Abstract
Two xylanases were isolated and purified from crude culture filtrate of Aspergillus sydowii SBS 45 after 9 days of growth on wheat bran containing 0.5% (w/v) birch wood xylan as the carbon source under solid-state fermentation. After a three-step purification scheme involving ammonium sulfate precipitation, gel filtration chromatography (Sephadex G-200), and anion exchange chromatography (DEAE-Sephadex A-50), xylanase I was purified 93.41 times, and xylanase II was purified 77.40 times with yields of 4.49 and 10.46, respectively. Molecular weights of xylanase I and II were 20.1 and 43 kDa, respectively, in sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Optimum temperature was 50 °C, and optimum pH was 10.0 for both xylanase I and II. The K m value of xylanase I for birch wood xylan was 3.18 mg ml−1 and for oat spelt xylan 6.45 mg ml−1, while the K m value of xylanase II for birch wood xylan was 6.51 mg ml−1 and for oat spelt xylan 7.69 mg ml−1. Metal ions like Al3+, Ba2+, Ca2+, Na+, and Zn2+ enhanced the activity of xylanase I and II at 10 mM concentration. Among the additives, l-tryptophan enhanced the activity of xylanase I and II at 10-, 20-, and 30-mM concentrations. Both xylanases appeared to be glycoproteins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Xylan is a major component of plant hemicellulose. After cellulose, it is the next most abundant renewable polysaccharide in nature. Xylan represents up to 30–35% of the total dry weight of land plants [1]. Xylan together with cellulose (1,4-β-glucan) and lignin make up the major polymeric constituents of plant cell walls [2]. Xylan is a heteropolysaccharide and is made up of a homopolymeric backbone chain of 1,4-linked β-d-xylopyranosyl units, which can be substituted to varying degrees with glucuronopyranosyl, 4-O-methyl-d-glucuronopyranosyl, α-l-arabinofuranosyl, acetyl, feruloyl, and/or p-coumaroyl side chain groups [2]. Because of heterogeneity and the complex chemical nature of plant xylan, its complete breakdown requires the action of a complex of several hydrolytic enzymes with diverse specificity and modes of action. Endo-1,4-β-d-xylanases (EC 3.2.1.8) randomly cleave xylan backbone, and β-d-xylosidases (EC 3.2.1.37) cleave xylose monomers from the nonreducing end of xylo-oligosaccharides and xylobiose, while the removal of side groups is catalyzed by α-l-arabinofuranosidases (EC 3.2.1.55), α-d-glucuronidases (EC 3.2.1.139), acetyl xylan esterases (EC 3.2.1.72), ferulic acid esterases (EC 3.1.1.73), and p-coumaric acid estrases (EC 3.1.1.–) [3].
Xylanolytic enzymes from microorganisms have attracted attention for the last few decades, particularly because of their biotechnological potential in various industrial processes such as food, feed, and pulp and paper industries [4]. Currently, the most effective application of xylanases is in the prebleaching of kraft pulp to minimize use of harsh chemicals in the subsequent treatment stages of pulp bleaching [5]. In baking, they improve elasticity and strength of the dough thereby increasing loaf volumes and texture of bread [6]. In the feed industry, the incorporation of xylanase into the rye-based diet of broiler chickens results in the reduction in intestinal viscosity, thereby improving both weight gain of chicks and their feed conversion efficiency [7]. Xylanases are used for the conversion of xylan in wastewater released from agricultural and food industries into xylose. They are also used for clarifying must and juices and for liquefying fruits and vegetables. Xylanases in conjunction with other enzymes are used for the generation of biological fuels such as ethanol and xylitol from lignocellulosics, for degumming of bast fibers such as flax, hemp, jute, and ramie, and deinking of waste newspapers, which are used in the paper-making process [4].
Fungi, actinomycetes, and bacteria are some of the most important xylanolytic enzyme producers. Many of these organisms have been found to produce multiple forms of xylanases. These may have diverse physico-chemical properties, structures, specific activities, and yields, as well as overlapping but dissimilar specificities, thereby increasing the efficiency and extent of hydrolysis and also the diversity and complexity of the enzymes. Species of Aspergilli and Trichodermi are examples of microorganisms, which can produce xylanase isoenzymes. The aim of the study is to purify and characterize xylanases from the culture filtrate of the fungus Aspergillus sydowii SBS 45.
Materials and Methods
Chemicals
Birch wood xylan and oat spelt xylan were obtained from Sigma (St. Louis, MO). Sephadex G-200 and diethylaminoethyl (DEAE)-Sephadex A-50 were obtained from Pharmacia (Uppsala, Sweden). Protein molecular mass standards (wide range) were obtained from Genei (Bangalore, India).
Organism and Growth Conditions
A. sydowii SBS 45 was isolated from the soil in our laboratory and was maintained on Sabouraud dextrose agar medium containing 0.1% (w/v) birch wood xylan. The plates were incubated at 30 °C for 7 days for spore production and then stored at 4 °C until use.
Fermentation Studies
Solid-state fermentation was carried out using wheat bran as the substrate. Wheat bran was moistened with mineral salt medium containing: KCl (0.5 g l−1), MgSO4.7H2O (0.5 g l−1), NaH2PO4 (0.5 g l−1), CaCl2 2H2O (0.01 g l−1), FeSO4 7H2O (0.01 g l−1), MnCl2 4H2O (0.01 g l−1), ZnSO4 7H2O (0.002 g l−1), soyabean meal (2.5 g l−1), and birch wood xylan (5.0 g l−1). The initial pH of the medium was adjusted to 7. Fermentation was carried out in 500-ml Erlenmeyer flask containing 20 g wheat bran moistened with 20 ml mineral salt solution. Sterilized media were inoculated with 1 ml of spore suspension containing 1 × 106 spores per milliliter. Fermentation was carried out at 30 °C for 9 days under static conditions.
Enzyme Assays
Xylanase activity was determined by taking 0.9 ml of 1% (w/v) birch wood xylan (prepared in 50 mM Na-citrate buffer, pH 5.3) and 0.1 ml of diluted enzyme, and the mixture was incubated at 50 °C for 5 min [8]. Reaction was stopped by the addition of 1.5 ml 3,5-dinitrosalicylic acid (DNS) reagent, and contents were boiled for 5 min [9]. After cooling, the color developed was read at 540 nm. The amount of reducing sugar liberated was quantified using xylose as the standard. One unit of xylanase activity was defined as the amount of enzyme required to release 1 μmol of xylose equivalents per minute.
Cellulase activity was determined by incubating 0.9 ml of 1% (w/v) carboxymethyl cellulose (prepared in 50 mM Na citrate buffer pH 5.3) and 0.1 ml of diluted enzyme at 50 °C for 15 min [10]. Reaction was stopped by the addition of 1.5 ml DNS reagent, and the contents were boiled for 5 min [9]. The color developed was read at 540 nm. The amount of reducing sugar liberated was quantified using glucose as the standard. One unit of cellulase activity was defined as the amount of enzyme required to release 1 μmol of glucose equivalents per minute.
β-Xylosidase activity was determined by incubating 0.9 ml of freshly prepared solution of 4 mM p-nitrophenyl-β-d-xylopyranoside prepared in sodium citrate buffer (50 mM pH 4.5) and 0.1 ml of diluted enzyme solution at 50 °C for 30 min [11]. The reaction was terminated by the addition of 1 ml of 2 M sodium carbonate solution. The color developed was read at 410 nm. One unit of β-xylosidase activity was defined as the amount of enzyme that catalyses the formation of 1 μmol of p-nitrophenol per minute under assay conditions.
Determination of Protein
Soluble protein was estimated using bovine serum albumin as the standard [12]. Five milliliters of alkaline copper reagent was added to 1 ml of properly diluted enzyme. After 10-min incubation, 0.5 ml Folin’s phenol reagent was added. After 30-min incubation in the dark, absorbance was measured at 660 nm.
Purification of Xylanase I and II
All purification steps were carried out at 4 °C. After incubation, the enzyme was extracted using sodium citrate buffer (50 mM, pH 5.3). The fermented slurry was filtered through cheesecloth, and the filtrate was centrifuged at 10,000 × g for 20 min at 4 °C. The supernatant was filtered through Whatman no. 1 filter paper, and the filtrate was treated with ammonium sulfate (30–90% w/v), and the protein precipitated was separated (10,000 × g for 30 min). The precipitate was resuspended in 50 mM sodium acetate buffer, pH 5.5, and dialyzed against the same buffer. The dialyzed sample was concentrated by polyethylene glycol (PEG) 20,000.
Concentrated protein was subjected to gel filtration on a Sephadex G-200 column (2.5 × 70 cm) equilibrated with 50 mM sodium acetate buffer, pH 5.5. Proteins were eluted using the same buffer at a flow rate of 20 ml h−1. Fractions of 5.0 ml were collected, and those showing highest xylanase activity were pooled and concentrated by PEG 20,000. The protein in each sample was determined by taking absorbance at 280 nm.
Fractions containing xylanase activity were pooled, concentrated, and loaded on to an anion exchange column (DEAE Sephadex A-50, 3.5 × 50 cm) equilibrated with 50 mM sodium acetate buffer, pH 5.5. Unbound proteins were eluted in equilibrating buffer, while bound proteins were eluted with a linear gradient of NaCl (0–1 M) in the same buffer. Flow rate was adjusted to 20 ml h−1, and fractions of 5.0 ml were collected. Fractions eluted in the NaCl gradient were dialyzed against 50 mM sodium acetate buffer, pH 5.5. Fractions showing xylanase activity were pooled and concentrated and stored at 4 °C for further studies.
Electrophoresis
Purified enzymes were subjected to native and sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) in 12.5% acrylamide gels [13]. Protein bands were stained with Coomassie brilliant blue G-250.
Biochemical Characterization
Effect of Temperature on the Activity and Stability of Xylanase I and II
Optimum temperature of xylanase I and II was determined by varying reaction temperature from 30 to 80 °C. Thermal stability of xylanase I and II was determined by monitoring residual activity after incubating enzymes at temperatures ranging from 30 to 80 °C for 4 h. Aliquots were taken after 1-, 2-, 3-, and 4-h intervals, and residual xylanase activity was determined.
Effect of pH on the Activity and Stability of Xylanase I and II
The pH optima were determined by monitoring activities of xylanase I and II in pH range 2–12. Various buffers used were 50 mM glycine–HCl (2–3), 50 mM sodium acetate (4–6), 50 mM Tris–HCl (7–9), and 50 mM glycine–NaOH (10–12). To monitor pH stabilities, xylanases were diluted in respective buffers and incubated at 30 °C. Aliquots were taken after 1-, 2-, 3-, and 4-h intervals, and residual xylanase activity was determined.
Kinetic Parameters
Kinetic parameters (K m and V max) were determined by incubating xylanase I and II with various concentrations of birch wood xylan (4 to 10 mg ml−1) and oat spelt xylan (2 to 10 mg ml−1) solutions prepared in 50 mM sodium citrate buffer, pH 5.3, and enzyme activity was estimated. K m and V max were calculated by linear regression from Lineweaver–Burk plots.
Substrate Specificity of Xylanase I and II
Specificity of xylanase I and II against birch wood xylan, oat spelt xylan, carboxymethyl cellulose, pectin, and starch were tested. Substrates (1% w/v) were prepared in 50 mM sodium citrate buffer, pH 5.3.
Effect of Metal Ions on the Activity of Xylanase I and II
Residual activity of xylanase I and II was assayed in presence of Al3+, Ba2+, Ca2+, Cu2+, Co2+, Cd2+, Fe3+, Mn2+, Na+, Hg2+, Ni2+, Pb2+, and Zn2+ at 10-, 20-, and 30-mM concentrations after incubating in respective metal ion solutions for 1 h at 30 °C.
Effect of Additives on the Activity of Xylanase I and II
Effect of various additives such as N-bromosuccinamide, dithiothreitol (DTT), β-mercaptoethanol, l-tryptophan, l-cysteine, sodium lauryl sulfate (SLS), and ethylenediamine tetraacetate (EDTA) was tested at 10-, 20-, and 30-mM concentrations. Residual activity was determined after incubating xylanase I and II in respective solutions for 1 h at 30 °C.
Total Carbohydrate Content of Xylanase I and II
Total carbohydrate content of xylanase I and II was determined using glucose as standard [14]. 1 ml of phenol solution was added to 1 ml of enzyme solution followed by the addition of 5 ml 96% sulfuric acid. After 10 min, the mixture was incubated at 25–30 °C for 20 min. The color developed was read at 490 nm.
Results and Discussion
Purification of Xylanase I and II
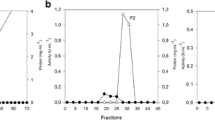
Summary of purification procedures of xylanase I and II are given in Table 1. Culture filtrate of A.sydowii SBS 45 grown on wheat bran exhibited high level of xylanolytic activity (37,440.88 U) with a total cellulase activity of 469.39 U and total β-xylosidase activity of 42.63 U. Total protein was 2,996.65 mg. Ammonium sulfate fraction (30–90%) when subjected to gel filtration chromatography on Sephadex G-200 resulted in one peak of xylanase activity (Fig. 1). Figure 2 shows profile of elution of xylanase I and II on DEAE Sephadex A-50. Two peaks of xylanase activity were obtained, one peak eluted in buffer, xylanase I, and the second peak eluted in the NaCl gradient (0.5–0.7 M), xylanase II. Specific activities of xylanase I and II were 1166.67 and 966.67, respectively. The production of multienzyme system of xylanases, in which each enzyme has a specific function, is one strategy for a microorganism to achieve effective hydrolysis of xylan [4].
Elution profile of xylanase I and II from Aspergillus sydowii SBS 45 on DEAE-Sephadex A-50. Equilibrating buffer was Na acetate, pH 5.5.The enzymes were eluted with a linear NaCl gradient in the same buffer. The flow rate and fraction size were 20 ml h−1 and 5.0 ml, respectively (circles, xylanase activity, U ml−1; triangles, protein absorbance at 280 nm)
The two purified enzyme preparations appeared homogenous, as they migrated as a single protein band under a nondenaturing condition (native PAGE) as well as a denaturing condition (SDS-PAGE; Fig. 3). The apparent molecular mass for xylanase I was 20.1 kDa and xylanase II was 43 kDa. Previously, it was reported that microbial xylanases are single-subunit proteins with molecular masses in the range of 8–145 kDa [15]. Isozymes with different molecular masses were reported by Segura and Fevre [16] from rumen fungus Neocallimastix frontalis where molecular masses of xylanase I and II were 45 and 70 kDa, respectively. Rana et al. [17] purified xylanase I and II from Humicola grisea va. thermoidea having molecular masses of 95 and 13 kDa, respectively.
SDS-PAGE (12.5%) of xylanase I and II from Aspergillus sydowii SBS 45. Lane 1,Crude culture filtrate; 2, ammonium sulfate precipitate; 3, gel filtration fraction; 4, xylanase I; 5, xylanase II; 6, molecular weight markers: myosin (205 kDa), phosphorylase b (97.4 kDa), bovine serum albumin (66 kDa), ovalbumin (43 kDa), carbonic anhydrase (29 kDa), soyabean trypsin inhibitor (20.1 kDa), lysozyme (14.3 kDa), aprotinin (6.5 kDa), and insulin (3 kDa)
Effect of Temperature on the Activity and Stability of Xylanase I and II
Optimum temperature of xylanase I and II was found to be 50 °C (Fig. 4). At 40 and 60 °C, xylanase I retained 50.45 and 99.4% of normal activity, while xylanase II retained 87.64 and 25.95% of normal activity.
After 4 h of incubation, both xylanase I and II retained 100% activity at 30 °C. It was observed that xylanase I retained 93.11% activity (Fig. 5) and xylanase II retained 94.16% activity after 4 h of incubation at 40 °C (Fig. 6). Xylanase I exhibited more thermal stability than xylanase II. Previous reports show that xylanase isolated from members of the genus Aspergillus showed maximal activity at 50 °C [18]. Kolenova et al. [19] purified Xyn B and Xyn C, which were most active at 50 °C from Schizophyllum commune. Sandrim et al. [20] purified xylanase I and xylanase II from Aspergillus caespitosus, and both xylanase exhibited the same optimum temperature of 50–55 °C.
Effect of temperature on the stability of xylanase I from Aspergillus sydowii SBS 45. Enzyme was incubated at 30–80 °C for 4 h. Aliquots were withdrawn after each hour up to 4 h, and residual activity was measured (squares, relative activity after 1 h [%]; circles, relative activity after 2 h [%]; triangles, relative activity after 3 h [%]; inverted triangles, relative activity after 4 h [%])
Effect of temperature on the stability of xylanase II from Aspergillus sydowii SBS 45. Enzyme was incubated at 30–80 °C for 4 h. Aliquots were withdrawn after each hour up to 4 h, and residual activity was measured (squares, relative activity after 1 h [%]; circles, relative activity after 2 h [%]; triangles, relative activity after 3 h [%];inverted triangles, relative activity after 4 h [%])
Effect of pH on the Activity and Stability of Xylanase I and II
The optimum activity of xylanase I and II was found to be at pH 10.0 in 50 mM glycine–NaOH buffer (Fig. 7). Xylanase I retained 100% activity at pH range 6–11, while xylanase II retained 100% activity at pH 3 and at a pH range of 6–11.
After 1 h, at 30 °C, 100% activity was retained by xylanase I at 4–11 pH range (Fig. 8), and xylanase II retained 100% activity at 5–11 pH range (Fig. 9). After 4 h, at 30 °C, more than 65% activity was retained by xylanase I at pH range 4–10, and more than 95% activity was retained by xylanase II at pH range 5–10.
Effect of pH on the stability of xylanase I from Aspergillus sydowii SBS 45. Xylanase I was incubated in 50 mM glycine–HCl (2–3), 50 mM sodium acetate (4–6), 50 mM Tris–HCl (7–9), and 50 mM glycine–NaOH (10–12) at 30 °C. Residual activity was measured after each hour up to 4 h (squares, relative activity after 1 h [%]; circles, relative activity after 2 h [%]; triangles, relative activity after 3 h [%]; inverted triangles, relative activity after 4 h [%])
Effect of pH on the stability of xylanase II from Aspergillus sydowii SBS 45. Xylanase II was incubated in 50 mM glycine–HCl (2–3), 50mM sodium acetate (4–6), 50 mM Tris–HCl (7–9), and 50 mM glycine–NaOH (10–12) at 30 °C. Residual activity was measured after each hour up to 4 h (squares, relative activity after 1 h [%]; circles, relative activity after 2 h [%]; triangles, relative activity after 3 h [%];inverted triangles, relative activity after 4 h [%])
Both xylanase I and II showed a broad pH activity profile. Mathrani and Ahring [21] reported thermophilic and alkaliphilic xylanase from Dictyoglomus isolates retained 100% activity at pH range 5.5–9.0. Xylanase isolated from Cephalosporium shown maximum activity at pH range 6.5–9.0 [22]. An alkali-tolerant xylanase from Aspergillus fischeri was reported to exhibit remarkable stability at pH 9.0 [23].
Hakulinen et al. [24] reported that stability at extreme pH values appeared to be characterized by a spatially biased distribution of charged amino acid residues. Enzymes stable in alkaline conditions were typically characterized by a decreased number of acidic residues and an increased number of arginines. Furthermore, a recent comparative structural study of family 11 enzymes suggests a correlation between pH activity/stability and the number of salt bridges, with acidophilic xylanases having much less of these interactions than their alkaliphilic homologs.
Kinetic Parameters
The K m value of xylanase I and II for birch wood xylan was 3.18 and 6.51 mg ml−1 and for oat spelt xylan was 6.45 and 7.69 mg ml−1, respectively. Bansod et al. [22] reported that K m values of xylanases lie in 0.5–9.6 mg ml−1 range. V max of xylanase I and II for birch wood xylan was 1,191 and 1,587 μmol min−1 mg−1 protein, respectively. V max of xylanase I and II for oat spelt xylan was 2,604 and 2,381 μmol min−1 mg−1 protein, respectively. Similar K m and V max values were reported in fungi like A. caespitosus [20], H. grisea var. thermoidea [25], Thermomyces lanuginosus CBS 288.54 [26], and T. lanuginosus SSBP [27].
Substrate Specificity of Xylanase I and II
Xylanase I and II showed strong specificity toward birch wood and oat spelt xylan (Table 2). No activity was detected against carboxy methyl cellulose, pectin, and starch. Magnuson and Crawford [28] purified a xylanase from Streptomyces viridosporus 77A, which shown strong specificity toward xylan preparations. No reaction was observed with other substrates tested. Similar results were reported in Thermoascus aurantiacus [29], Aeromonas caviae ME-1 [30], and Pencillium chrysogenum [31].
Effect of Metal Ions on the Activity of Xylanase I and II
At 10-mM concentration, Al3+, Ba2+, Ca2+, Na+, and Zn2+ enhanced the activity of xylanase I and II (Table 3), while at 20-mM concentration, Al3+ and Ba2+ retained 100% activity. At 30 mM, all metal ions were inhibitory particularly Pb2+.
Stimulatory effect of Al3+, Ba2+, Ca2+, and Na+ was reported in A. caespitosus [20], Streptomyces exfoliatus [32], and Talaromyces byssochlamydoides YH-50 [33]. Gessesse [34] reported the enhancement effect of Na+, Ca2+, and Zn2+ and the inhibitory effect of Pb2+ on xylanase isolated from an alkaliphilic Bacillus sp.
Effect of Additives on the Activity of Xylanase I and II
Among additives, l-tryptophan stimulated the activity of xylanase I and II at 10-, 20-, and 30-mM concentrations, while β-mercaptoethanol and DTT stimulated the activity of xylanase I at all concentrations (Table 4). Ximenes et al. [35] purified a xylanase from Acrophialophora nainiana and was activated by thiol containing reagents like l-cysteine and β-mercaptoethanol and l-tryptophan, indicating the presence of cysteine and tryptophan residues at the active site. Kang et al. [36] also reported the involvement of cysteine and tryptophan residues in the maintenance of tertiary structure of the active sites in xylanases.
SLS at 30-mM concentration inhibited xylanase I activity by 99% and enhanced xylanase II activity by 96.33%. Bastawde [37] reported that a concentration of 5 × 10–3 M SDS was found to be inhibitory for three different endoxylanases from Streptomyces sp. 3137, while Yoshioka et al. [33] reported both stimulatory and inhibitory activities of SDS in T. byssochlamydoides YH-50.
N-Bromosuccinamide, an amino acid modifier, inhibited activities of xylanase I and II by 78.61 and 79.95%, and EDTA inhibited by 94.08 and 71.13%, respectively, at 30-mM concentration. Belancic et al. [38] studied the effect of various metal ions and additives (1 mM) on xylanases from Pencillium purpurogenum and found that EDTA inhibits 30–50% of xylanase activity, while N-bromosuccinamide and SDS inactivated both enzymes completely.
Total Carbohydrate Content of Xylanase I and II
Both xylanases appeared to be glycoproteins. Xylanase I and II had 9.75 and 16.10 μg ml−1 glucose residues, respectively. Thus, the carbohydrate content of xylanase I and II were 40.63 and 53.67%, respectively. The glycoprotein content of endoxylanases from Aspergillus fumigatus was between 46.4 and 68% [39].
The occurrence of glycosylated enzymes is a common phenomenon among eukaryotic xylanases [2]. Carbohydrate moieties are covalently linked with protein as dissociable complexes with various xylanases [40]. Glycosylation has been implicated in the stabilization of glycanases against extreme environments [41].
Conclusions
Two xylanases I and II produced by A. sydowii SBS 45 were purified to homogeneity. Both enzymes were well characterized. They were low-molecular-weight proteins—especially xylanase I. They exhibited a broad range of pH activities and stabilities and showed great tolerances to metal ions. Both enzymes were highly specific to substrate xylan. Low molecular weights and high pH stabilities make them ideal for biobleaching of kraft pulps.
References
Joseleau, J. P., Comtat, J., & Ruel, K. (1992). In J. Visser, G. Beldman, M. A. Kusters-van Someren, & A. G. J. Voragen (Eds.), Xylans and xylanases (pp. 1–15). Amsterdam: Elsevier.
Kulkarni, N., Shendye, A., & Rao, M. (1999). FEMS Microbiology Reviews, 23, 411–456.
Collins, T., Gerday, C., & Feller, G. (2005). FEMS Microbiology Reviews, 29, 3–23.
Beg, Q. K., Kapoor, M., Mahajan, L., & Hoondal, G. S. (2001). Applied Microbiology Biotechnology, 56, 326–338.
Bajpai, P. (1999). Biotechnology Progress, 15, 147–157.
Maat, J., Roza, M., Verbakel, J., Stam, H., daSilra, M. J. S., Egmond, M. R., et al. (1992). In J. Visser, G. Beldman, M. A. Kusters-van Someren, & A. G. J. Voragen (Eds.), Xylans and xylanases (pp. 349–360). Amsterdam: Elsevier.
Bedford, M. R., & Classen, H. L. (1992). In J. Visser, G. Beldman, M. A. Kusters-van Someren, & A. G. J. Voragen (Eds.), Xylans and xylanases (pp. 361–370). Amsterdam: Elsevier.
Bailey, M. J., Biely, P., & Poutanen, K. (1992). Journal of Biotechnology, 23, 257–270.
Miller, G. L. (1959). Analytical Chemistry, 31, 426–428.
Ghose, T. K. (1987). Pure and Applied Chemistry, 59(2), 257–268.
Lachke, A. H. (1988). Methods in Enzymology, 160, 679–684.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Journal of Biological Chemistry, 193, 265–275.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Analytical Chemistry, 26, 350.
Sunna, A., & Antranikian, G. (1997). Critical Reviews in Biotechnology, 17, 39–67.
Segura, B. G., & Fevre, M. (1993). Applied and Environmental Microbiology, 59, 3654–3660.
Rana, B. K., Johri, B. N., & Thakur, I. S. (1996). World Journal of Microbiology & Biotechnology, 12, 12–15.
Duarate, J. C., & Costa-Ferreira, M. (1994). FEMS Microbiology Reviews, 13, 377–386.
Kolenova, K., Vrsanska, M., & Biely, P. (2005). Enzyme and Microbial Technology, 36(7), 903–910.
Sandrim, V. C., Rizzatti, A. C. S., Terenzi, H. F., Jorge, J. A., Milagres, A. M. F., & Polizeli, M. L. T. M. (2005). Process Biochemistry, 40, 1823–1828.
Mathrani, I. M., & Ahring, B. K. (1992). Applied Microbiology Biotechnology, 38, 23–27.
Bansod, S. M., Datta, C. M., Srinivasan, M. C., & Rele, M. V. (1993). Biotechnology Letters, 15, 965–970.
Raj, K. C., & Chandra, T. S. (1996). Microbiology Letters, 145, 457–461.
Hakulinen, N., Turunen, O., Jamis, J., Leisola, M., & Rouvinen, J. (2003). European Journal of Biochemistry, 270, 1399–1412.
Monti, R., Terenzi, H. F., & Jorge, J. A. (1991). Canadian Journal of Microbiology, 37, 675–681.
Li, X. T., Jiang, Z. Q., Li, L. T., Yang, S. Q., Feng, W. Y., Fan, J. Y., et al. (2005). Bioresource Technology, 96(12), 1370–1379.
Lin, J., Ndlovu, L. M., Singh, S., & Pillay, B. (1999). Biotechnology and Applied Biochemistry, 30, 73–79.
Magnuson, T. S., & Crawford, D. L. (1997). Enzyme and Microbial Technology, 21, 160–164.
Tan, L. U. L., Mayers, P., & Saddler, J. N. (1987). Canadian Journal Microbiology, 33, 689–692.
Kubata, B. K., Suzuki, T., Horitsu, H., Kawai, K., & Takamizawa, K. (1994). Applied and Environmental Microbiology, 60(2), 531–535.
Haas, H., Herfurth, E., Stoffler, G., & Rendl, B. (1992). Acta Biochimica et Biophysica Sinica, 1117, 279–286.
Sreenath, H. K., & Joseph, R. (1982). Folia Microbiologica, 27, 107–115.
Yoshioka, H., Nagato, N., Chavanich, S., Nilubol, N., & Hayashida, S. (1981). Agricultural Biological Chemistry, 45(11), 2425–2432.
Gessesse, A. (1998). Applied and Environmental Microbiology, 64(9), 3533–3535.
Ximenes, F. A., Sousa, M. V., Puls, J., Silva, F. G., & Filho, E. X. F. (1999). Current Microbiology, 38, 18–21.
Kang, M. K., Maeng, P. J., & Rhee, Y. H. (1996). Applied and Environmental Microbiology, 62(9), 3480–3482.
Bastawde, K. B. (1992). World Journal Microbiology & Biotechnology, 8, 353–368.
Belancic, A., Scarpa, J., Peirano, A., Diaz, R., Steiner, J., & Eyzaguirre, J. (1995). Journal of Biotechnology, 41, 71–79.
Flannigan, B., & Sellars, P. N. (1977). Transaction of the British Mycological Society, 69, 316–317.
Wong, K. K. Y., Tan, L. U. L., & Saddler, J. N. (1988). Microbiological. Reviews, 52, 305–317.
Merivuori, H., Sands, J. A., & Montenecourt, B. S. (1985). Applied Microbiology and Biotechnology, 23, 60–66.
Acknowledgments
We acknowledge the Council of Scientific and Industrial Research, New Delhi, India, for awarding Senior Research Fellowship to Suprabha G. Nair.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nair, S.G., Sindhu, R. & Shashidhar, S. Purification and Biochemical Characterization of Two Xylanases from Aspergillus sydowii SBS 45. Appl Biochem Biotechnol 149, 229–243 (2008). https://doi.org/10.1007/s12010-007-8108-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-8108-9