Abstract
In order to construct a strain that converts sugar mixture and resist/metabolize inhibitors in lignocellulosic dilute-acid hydrolysate, the biotechnology of inactive intergeneric fusion between Saccharomyces cerevisiae and Pachysolen tannophilis was performed. Fusant 1 was successfully obtained as a hybrid strain, which was screened out by xylose and mixed sugar (xylose and glucose) fermentation. This strain showed good abilities of ethanol production, ethanol tolerance, and resistance to the toxic inhibitors presenting in the hydrolysate. The maximum volumetric yield of ethanol and yield of xylitol in mixed sugar was 9.52 g/l and 0.44 g/g, respectively. The results indicated that the constructed strain Fusant 1 was a good producer for ethanol and xylitol from lignocellulosic dilute-acid hydrolysate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As an environment-friendly energy, ethanol can be derived from a renewable resource—lignocellulosic biomass, such as agricultural and forestry residues, waste paper, and industrial wastes.
Lignocellulose, which is mainly composed of cellulose, hemicellulose, and lignin, is often hydrolyzed by acid [1–3]. Such lignocellulosic hydrolysate contains various sorts of monosaccharides, such as glucose, xylose, mannose, galactose, and arabinose, and some kinds of oligosaccharides that can be converted to ethanol and other chemicals by fermentation.
An important factor for efficient ethanol production is the high-yield, high-rate fermentation of hydrolysate to ethanol. The demands on the microorganisms that carry out this process are more complicated than those for ethanol production from grains, which can be easily hydrolyzed to hexoses or their disaccharides. For example, Saccharomyces cerevisiae is considered to be the most effective ethanol producer, but substantial amounts of pentose generated by hydrolysis of hemicelluloses cannot be fermented by wild-type S. cerevisiae. Additionally, the process of dilute-acid hydrolysis of lignocelluloses generates numerous toxic compounds, such as acetic acid, furfural, and 5-hydroxymethyl-furfural (5-HMF), which inhibit microbial growth [4]. Most xylose-fermenting strains such as Pachysolen tannophilis, Pichia stipitis, Candida shehatae, and Candida parapsilosis cannot tolerate the inhibitors. The capability of these yeasts to ferment pentoses from dilute-acid hydrolysate is hindered by the presence of inhibitors. Therefore, tolerance to these toxic compounds and ethanol is a prerequisite for the fermentation of lignocellulosic hydrolysate.
Based on current researches, S. cerevisiae appears to be the most promising metabolic-engineering platform for bioethanol production from lignocellulosic hydrolysate because of its advantages of high ethanol production from hexoses and high tolerance of ethanol and inhibitors, as well as ability to grow in strict anaerobic condition. The obvious disadvantage of S. cerevisiae is the inability of metabolizing xylose. At present, metabolic engineering is usually applied to modify this yeast for utilizing xylose.
In this research, another method is considered and applied to modify this yeast in order to acquire a hybrid strain that could use xylose and glucose to produce ethanol and have the resistance of inhibitors. S. cerevisiae and P. tannophilis were fused by the method of inactive intergeneric protoplast fusion through polyethylene glycol (PEG) induced. One parental strain S. cerevisiae, stored in our lab, has a good ability of glucose fermentation and good tolerance of inhibitors and ethanol; another parental strain, P. tannophilis, is capable of converting xylose to ethanol. We expected to establish a strain, which combine the advantages of both parental strains by protoplast fusion method. The fusant should have the abilities of converting both glucose and xylose to ethanol and have the tolerance of ethanol and inhibitors in lignocellulosic hydrolysate.
Protoplast fusion is a non-specific recombination technique used for transfer cytosolic organelles including genetic material. This technique is generally regarded as a technique of some potential with regard to improving yeasts of industrial application [5–7]. The technique has indicated that sexual barriers, preventing genetically unrelated yeast strains from mating, may be obviated to a certain extent by cell wall digestion, thus facilitating total or partial exchange of genetic components [8–11]. After fusion, the cytosol and organelles of the parental strains were fused and exchanged; the genomes of the parental strains would contact with each other as well. This kind of exchange greatly exceeds the changes made by the genetic engineering method. Although the possibility of obtaining a fusant that owns both advantageous properties from parental strains is not high, this breeding method still supply us a new prospect.
This study aimed to investigate the feasibility of obtaining hybrids of S. cerevisiae and P. tannophilis, which would show the improved yield of ethanol from lignocellulosic hydrolysate.
Materials and Methods
Materials
Strains and Culture Condition
S. cerevisiae 2.0251 was provided by the China General Microbiological Culture Collection Center. P. tannophilis ATCC 2.1662 was obtained from China Industrial Cultures Center. The two parental strains, 2.0251 and 2.1622, were stored on a medium of the following compositions: 10 g/L glucose, 10 g/L peptone, 5 g/L yeast extract, and 20 g/L agar.
Enzymes and Reagents
DNA extraction kit was purchased from Shanghai Huashun Engineering Co., Ltd., Shanghai, China. EDTA-Na2, snailase, β-mercaptoethanol, 2-iodacetamide, and PEG (MW-6000) were purchased from Beijing DingGuo Biotech Co., Ltd., Beijing, China. All other reagents were commercial products of analytical grade from standard suppliers.
Methods
Protoplast Preparation
Cell wall lysis was done by enzymatic digestion in the presence of an isotonic buffer having optimal pH and containing divalent cations and chelating agents. The two important parameters for proper generation of protoplast are the age of cells and the contact time with lytic enzyme. For the production of cells for protoplasts formation, the cells were re-inoculated into yeast extract peptone dextrose (YEPD) medium and incubated until the middle log phase was reached. Then, the two kinds of yeasts were harvested and suspended in 0.2 M phosphate-buffered saline (PBS) (KH2PO4/K2HPO4) buffer (pH 5.8) containing 0.2% (w/v) EDTA-Na2 and 0.06% (v/v) β-mercaptoethanol for 20 min at 30 °C. Being washed with PBS, the cells were obtained by centrifugation (4,500 rpm, 5 min), and the pellets were incubated at 30 °C for 1 h with 2.0% (w/v) snailase to make protoplasts. After this treatment, regeneration of protoplasts was done to check the viability.
Fusion and Regeneration
Chemo-fusion technique was carried out between the two viable protoplasts using a fusogen in the presence of divalent cations at optimal pH and under suitable-related conditions. The most commonly used fusogen was PEG. The divalent ion used in this experiment was calcium. A fusion solution composed of 35% PEG, 0.01 mol/L CaCl2, and KH2PO4/K2HPO4 buffer (pH 5.8 with 0.8 mol/L potassium chloride) proved to be the most efficient one in this case. The two kinds of protoplasts were collected and inactivated, respectively [12, 13]. The cells of 2.0251 were treated with 0.6% (w/v) 2-iodacetamide for 3 min to inactivate [13], while cells of 2.1622 were heated to 55 °C for 40 min [14]. The inactivated protoplasts of two parental strains were obtained and mixed together at a cell ratio of about 1:1 in the cell numbers. Being washed with 0.4 mol/L calcium chloride, the mixture was suspended in 1 mL freshly prepared fusion solution containing a certain concentration of pre-warmed PEG-6000 and Ca2+ for 20 min at 30 °C. The fused protoplasts were then plated onto YEPD solid medium and incubated at 30 °C for 2 days. Then, the regenerated colonies were picked out and transferred to YEPD medium for further screening.
Analysis of Presumed Fusant Colonies
The regenerated colonies were isolated and then screened out by xylose and mixed sugar (xylose and glucose) fermentation. Those fusants, which presented good abilities for xylose and glucose consumption, were selected out. Determinations of cell size and DNA content were performed to confirm the hybridity of these fusants. Then, tests of fermentation stability and tolerance of ethanol and inhibitor were identified, respectively.
Softwood Hydrolysate
The hydrolysate used in the experiment was provided by the Department of Energy Chemical Engineering, East China University of Science and Technology, Shanghai, China. Softwood chips were immersed in 2% hydrochloric acid and 0.5% ferrous chloride (v/v) at 170 °C for 30 min. The liquid phase from the acid hydrolysis was recovered. The hydrolysate contained about 37.5 g/L of monomeric sugar, approximately 75% of which was glucose. It also contained the inhibitors furfural (2.2 g/L) and 5-HMF (1.6 g/L). The final pH for the solution was 1.0. There was no detoxification applied to the hydrolysate. Sodium hydroxide was added to adjust the pH of hydrolysate to 5.5 before fermentation.
Cell counting
The optical density of culture, which indicates the cell concentration in a certain range, was measured by using spectrophotometer at 600 nm. The amount of cells alive was counted by using hemocytometer after stained with 0.05% methylene blue.
Genome Extraction from the Strains
The DNA extraction of all the strains was performed by using Yeast DNA Extraction Kit according to the manual book.
Analytical Procedures
The culture samples after fermentation were analyzed by gas chromatography equipped with a chromos orb 105 column and a flame ionization detector for ethanol concentration. The sugar concentrations of xylose and glucose were determined by high-performance liquid chromatography (Knouer, Berlin, Germany) equipped with an ammonia analysis column and a refractive index detector. Acetonitrile was used as the mobile phase at a flow rate of 0.8 mL/min.
Results and Discussions
Results of Fusion
Totally, 45 isolations were collected from the YEPD regeneration plates. After one screening, 25 strains were removed from the fusant list by morphological observation.
Fermentation of xylose
The xylose fermentation with the 20 fusants left was undertaken. The 20 fusants and two parental strains were inoculated into YEPD liquid medium (100/250 mL) in Erlenmeyer flasks, which were kept at 30 °C on a rotary shaker (80–90 rpm) for 96 h. Xylose utilization and ethanol production of these strains were determined. The results are shown in Table 1.
The ethanol yields produced by Fusants 1, 30, 19, 2, 37, 8, 9, 14, 16, and 34 were higher than that produced by 2.1622. The ethanol-producing ability of these fusants from xylose was increased by the fusion treatment.
Xylose utilization rate of 72% was reached by Fusant 1, which was higher than that of other fusants. Fusant 1 utilized xylose even more efficiently than 2.1622. The final ethanol concentration by Fusant 1 was 3.44 g/L, which was two times higher than 2.1622.
Additionally, xylitol was detected in the medium of Fusant 1. It was indicated that xylose was partially converted to xylitol by Fusant 1. These results showed that Fusant 1 proved to have the best ability to utilize xylose. The fusant was acquired with the ability from parental strain by the fusion treatment. What is more, the ability was increased.
Fermentation of Mixed Sugar
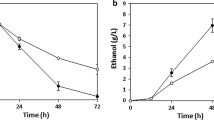
Fusant 1 and two parental strains were inoculated into mixed sugar medium (30 g/L glucose, 20 g/L xylose, 20 g/L peptone, and 10 g/L yeast extract) in Erlenmeyer flasks (100/250 mL), which were incubated at 30 °C on a rotary shaker (80–90 rpm). The profiles are shown in Fig. 1.
Glucose in the medium was used up within 24 h by all the three strains. The similar ethanol concentrations of 11.76 and 11.2 g/L by Fusants 1 and 2.0251 were achieved, respectively, while that of 2.1622 was 8.8 g/L at same time (Fig. 1a). Xylose in the media was used earlier by Fusant 1 than 2.1622 (Fig. 1b), it implied that glucose and xylose could be used by Fusant 1 simultaneously, which might result in the higher concentration of ethanol than 2.0251.
The final concentration of ethanol was 8.18, 9.92, and 10.24 g/L by 2.1622, 2.0251, and Fusant 1, respectively, during the course. A significant xylitol was found in the concentration of 8.8 and 1.9 g/L by Fusants 1 and 2.1622, respectively (Fig. 1c). Therefore, Fusant 1 had not only the ability of 2.0251 in producing ethanol from glucose but also the ability of 2.1622 in converting xylose to xylitol.
Determination of Cell Size and DNA Content
The morphological observation and molecular analysis were carried out to further confirm the hybridity of Fusant 1. The results of cell size and DNA content of Fusant 1 were illuminated in Tables 2 and 3.
The average DNA content of the Fusant 1 was 0.80 × 10−7 µg/mL, which is larger than that of 2.1622 but less than half of the sum of the two parental strains (1.26 × 10−7 µg/ml). These results showed that the two cells that fused had altered genomes. After fusion, the genetic material of the hybrid cell was not distributed equally. The Fusant 1 received a majority of genetic material from 2.1622 and a small amount of that provided by another parental strain 2.0251.
Analysis of the Fusant
Ethanol Tolerance
Fusant 1 and two parental strains were inoculated to YEPD liquid medium (30/250 mL) in Erlenmeyer flasks. After incubation at 30 °C on a rotary shaker (150 rpm) for 24 h, they were re-inoculated into YEPD liquid media with different concentrations of ethanol. The cultures were incubated at 30 °C for 96 h. Then, the cell concentration was measured. The results are shown in Fig. 2.
Ethanol tolerance is an important feature as an ethanol producer. The results showed that the growth of Fusant 1 was strongly prohibited by an ethanol concentration of about 8% (Fig. 2a), which was higher than that of about 5% with parental strain 2.1622 (Fig. 2b). On the contrary, 2.0251 grew well on the YEPD with an ethanol concentration of 10% (Fig. 2c).
Inhibitor Tolerance
The harsh conditions that prevail during the acid pre-treatment and hydrolysis of lignocellulosic biomass result in the release of many compounds that inhibit yeast growth and ethanol production.
The number and identity of these toxic compounds varies with the nature of the raw [4]. Usually, there are two main approaches to limit the impact of inhibitors on the fermentation process. One is to introduce additional chemical, physical, or biological process steps for the removal or inactivation of inhibitors. The other is to improve the tolerance of ethanol-fermentingyeasts to the inhibitors. Obviously, physical and chemical methods of detoxification are effective but cost-prohibitive. In contrast, biological methods focused on either in situ inactivation/metabolism of inhibitors or the development of more stress-resistant yeast are relatively inexpensive.
Fusant 1 was inoculated to YEPD liquid medium (30/250 mL) in Erlenmeyer flasks. After incubated at 30 °C on a rotary shaker (150 rpm) for 24 h, it was re-inoculated to a liquid medium with different concentrations of lignocellulosic dilute-acid hydrolysate containing inhibitors, such as furfural, 5-HMF, acetic acid, etc. The three cultures were incubated at 30 °C for 96 h, and the cell concentration was evaluated (Fig. 3).
Growth profiles of Fusant 1 (a), P. tannophilis 2.1622 (b), and S. cerevisiae 2.0251 (c) in YEPD with different hydrolysate concentrations. The concentrations of furfural in the diluted hydrolysate of 30%, 40%, and 50% (v/v) were equivalent to 0.66, 0.88, and 1.1 g/L, respectively. The concentrations of 5-HMF in the diluted hydrolysate of 30%, 40%, and 50% (v/v) were equivalent to 0.48 g, 0.64, and 0.8 g/L, respectively
Because the tolerance of both parental stains to furfural and 5-HMF is not good, the growth of these two parental strains were inhibited in the dilute of hydrostats with a concentration of more than 50% (v/v). The 2.0251 had a good tolerance to acetic acid, but it cannot resist furfural and 5-HMF. In our research, it was found that those two inhibitors were the key factors that inhibited the cell growth of the yeast. Therefore, furfural and 5-HMF were used as test indicators to demonstrate the tolerance of Fusant 1 to the inhibitors.
Compared with its parental strains (showed in Fig. 3), the amount of live cells was larger. This phenomenon indicated that Fusant 1 presented a better tolerance to furfural and 5-HMF.
Stability of Fusant 1
To ascertain whether fusant 1 is genetically stable, it was passed for 15 generations, and the ethanol and xylitol production in mixed sugar medium for every four generations was measured. All the generations demonstrated similar ability as the original Fusant 1, suggesting that fusant 1 is genetically stable. (Showed in Fig. 4)
Conclusion
Protoplast fusion provided opportunities for bringing together genomes of taxonomically divergent species that cannot be combined sexually due to crossability barriers. Inactive intergeneric protoplast fusion between S. cerevisiae 2.0251 and P. tannophilis 2.1622 was achieved.
The protoplasts of 2.0251 were fused with the protoplasts of 2.1622 by 35% PEG (MW-6000) and 0.01 mol/L potassium chloride for 20 min. The fusion frequency was about 7.52 × 10−6. Finally, 45 hybrids were obtained by protoplast fusion. Fusant 1 was screened out through the xylose-fermentation and the mixed sugar (xylose and glucose) fermentation. The strain showed better fermenting ability in 30 g/L glucose and 20 g/L xylose fermentation. The volumetric yield of ethanol was 9.52 g/L after 72 h and was improved by about 19% than parental strain 2.1622. The volumetric yield of xylitol was up to 0.44 g/g, which is five times that of 2.1622. The cell size and DNA content were determined, and the stability of fermentation of the hybrid was also tested. All the results showed that Fusant 1 is indeed an intergeneric hybrid. The tests of other capabilities of Fusant 1 such as ethanol tolerance and inhibitor tolerance showed that this strain exceeded parental strain 2.1622.
In summary, we obtained the hybrid strain, Fusant 1, with xylose and glucose utilizing abilities by inactive intergeneric protoplast fusion between S. cerevisiae and P. tannophilis. This hybrid strain can convert xylose and glucose to xylitol and ethanol, respectively, and is genetically stable as well. Meanwhile, its tolerance of inhibitors and ethanol showed the promising prospect for industrial application.
References
Aristos, A., & Merja, P. (2000). Metabolic engineering applications to renewable resource utilization. Current Opinion in Biotechnology, 11, 187–198. doi:10.1016/S0958-1669(00)00085-9.
Saha, B. C. (2003). Hemicellulose bioconversion. Journal of Industrial Microbiology, 30, 279–291. doi:10.1007/s10295-003-0049-x.
Satoshi, K., Atsuko, M., Hideki, F., & Akihiko, K. (2006). Ethanol fermentation from lignocellulosic hydrolysate by a recombinant xylose- and cellooligosaccharide-assimilating yeast strain. Applied Microbiology and Biotechnology, 72, 1136–1143. doi:10.1007/s00253-006-0402-x.
Klinke, H. B., Thomsen, A. B., & Ahring, B. K. (2004). Inhibition of ethanol-producing yeast and bacteria by degradation products during pre-treatment of biomass. Applied Microbiology and Biotechnology, 66, 10–26. doi:10.1007/s00253-004-1642-2.
Heluane, H. (1993). Characterization of hybrids obtained by protoplast fusion between Pachysolen tannophilis and Saccharomyces cerevisiae. Applied Microbiology and Biotechnology, 40, 98–100. doi:10.1007/BF00170435.
Jhannsan, E., Eagal, L., & Brendenhann, G. (1985). Protoplast fusion used for the construction of presumptive ploypiods of the D-xylose fermenting yeast Candida shehatae. Current Genetics, 9, 313–319. doi:10.1007/BF00419961.
Kordowska, W. M., & Targonski, Z. (2001). Application of Saccharomyces cerevisiae and Pichia stipitis karyoduction to the production of ethanol from xylose. Acta Microbiologica Polonica, 50, 291–299.
Ferencezy, L., Kevei, F., & Zsolt, J. (1974). Fusion of fungal Protoplast. Nature, 248, 793–794. doi:10.1038/248793a0.
Kao, K. N., & Michayluk, M. R. A. (1974). Method for high frequency intergeneric fusion of plant protoplasts. Planta, 115, 355–367. doi:10.1007/BF00388618.
Perberdy, J. F. (1980). Protoplast fusion—a tool for genetic manipulation and breeding industrial microorganisms. Enzyme and Microbial Technology, 2, 23–29. doi:10.1016/0141-0229(80)90004-6.
Zimmermann, U., & Pilwat, G. (1978). The relevance of electric field induced changes in the membrane structure to basic membraneresearch and clinical therapeutics and diagnosis. In: Abstract of the 6th International Biophysics Congress Kyoto, IV-19-(H):140
Ferenczy, L. (1984). Fungal protoplast fusion. In R. F. Beers Jr, & E. G. Bassett (Eds.), Cell fusion: Gene transfer and transformation. New York: Raveb Oressam.
Wright, W. E. (1978). The isolation of heterokaryons and hybrids by a selective system using irreversible biochemical inhibitors. Experimental Cell Research, 112, 395–407. doi:10.1016/0014-4827(78)90222-7.
Fodor, K. (1978). Journal of Bacteriology, 135(1), 68–70.
Acknowledgments
We would like to thank the Ministry of Science and Technology for financial support (nos. 2001AA514024 and 2002AA514010). We gratefully acknowledge the Department of Energy Chemical Engineering (East China University of Science and Technology, Shanghai, China) for providing the hydrolysate used in the experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Yan, F. Bai, and S. Tian contributed equally to this research work.
Rights and permissions
About this article
Cite this article
Yan, F., Bai, F., Tian, S. et al. Strain Construction for Ethanol Production from Dilute-Acid Lignocellulosic Hydrolysate. Appl Biochem Biotechnol 157, 473–482 (2009). https://doi.org/10.1007/s12010-008-8343-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8343-8