction times in the present study.
Glucose conversion profiles for 30 and 37 °C were very similar, although half of the value was obtained at 45 °C. This result may be interesting because the enzymatic hydrolysis process can be designed in various ways. The steps following pretreatment, i.e., hydrolysis and fermentation, can be run separately (SHF) or simultaneously (SSF). The advantage of SHF is the ability to carry out each step under optimal conditions, e.g., enzymatic hydrolysis at 40–50 °C and fermentation at about 30 °C. In SSF, the fermenting microorganism immediately consumes the glucose produced, which is an advantage avoiding enzyme inhibition. Ethanol, the product of fermentation, can also act as an inhibitor in hydrolysis but not as strongly as cellobiose or glucose. The temperature used in SSF, of about 30–37 °C, is a compromise solution, but the development of thermotolerant organisms is expected to improve the performance of SSF [10, 20].
The effect of enzyme load and solid percentage on the saccharification of pretreated cashew apple bagasse (CAB-H and CAB-OH) is shown in Fig. 2. As expected, glucose concentration increased with increasing enzyme loads. At 45 °C, 19.55 ± 0.08 g L−1 (CAB-H, 16% w/v) and 52.42 ± 0.61 g L−1 (CAB-OH, 16% w/v) of glucose was obtained when 30 FPU/g bagasse was used, an increase of 35.1% (CAB-H) and 17.3% (CAB-OH) on sugar concentration, compared with the results obtained by using 15 FPU/g bagasse. Furthermore, higher glucose concentration was achieved when 16% (w/v) of CAB-H or CAB-OH was used, rather than 2% (w/v). Other authors [4] also observed that increasing solid percentage had a positive effect on glucose production when using sugarcane bagasse.
Figure 3 shows the effect of solid percentage and enzyme load on glucose conversion, the amount of glucose produced by weigh of bagasse. Conversions of 82 ± 2 mg per gram CAB-H and 730 ± 20 mg per gram CAB-OH were achieved by using 2% (w/v) solid percentage and 30 FPU/g bagasse during hydrolysis at 45 °C. This represents 4.4% and 73%, respectively, of total pretreated cashew apple bagasse. When 15 FPU/g was used instead, the conversion was 44 ± 2 mg per gram CAB-H and 450 ± 50 mg per gram CAB-OH, approximately two times smaller.
By comparing Figs. 2 and 3, it can be observed that, although glucose concentration was higher when using 16% of pretreated CAB-OH, glucose conversion was higher when using 2% of pretreated CAB-OH. A relatively large difference in the cellulose conversion was observed between 2% and 16% (w/v) in the enzymatic hydrolysis step (Fig. 3b). The same result was observed by other authors [10] who showed that an increase in substrate concentration of pretreated softwood from 7.5% to 18% decreased the cellulose conversion, for a cellulase loading up to 36 FPU g−1 cellulose [10]. On the other hand, concentration and conversion of glucose were higher with 16% (w/v) solid percentage using CAB-H. However, concentration and conversion of glucose increased by 92% and 32%, respectively, compared with the assays using 2% (w/v). This negative influence of solid percentage on glucose conversation can be explained by enzyme inhibition, more precisely product inhibition (glucose and cellobiose), caused by the increase on glucose concentration during hydrolysis [21].
Cellulose chain is attacked by cellulases with the formation of cellobiose. Cellobiose cleavage to glucose is catalyzed by β-glucosidase, an enzyme present in the Celluclast [7] which also reduces inhibition of cellulases by cellobiose. Thus, the presence of a sufficient amount of β-glucosidase is important to obtaining high yields of glucose in the biomass-to-ethanol process. To increase the degree of cellulose conversion, higher concentrations of cellulases were required (Figs. 2 and 3), in accordance with the results of other authors obtained with different types of lignocellulosic materials, such as dilute acid pretreated wheat straw [10] and spruce, that has been shown to inhibit enzymatic hydrolysis [10]. In contrast, high degree of hydrolysis (73%) of cashew apple bagasse treated with NaOH (CAB-OH) has been achieved at low substrate (2% w/v) and high cellulase concentration (30 FPU g−1 bagasse). According to Tengborg et al. [10], such activity is still very high, regarding enzyme production costs nowadays. Nevertheless, these data are necessary to economically optimize some process operational conditions which will influence the capital and operating costs, such as residence time, enzyme concentration, and yield.
Based on the obtained results, CAB pretreatment with diluted acid followed by treatment with NaOH had the best performance on enzymatic hydrolysis. According to other authors [4], this result could be explained by the increase of accessibility of enzymes to cellulose of CAB as a result of the partial removal of lignin promoted by NaOH. This pretreatment improved glucose concentration by 10-fold when compared to the analog process using CAB-H.
Fermentation of Cashew Apple Bagasse Hydrolyzates
The fermentability of the hydrolyzates, obtained after enzymatic saccharification of the two pretreated bagasses (CAB-H and CAB-OH) at 45 °C, using 30 FPU/g bagasse for 72 h, was evaluated using S. cerevisiae. Fermentation was conducted by using 10 g L−1 of inoculum, and the kinetics profiles are presented in Fig. 4a,b. S. cerevisiae was able to grow and to produce ethanol when cultivated in CAB-OH and CAB-H hydrolyzates without any nutritional supplements, with the consumption of all glucose available but no xylose consumption. Although traditionally S. cerevisiae ferments glucose to ethanol rapidly and efficiently, it cannot ferment other sugars such as xylose and arabinose to ethanol [11]. Usually, medium components added to the hydrolyzate influence kinetics and economy of the process [22], but in this study, the nutrients present in the hydrolyzates were sufficient to allow growth and ethanol production. Nevertheless, the addition of other sources of nutrients may increase ethanol yield.
When using CAB-OH hydrolyzate (initial glucose concentration of 52.4 g L−1), ethanol concentration and productivity was 20.0 ± 0.2 g L−1 and 3.33 g L−1 h−1, respectively, in 6 h (Fig. 4a). An ethanol concentration and productivity of 8.2 ± 0.1 g L−1 and 2.7 g L−1 h−1, respectively, was achieved in 3 h using CAB-H hydrolyzate (initial glucose concentration of 17.4 g L−1). After this time, no glucose was left in the medium (Fig. 4b). The volumetric productivities obtained here are not so far from the values obtained in the conventional ethanol fermentation of sucrose, 5.0–8.0 g L−1 h−1 [4] or cashew apple juice, 3.0–9.7 g L−1 h−1 [23]. Ethanol yields of 0.38 g/g glucose and 0.47 g/g glucose, using pretreated CAB-OH and CAB-H hydrolyszates, respectively, were obtained. Furthermore, an ethanol yield, based on the amount of pretreated bagasse, of 0.12 g/g CAB-OH and 0.6 g/g CAB-H was obtained. Saha et al. [7] used rice hull hydrolyzates for ethanol production by Recombinant Escherichia coli Strain FBR5 and obtained an ethanol yield of 0.43 g/g available sugars (glucose, xylose, arabinose, and galactose) and 0.13 g/g rice hull, which is close to the results obtained in this work for CAB-OH. Other authors [4] studied ethanol production from the celluligninG hydrolyzate (sugarcane bagasse) by S. cerevisiae and obtained a final ethanol concentration of 30.0 g L−1 and productivity of 3.0 g L−1 h−1 in 10 h of fermentation.
Ethanol yield is an important process parameter with regard to economy both because the cost of the raw material, which constitutes a major part of the total production cost, and also because the processing costs are typically associated with the amount of material passing through the process and not the amount of product made [22]. When peduncle is industrially processed for the production of juice, 40% (w/w) of bagasse is produced, which is not used for human consumption and is usually discarded by the local industry. These facts, together with its composition, turns cashew apple bagasse into an interesting and inexpensive ($ 0.50/kg) substrate for several potential applications [5, 24]. These facts together with the results obtained during the fermentation of CAB-OH hydrolyzate, after dilute acid pretreatment, indicates that cashew apple bagasse stands as an alternative raw material for the production of ethanol from lignocellulosic residues.
Conclusion
The conditions of hydrolysis that yielded the highest glucose concentration, 52 g L−1, were: 45 °C, enzyme load of 30 FPU/g bagasse, and solid percentage of 16% (w/v), using cashew apple juice after dilute acid pretreatment followed by lignin removal by NaOH. It is possible to conclude that the hydrolyzate produced in the enzymatic hydrolysis of CAB is easily fermented by S. cerevisiae yeast for the production of ethanol, resulting in a concentration of 20 g L−1 in 6 h of fermentation. Ethanol yield of 0.38 and 0.47 g/g glucose from pretreated CAB-OH and CAB-H hydrolyzates, respectively, were achieved. Moreover, an ethanol yield, based on the amount of pretreated bagasse, of 0.12 g/g CAB-OH and 0.6 g/g CAB-H was obtained. Therefore, the fermentation CAB-OH hydrolyzate stands as an alternative process for fuel ethanol production from lignocellulosic residues.
References
Hahn-Hägerdal, B., Galbe, M., Gorwa-Grauslund, M. F., Lidén, G., & Zacchi, G. (2006). Trends in Biotechnology, 24(12), 549–556. doi:10.1016/j.tibtech.2006.10.004.
Santos, R. P., Santiago, A. A. X., Gadelha, C. A. A., Cajazeiras, J. B., Cavada, B. S., Martins, J. L., et al. (2007). Journal of Food Engineering, 79, 1432–1437. doi:10.1016/j.jfoodeng.2006.04.040.
Instituto Brasileiro de Pesquisa e Estatística in www.ibge.gov.br accessed in October, 21st, 2008.
Vásquez, M. P., Silva, J. N. C., Souza, M. B. Jr., & Pereira, N. Jr. (2007). Applied Biochemistry and Biotechnology, 136–140, 141–154. doi:10.1007/s12010-007-9046-2.
Ferreira, A. C. H., Neiva, J. N. M., Rodríguez, N. M., Lobo, R. N. B., & Vasconcenlos, V. R. (2004). Revista Brasileira de Zootecnia, 33, 1380–1385.
Buchaman, B., Gruissem, W., & Jones, R. L. (2001). Biochemistry and molecular biology of plants (3rd ed.). Rockville, MD: Courier Companies, Inc.
Saha, B. C., Iten, L. B., Cotta, M. A., & Wu, Y. V. (2005). Process Biochemistry, 40, 3693–3700. doi:10.1016/j.procbio.2005.04.006.
Saha, B. C., & Cotta, M. A. (2006). Biotechnology Progress, 22, 449–453. doi:10.1021/bp050310r.
Bradshaw, T. C., Alizadeh, H., Teymouri, F., Balan, V., & Dale, B. E. (2007). Applied Biochemistry and Biotechnology, 136–140, 395–405. doi:10.1007/s12010-007-9067-x.
Tengborg, C., Galbe, M., & Zacchi, G. (2001). Biotechnology Progress, 17, 110–117. doi:10.1021/bp000145+.
Saha, B. C. (2003). Journal of Industrial Microbiology & Biotechnology, 30, 279–291. doi:10.1007/s10295-003-0049-x.
Santos, D. T., Sarrouh, B. F., Rivaldi, J. D., Converti, A., & Silva, S. S. (2008). Journal of Food Engineering, 86, 542–548. doi:10.1016/j.jfoodeng.2007.11.004.
Dizhbite, T., Zakis, G., Kizima, A., Lazareva, E., Rossinskaya, G., Jurkjane, V., et al. (1999). Bioresource Technology, 67, 221–228. doi:10.1016/S0960-8524(98)80004-7.
Cardona, C. A., & Sáncheza, O. J. (2007). Bioresource Technology, 98(12), 2415–2457. doi:10.1016/j.biortech.2007.01.002.
Sassner, P., Galbe, M., & Zacchi, G. (2006). Enzyme and Microbial Technology, 36, 756–762. doi:10.1016/j.enzmictec.2005.12.010.
Ghose, T. K. (1987). Pure and Applied Chemistry, 59(2), 257–268. doi:10.1351/pac198759020257.
Atala, D. I. P., Costa, A. C., Maciel, R., et al. (2001). Applied Biochemistry and Biotechnology, 91–3, 353–365. doi:10.1385/ABAB:91-93:1-9:353.
Tengborg, C., Galbe, M., & Zacchi, G. (2001b). Enzyme and Microbial Technology, 28, 835–844. doi:10.1016/S0141-0229(01)00342-8.
Dien, B. S., Li, X. L., Iten, L. B., Jordan, D. B., Nichols, N. N., O’Bryan, P. J., et al. (2006). Enzyme and Microbial Technology, 39, 1137–1144. doi:10.1016/j.enzmictec.2006.02.022.
Öhgren, K., Bengtsson, O., Gorwa-Grauslund, M. F., Galbe, M., Hahn-Hägerdal, B., & Zacchi, G. (2006). Journal of Biotechnology, 126, 488–498. doi:10.1016/j.jbiotec.2006.05.001.
Lynd, L. R., Weimer, P. J., Van Zyl, W. H., & Pretorious, I. S. (2002). Microbiology and Molecular Biology Reviews, 66(3), 506–577. doi:10.1128/MMBR.66.3.506-577.2002.
Olsson, L., & Bärbel, H. (1996). Applied Microbiology, 18, 311–331.
Pinheiro, A. D. T., Rocha, M. V. P., Macedo, G. R., & Gonçalves, L. R. B. (2008). Applied Biochemistry and Biotechnology, 148(1–3), 227–234. doi:10.1007/s12010-007-8118-7.
Rocha, M. V. P., Souza, M. C. M., Benedicto, S. C. L., Bezerra, M. S., Macedo, G. R., Pinto, G. A. S., et al. (2007). Applied Biochemistry and Biotechnology, 136–140, 185–194. doi:10.1007/s12010-007-9050-6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocha, M.V.P., Rodrigues, T.H.S., de Macedo, G.R. et al. Enzymatic Hydrolysis and Fermentation of Pretreated Cashew Apple Bagasse with Alkali and Diluted Sulfuric Acid for Bioethanol Production. Appl Biochem Biotechnol 155, 104–114 (2009). https://doi.org/10.1007/s12010-008-8432-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8432-8