Abstract
Hypocrellin A production by Shiraia sp. SUPER-H168 was studied under solid-state fermentation. Corn was found to be the best substrate after evaluating eight kinds of agro-industrial crops and residues. The optimized solid-state fermentation conditions were as follows: inoculum size 3 × 106 spores, substrate particle size 0.8–1 mm, initial moisture content 50%, and temperature 30 °C. Six kinds of external carbon source and seven kinds of external nitrogen source were evaluated, respectively, for HA production. Glucose and NaNO3 were the best. The combination of them was optimized by the response surface method. The optimum compositions of the supplementary glucose and NaNO3 were 1.65 g/100 g and 0.43 g/L, respectively. Hypocrellin A production reached 4.7 mg/g.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypocrellin compounds, traditionally used as medicinal agents in Asia, are perylene quinonoid pigments. They have been isolated and characterized from parasitic fungi Shiraia bambusicola and Hypocrella bambuase recently [1, 2]. Because hypocrellins showed unique antiviral, antidepressant, and antiretroviral properties, they drew much attention for their potential medical applications lately [3, 4].
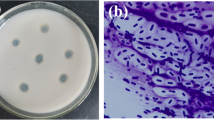
Until now, four kinds of hypocrellin (A, B, C, D) have been isolated; they had the similar structures and properties [5, 6]. Hypocrellin A (HA, Fig. 1a), the highest content of hypocrellins in the stromata of S. bambusicola and H. bambuase [2], has been known as a kind of excellent photosensitizer [5, 7]. It can accumulate selectively in cancer cells and kill tumor cells efficiently [8, 9]. It also has an inhibitory effect on human immunodeficiency virus type 1 [10].
HA has been mainly obtained from the stromata of S. bambusicola (Fig. 1b) so far. S. bambusicola is a parasitic fungus on twigs of several genera of bamboos. It belongs to the family Phaeosphaeriaceae [11]. The number of S. bambusicola is so limited that it cannot be widely used. A few strains have been used for hypocrellin production; however, the production was relatively low in general. Li et al. reported that the yield of hypocrellin under the solid-state fermentation (SSF) was about 0.2 mg/g [12]. Yang et al. found that the strain S. bambusicola UV-62 produced hypocrellin by submerged fermentation, and the production could reach 0.0196% [13]. In our previous works, we had isolated and identified a new HA producing strain, Shiraia sp. SUPER-H168 [14]. It can efficiently produce HA in SSF. HA is a kind of secondary metabolite with a complex unknown metabolic pathway. It has been shown that many secondary metabolites could be produced solely during growth on solid substrates. Thus, SSF will be a suitable method to produce HA with high productivities and low production costs. The objectives of this study are (1) to screen potential agro-industrial crops and residues for producing hypocrellin A by Shiraia sp. SUPER-H168 and (2) to optimize the solid-state fermentation conditions for high yield hypocrellin A production.
Materials and Methods
Organism, Media, and Solid-State Fermentation Conditions
Shiraia sp. SUPER-H168 was a stock culture of the Laboratory of Biochemistry, School of Biotechnology, Jiangnan University, Wuxi, Jiangsu Province, China [14].
To prepare a spore suspension, the strain was cultured on potato dextrose agar (PDA) slants in the dark at 30 °C for 18 days. The black massive spores were collected and homogenized aseptically in a Sorvall Ominimixer with sterile distilled water for 10 min, and spore suspension was used as inoculum.
SSF was carried out in conical flasks (250 ml) with 30 g of dry substrate, moistened with salt solution containing (g/L): K2HPO4 1 g, KCl 0.5 g, MgSO4·7H2O, 0.5 g, FeSO4 0.01 g. Each flask was sterilized at 121 °C for 20 min. After sterilization, each flask was inoculated with the spore suspension previously prepared and incubated for 18 days. Rice, corn, wheat, rice bean, mung bean, soy bean, wheat bran, and soybean meal were used as substrate, respectively, to evaluate HA production.
HA Assay
Fermented material was dried at 60 °C and porphyrized to pass through a 0.2-mm sieve. Two-gram sample was refluxed with 50 ml methanol in a water bath at 70 °C for 2 h. The extract was filtered through 0.45-μm membrane filter and diluted to 100 ml. After that, HA was determined by reverse-phase high-performance liquid chromatography (HPLC). The HPLC system (PerkinElmer Series 200 HPLC Systems, CA, USA) was equipped with a reversed phase column (C18 analytical column , Hedera ODS-2, 250 × 4.6 mm, 5 μm, Hanbon Science & Technology Co. Ltd., China) and ran isocratically by using methanol/water (72:28) as the mobile phase, with flow rate at 1 ml/min and temperature at 30 °C. HA standard (LKT Laboratories, Inc.) solution (12.5, 25, 50, 100, 200, 250 mg/L) was detected at 300 nm. The standard curve was established according to the peak areas to evaluate HA production.
Fungal Biomass Estimation
The fungal biomass was determined by the Elson–Morgan photometry method based on glucosamine content [15].
Protein and Starch Estimation
Substrates were oven-dried at 60–80 °C to a constant weight. The crude protein of the substrate was measured by standard Kjeldahl method [16]. A total starch analysis kit was used for determining the total starch in substrate [17]. The kit (AA/AMG 9/97) was from Megazyme International Ltd.
Effect of Substrates
Eight kinds of agro-industrial crops and residues (rice, corn, wheat, rice bean, mung bean, soy bean, wheat bran, and soybean meal) were studied to select a suitable substrate for SSF.
Effects of external carbon and nitrogen source on HA production were studied, respectively. Xylose, lactose, fructose, sucrose, maltose, and glucose were added into the solid substrate, respectively. The final concentration of external carbon was 2/100 g.
NaNO3, NH4Cl, NH4NO3, urea, beef extract, peptone, and yeast extract were added to the solid substrate, respectively, to evaluate their effects on the yield of HA. The final concentration of external nitrogen source was 0.00588 mol/100 g (based on the N content of different source).
The control experiments were carried out without supplementation.
Effect of Incubation Conditions
The various process parameters were evaluated for HA production as follows: inoculum size (1, 2, 3, 4, 5, and 6 × 106 spores), particle size (0.4–0.6, 0.6–0.8, 0.8–1.0, 1.0–1.2, 1.2–1.4, and 1.4–1.8 mm), initial moisture content (30%, 40%, 50%, 60%, and 70%), temperature (24, 26, 28, 30, 32, and 34 °C), and pH (4, 5, 6, 7, 8, and 9).
The corns were crushed in a grinder and passed through sieves to get different particle size distribution corns. Spores were eluted from the PDA plates with sterile water, and their numbers were counted with colony counting method [18]. The other conditions were kept at their optimal level with corn as a substrate when a certain incubation condition was being studied.
Optimization of Medium with the Response Surface Method
After studying the effect of different external carbon and nitrogen sources (see “Effect of External Carbon Source and Nitrogen Source”), glucose and NaNO3 were found to be the most suitable external nutrition. The response surface method (RSM) was applied to optimize the culture medium of external carbon source and nitrogen source for improving HA production. Central composite design [19] was used as an experimental design for the combination of external glucose and NaNO3. A full quadratic model was used to fit the experimental data, and then analysis of variance (ANOVA) was performed. The R 2 value, the residual error, the pure error, and the lack-of-fit were calculated to evaluate the model. The RSM calculation and graph were done by the software NCSS 2007 and Matlab 7.0, respectively.
Results and Discussion
Selection of Substrate
Typically, the selection of suitable substrate was very important for products development in solid-state fermentation [20, 21]. Several agro-industrial crops and residues were used as substrate to evaluate HA production. The results were listed in the Table 1.
It was indicated by the results (in Table 1) that all the substrates well supported the growth of Shiraia sp. SUPER-H168. However, HA production did not show close relationship with biomass. Highest HA production was achieved with corn. Rice, wheat, and rice bean had similar HA production. Mung bean and wheat bran led to low HA production. Shiraia sp. SUPER-H168 could not produce HA on soybean and soybean meal.
Much research had proven that the C/N ratio was an important factor for fungal secondary metabolism [22, 23]. The C/N ratio influenced the microbial metabolic process and metabolite. According to the results shown in Table 1, C/N ratio influenced HA production considerably. When the C/N ratio was about 1, Shiraia sp. SUPER-H168 grew normally, but no HA was produced. The fungi should get enough biomass before the secondary metabolites was synthesized. Therefore, when Shiraia sp. SUPER-H168 grew well under low C/N ration condition, it might not have sufficient carbon source to synthesize HA. HA production from corn, wheat, rice bean, and wheat bran decreased as the C/N ratio decreased. Mung bean was exceptional: its C/N ratio was similar to rice bean’s, but its HA production, however, was at a very low level for some unknown reasons. The fungal biomass did not correlate with HA production in this study. For example, the strain Shiraia sp. SUPER-H168 could grow well on the substrate of soybean meal; however, no HA was detected. The C/N ratio did not show significant effect on the fungal biomass.
Considering the price of substrate and HA yield, we selected corn as the best substrate out of the eight substrates tested.
Effect of Inoculum
In the present study, we tested the HA production with varied inoculum size. Figure 2 showed that when inoculum size was 106 spores, the HA production reached to its highest level, i.e., 2.5 ± 0.1 mg/g.
The size of inoculum is an important role for production of metabolites under SSF. Low level of inoculum may not be sufficient for initiating growth and HA producing. However, oversized inoculum may decrease HA production because of over depletion of nutrients [24]. Normally, the suitable inoculum size is between 104 and 106 spores/g [25–27]. Our result was in the reasonable range.
Effect of Substrate Particle Size
Effect of corn particle size was another important factor that might influence the HA production. In our experiments (Fig. 3), when particle size ranged from 0.8 to 1 mm, HA production was the highest value at 3.38 ± 0.28 mg/g. The smaller or bigger substrate particles would decrease the yield of HA.
Too small substrate particles easily cause the substrate to agglomerate. It may result in poor microbial growth because of insufficient aeration and bad heat transfer [28, 29]. On the other hand, the larger particles will make it difficult for the hyphae to penetrate the substrate, although it can provide high aeration intensity [30]. Therefore, the suitable particle size is important to find a balance between surface area and porosity for HA production.
Effect of Initial Moisture Content
Six moisture levels ranging from 20% to 70% were established to study their effect on HA production. Figure 4 showed that the highest production (3.52 ± 0.14 mg/g) was attained when the initial moisture level was 50%.
The initial moisture level of media is important for the SSF process. If the moisture content is too low, the growth of microorganism will be hindered by low mass transfer efficiency [31]. High-moisture substrates can reduce the porosity of the corn. The voids may not contain enough oxygen to sustain the growth of cells [32]. Either low or high initial moisture can decrease the final HA production.
Effect of Temperature and Initial pH
Different culture temperatures resulted in different HA productions. Shiraia sp. SUPER-H168 could produce HA at 24 to 34 °C. It was shown by the experiment results (Fig. 5) that at the fermentation temperature of 30 °C, the maximum concentration of HA in media could be obtained. The optimal temperature.of Shiraia sp. SUPER-H168 was slightly higher than previously reported [12]. Yang recorded an optimal temperature of 25 °C for submerged fermentation [13].
In our study, HA production by Shiraia sp. SUPER-H168 was not sensitive to pH. The highest HA production was observed when Shiraia sp. SUPER-H168 was cultured at pH 7, as our experiment results indicated (Fig. 6).
As the original pH of the corns ranged between 5.8 and 6, it was not necessary to adjust the pH for HA production in solid-state fermentation. SSF was less affected by pH than submerged fermentation [33].
Effect of External Carbon Source and Nitrogen Source
Studies of supplementation of carbon sources were shown in Fig. 7. Supplementation of xylose resulted in the decrease of HA production. Additional lactose and fructose had little effect. Sucrose, maltose, and glucose promoted the HA production. Maximum HA production (3.9 mg/g) was observed when the substrate was supplemented with glucose.
The influence of external nitrogen source was visualized in Fig. 8. NaNO3 could significantly increase HA production to 4.1 mg/g. Beef extract and yeast extract also had positive effect. However, NH4Cl, NH4NO3, urea, and peptone significantly decreased HA production.
Sometimes, the agro-industrial crops substrates are already rich enough to supply the nutrients required for some fungal growth and production [34]. However, in some cases, external carbon or nitrogen can enhance the production of metabolites because additional nutrition may change the fungal metabolism mechanism [35].
From the above results, glucose and NaNO3 were the best external carbon and nitrogen sources for HA production. They might enhance the initial Shiraia sp. SUPER-H168 growth and improve the C/N ratio. The external carbon and nitrogen had limited effect on global C/N ratio. But, they could be utilized by mycelium directly, and moreover, they might regulate the local C/N ratio when the substrate was decomposing gradually by the enzymes of Shiraia sp. SUPER-H168.
In order to work out the best combination of glucose and NaNO3, RMS were used to optimize it. The experimental design and results were listed in Table 2.
The final estimated response model equation was
where Y was the response factor (HA production, mg/g) and X 1, X 2 represented the real values of the independent factors—glucose (g/100 g), NaNO3 (g/100 g).
The ANOVA was presented in Table 3.
The ANOVA table showed that R 2 of the final model was 0.913. F value (14.64) for the overall regression was significant at a 5% level. The lack-of-fit was insignificant at a 5% level. The linear and the quadratic items were significant. The statistical results indicated a good fit between the model and the experimental data.
Figure 9 plotted the theoretical combination of glucose and NaNO3 vs. production. The optimum compositions of the supplementary glucose and NaNO3 were 1.65 g/100 g and 0.43 g/L, respectively. The theoretical maximum HA production was 4.6 mg/g. In the confirmed experiments, HA production reached 4.7 ± 0.2 mg/g. In addition, we could find through the response surface that combinations of glucose and NaNO3 nearby the left lower part of the center were security to improve HA production.
So far, several strains of S. bambusicola had been screened, but most of them could not produce HA [36, 37]. The reported highest yield of SSF was 217 color value/g dry mass (about 0.2 mg/g) [12]. Also, Yang had produced the hypocrellin by submerged fermentation with production of 0.0196% for 5 days [13]. HA production of this study was the highest to date.
Time Course of HA Production under SSF
Fermentation time courses under optimized and non-optimized conditions were given in Fig. 10. Both time courses showed similar fermentation process trend. Initially, HA production was very low, and after a lag phase of almost 3 days, HA production gradually increased. After about 18 days of incubation, HA reached the maximum yield. The time course showed that the optimized fermentation conditions did not change the HA producing process.
In many SSF processes, when the products reach the highest yield, further increase in incubation time can decrease the yield due to the degradation of metabolites [38, 39]. However, HA is stable below 80 °C. Also, it cannot be decomposed by microorganism during the SSF process because of its activity against microorganisms [40]. Increase in incubation time will not decrease HA production.
Conclusion
In the paper, optimization of HA production by Shiraia sp. SUPER-H168 was presented. The experiment results suggested that the suitable substrate was corn. The particle size, temperature, and initial pH did not have a major effect on the HA production. The inoculum size, C/N ratio, initial moisture content, and the addition of extra carbon and nitrogen sources exerted a marked influence on the yield of HA. The HA productivity was the highest in the existing literatures. Therefore, SSF provided a promising approach to produce HA with the high production but low production cost. The process of HA production in laboratory scale had the potential to scale up. It laid the foundation for a large-scale application of HA.
References
Wan, X. Y., & Chen, Y. T. (1981). Chinese Science Bulletin, 26, 1040–1042.
Li, C., Wang, H. Q., Xie, J. L., Ling, N. Y., Wan, D. H., & Chen, Y. T. (2000). Chinese Traditional and Herbal Drugs, 31, 250–251.
Diwu, Z. J. (1995). Photochemistry and Photobiology, 61, 529–539.
Ma, G. Y., Khan, S. I., Jacob, M. R., Tekwani, B. L., Li, Z., Pasco, D. S., et al. (2004). Antimicrobial Agents and Chemotherapy, 48, 4450–4452.
Kishi, T., Tahara, S., Taniguchi, N., Tsuda, M., Tanaka, C., & Takahashi, S. (1991). Planta Medica, 57, 376–379.
Fang, L. Z., Qing, C., Shao, H. J., Yang, Y. D., Dong, Z. J., Wang, F., et al. (2006). Journal of Antibiotics, 59, 351–354.
Wu, H. M., Lao, X. F., Wang, Q. W., & Lu, R. R. (1989). Journal of Natural Products, 52, 948–951.
Fu, N. W., & Chu, Y. X. (1989). Acta Pharmacologica Sinica, 10, 371–373.
Zhang, J., Cao, E. H., Li, J. F., Zhang, T. C., & Ma, W. J. (1998). Journal of Photochemistry and Photobiology B, 43, 106–111.
Hirayama, J., Ikebuchi, K., Abe, H., Kwon, K. W., Ohnishi, Y., Horiuchi, M., et al. (1997). Photochemistry and Photobiology, 66, 697–700.
Cheng, T. F., Jia, X. M., Ma, X. H., Lin, H. P., & Zhao, Y. H. (2004). Journal of Basic Microbiology, 44, 339–350.
Li, D. X., Zhao, J., He, Y., & Yang, Z. R. (2003). Journal of Sichuan University (Natural Science Edition, in Chinese), 40, 139–143.
Yang, H. L., Xiao, C. X., Ma, W. X., & He, G. Q. (2009). Dyes and Pigments, 82, 142–146.
Liang, X. H., Cai, Y. J., Liao, X. R., Wu, K., Wang, L., Zhang, D. B., et al. (2009). Microbiological Research, 164, 9–17.
Elson, L. A., & Morgan, W. T. (1933). Biochemical Journal, 27, 1824–1828.
Owusu-Apenten, R. K. (2002). Food protein analysis: Quantitative effects on processing. New York: Marcel Dekker.
Meares, C. A., Bogracheva, T. Y., Hill, S. E., & Hedley, C. L. (2004). Starch-Starke, 56, 215–224.
García-Armesto, M. R., Prieto, M., García-López, M. L., Otero, A., & Moreno, B. (1993). Microbiologia, 9, 1–13.
Douglas, C. M. (2000). Design and analysis of experiments (5th ed.). New York: John Wiley & Sons.
Raimbault, M. (1998). Electronic Journal of Biotechnology, 3, 174–188.
Pandey, A., Soccol, C. R., Nigam, P., Soccol, V. T., Vandenbegh, L., & Mohan, R. (2000). Bioresearch Technology, 74, 81–87.
Elson, M. K., Schisler, D. A., & Jackson, M. A. (1998). Mycologia, 90, 406–413.
Sasaki, N., Suehara, K., Kohda, J., Nakano, Y., & Yang, T. (2003). Journal of Bioscience and Bioengineering, 96, 47–52.
Sabu, A., Augur, C., Swati, C., & Pandey, A. (2006). Process Biochemistry, 41, 575–580.
Shankara, S. K., & Mulimani, V. H. (2007). Bioresearch Technology, 98, 958–961.
Tunga, R., Banerjee, R., & Bhattacharyya, B. C. (1998). Bioprocess Engineering, 19, 187–190.
Park, Y. S., Kang, S. W., Lee, J. S., Hong, S. I., & Kim, S. W. (2002). Applied Microbiology and Biotechnology, 58, 761–766.
Valera, H. R., Gomes, J., Lakshmi, S., Gururaja, R., Suryanarayan, S., & Kumar, D. (2005). Enzyme and Microbial Technology, 37, 521–526.
Yua, J. L., Zhang, X., & Tan, T. W. (2008). Fuel Processing Technology, 89, 1056–1059.
Pandey, A., Soccol, C. R., & Mitchell, D. (2000). Process Biochemistry, 35, 1153–1169.
Raghavarao, K. S. M. S., Ranganathan, T. V., & Karanth, N. G. (2003). Biochemical Engineering Journal, 13, 127–135.
Adinarayana, K., Prabhakar, T., Srinivasulu, V., Rao, M. A., Lakshmi, P. J., & Ellaiah, P. (2003). Process Biochemistry, 39, 171–177.
Couto, S. R., & Sanroman, M. A. (2006). Journal of Food Engineering, 76, 291–302.
Babitha, S., Soccol, C. R., & Pandey, A. (2007). Bioresearch Technology, 99, 1554–1560.
Virupakshi, S., Gireesh, B. K., Gaikwad, S. R., & Naik, G. R. (2005). Process Biochemistry, 40, 431–435.
Morakotkarn, D., Kawasaki, H., & Seki, T. (2007). FEMS Microbiology Letters, 266, 10–19.
Cheng, L. S., & Wang, J. Z. (1985). Acta Biologiae Experimentalis Sinica (in Chinese), 18, 89–90.
De Carvalho, J. C., Pandey, A., Oishi, B. O., Brand, D., Rodriguez-Leon, J. A., & Soccol, C. R. (2006). Biochemical Engineering Journal, 29, 262–269.
Hudson, J. B., Zhou, J., Chen, J., Harris, L., & Towers, G. H. (1994). Photochemistry and Photobiology , 60, 253–255.
Ma, G., Khan, S. I., Jacob, M. R., Tekwani, B. L., Li, Z., Pasco, D. S., et al. (2004). Antimicrob. Agents Chemother, 48, 4450–4452.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, Y., Liang, X., Liao, X. et al. High-Yield Hypocrellin A Production in Solid-State Fermentation by Shiraia sp. SUPER-H168. Appl Biochem Biotechnol 160, 2275–2286 (2010). https://doi.org/10.1007/s12010-009-8728-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8728-3