Abstract
Cellulase distribution between residual substrate and supernatant in the process of enzymatic hydrolysis of steam-exploded wheat straw was investigated. Subsequently, a novel stepwise recovery strategy with three successive steps was adopted to recover cellulase adsorbed to the residual substrate. The results showed that cellulase protein in the supernatant increased as the hydrolysis time increased. When hydrolysis ended, the cellulase remaining on the residual substrate accounted for 33–42% of the original added cellulase according to the different cellulase loading. To obtain the maximum cellulase recovery rate, the residual substrate was dealt with in three successive steps: washed with sodium acetate buffer (step 1), shaken with sodium acetate buffer (step 2), and then treated with 0.0015 mol/L, pH 10 Ca(OH)2 (step 3). The total cellulase protein recovered by the three steps reached 96.70–98.14%. The enzyme activity of cellulase recovered by the first two steps was kept well. The ratios of the specific activity between the recovered cellulase and the original were 89–96%, which was by far higher than that using step 3 (the value was 48% ∼ 56%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The cellulase is the main cost component in the bioconversion process of lignocellulosics [1, 2]. A reduction in the cost of the cellulase is, therefore, necessary to make the process more economically attractive. One avenue that has been suggested as a means of lowering the cost is the recovery of the cellulase.
Many reporters have defined the first step in cellulose hydrolysis as adsorption of cellulase onto the substrate. As cellulose hydrolysis proceeds, a portion of the adsorbed cellulase is gradually released into the supernatant [3, 4]. In the process of hydrolysis, the cellulase is distributed between the residual substrate and the supernatant [5]. Thus, cellulase can be recovered from either phase. For the cellulase present in the supernatant, the simplest recovery method is re-adsorption onto fresh substrate [6]. It is difficult to recover the portion of cellulase on the residual substrate, which accounts for 40–90%, according to temperature, ratio of enzyme and substrate, time of hydrolysis [5, 7]. Nowadays, one-step strategies are adopted by many researchers to recover the cellulase remaining adsorbed to the residue substrate by using detergents [8], alkali [9], glycerol, urea [10], and phosphate or acetate buffers of various pH [11]. Although easy to perform and also could acquire the maximum recovery rate of cellulase protein, these one-step recovery methods tend to inactivate the recovered cellulase by using the high concentration of desorbents or severe operation conditions. To date, the best result has been obtained by Otter et al. [9] using a one-step method with alkali and Tween-80 to recover only about 65% of the adsorbed Avicelase activity.
The present study firstly investigated the distribution of cellulase between the residual substrate and supernatant during the course of enzymatic hydrolysis of steam-exploded wheat straw. Then, a stepwise strategy using three successive steps to recover the cellulase remaining adsorbed to the residual substrate was investigated.
Materials and Methods
Steam-exploded Wheat Straw and Cellulase
Steam-exploded wheat straw was prepared by putting the wheat straw (3–4 cm, containing 15% water) into the steam-exploded vessel at 1.5 MPa for 10 min [12]. The composition of steam-exploded wheat straw, expressed as the average of the three replicate determinations and expressed in terms of weight percent, was as follows: cellulose, 38.65%; xylan, 13.59%; lignin, 23.19%. Cellulase from Penicillium decumbens JUA10 was used in this work [13].
Hydrolysis Procedure
Hydrolysis was carried out at a substrate concentration of 1/6 (dry weight [DW]/v) in 50 mM sodium acetate buffer (pH 4.8) with cellulase dose of 5, 15, and 50 IU filter paper activity (FPA)/(g substrate), respectively. The hydrolysis system was incubated at 50°C with agitation at 180 rpm. The whole hydrolysis time was 168 h for 15 and 50 IU FPA/(g substrate) systems and 288 h for 5 IU FPA/(g substrate) system. Twenty-five IU FPA was supplemented to the system of 5 IU FPA/(g substrate) at the168th hour to completely hydrolyze the cellulose.
Samples were removed at different times, chilled with ice to stop the hydrolysis reaction, followed by filtration to remove the insoluble materials (kept in 0°C). The reducing sugar concentration in the supernatant was determined by DNS method [14]. The percent conversion of the substrate was calculated from the reducing sugar content, as a percentage of the theoretical reducing sugar available in the substrate. Total protein in the supernatant was measured by the Bradford Assay using bovine serum albumin as the standard. The optical density (OD) of 595 nm was detected. The amount of cellulase protein adsorbed to the residual substrate was calculated by subtracting the amount of protein in the supernatant from the total added protein.
To investigate the loss of cellulase activity during hydrolysis, the system without substrate and other conditions as described above was set as the control.
Desorption Procedure of Cellulase Adsorbed to the Residual Substrate
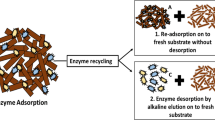
The residual substrates obtained from different systems with cellulase doses of 5, 15, and 50 IU FPA/(g substrate) were dealt with using the three successive steps as shown in Fig. 1. Three replicates were set for every recovery step.
-
Step 1
Washed by sodium acetate buffer (100 mM, pH 4.8, preheated to 50°C);
-
Step 2
Shaken with sodium acetate buffer (100 mM, pH 4.8) at 50°C for 160 min;
-
Step 3
Treated with Ca(OH)2 (0.0015 mol/L, pH 10.0, 30 mL) at 50°C for 5 min.
The ratio of solid to liquid in the three recovery steps above was 1:5.
The liquid solutions obtained from the three steps were assayed in terms of protein content and FPA.
Cellulase Activity Determination
FPA, CMCase, and cellobiase were measured as described by Ghose [15]. One international unit (IU) of activity was defined as the amount of enzyme required to produce one micromole reducing sugar per minute.
Results and Discussion
Cellulase Adsorption During Hydrolysis
In different systems with cellulase doses of 5, 15, and 50 IU FPA/(g substrate), the proportion of the added cellulase protein present in the supernatant during the enzymatic hydrolysis of steam-exploded wheat straw was determined (Fig. 2). For the system of 5 IU FPA/(g substrate), the cellulose in the substrate was not hydrolyzed completely after 168 h. Cellulase of 25 IU FPA was, thus, supplemented and the reaction time was prolonged to 288 h. It could be seen from Fig. 2 that the adsorbed protein was gradually released into the supernatant as the hydrolysis time increased. The quantity of protein reached the maximum when the hydrolysis reaction ended. However, the peak times were different for different cellulase doses. About 90% of the added protein was present in the supernatant when the hydrolysis times were extended to 96 and 20 hours for the systems with cellulase doses of 15 and 50 IU FPA/(g substrate), respectively. In terms of the proportion of cellulase activity adsorbed to the residual to the original added, the values were 67, 58, and 64% for the systems with cellulase doses at 5, 15, and 50 IU FPA/(g substrate). It should be noted that for the system of 5 IU FPA/(g substrate), the complemented cellulase was included when calculating the proportion.
In our study, except cellulase dose, other conditions such as substrate, cellulase preparation, and hydrolysis temperature and so on were consistent, and the components of the residual were almost similar (data not shown). Thus, it could be easily inferred that adsorbing action of cellulase protein to the residual substrate should has no difference. Consequently, the cellulase quantity adsorbed to the residual should be the same. Whereas, the results showed that the absolute quantity of cellulase adsorbed to the residual substrate were 27.67, 48.33, and 69.36 mg for cellulase doses at 5, 15, and 50 IU FPA/(g substrate), respectively. To recover the remaining cellulase associated with the residual substrate, a further study was performed.
Desorption of Cellulase Adsorbed to the Residual Substrate
Three successive steps, i.e., washed with sodium acetate buffer (step 1) shaken with the buffer (step 2), and then treated with Ca(OH)2 (step 3), were adopted to desorb the cellulase adsorbed to the residual substrate.
In step 1, more cellulase protein was released as the volume of sodium acetate buffer increased. When using sodium acetate buffer at a volume of 60 mL, the desorption rate reached the maximum with 6.79% for 5 IU FPA/(g substrate) and 22.41% for the for 15 IU FPA/(g substrate). For the system of 50 IU FPA/(g substrate), the maximum desorption rate was achieved at a buffer volume of 80 mL sodium acetate, and the value is 40.68% (Fig. 3).
After being washed by sodium acetate buffer, the residual substrates were further mixed by sodium acetate buffer under shaking conditions (step 2). The desorption rate fluctuated slightly during the shaking process. The dynamic balances of the adsorption-desorption of cellulase were obtained in a short time. For the systems with cellulase doses of 5 and 15 IU FPA/(g substrate), the recovery rates of cellulase protein reached at buffer volume of 40 mL. At a buffer volume of 80 mL, the recovery rates of cellulase protein reached the peak for the system of 50 IU FPA/(g substrate) (Fig. 4).
By subtracting the total protein recovered through the two steps above from the total added protein, some cellulase protein still remained on the residual substrate. The residual substrates were then further treated with Ca(OH)2 (0.0015 mol/L, pH 10, 30 mL, 5 min) so as to recover the remaining portion of cellulase.
Table 1 shows that cellulase adsorbed to the residual substrate after hydrolysis could be gradually released by three successive steps. The cellulose protein recovery rates were significantly different among three successive steps, except for between steps 1 and 2 when cellulase dose of 5 IU FPA/(g substrate). For the system of 5 IU FPA/(g substrate), the cellulase protein recovery rate was 6.8–7.2% by each of the first two steps, and accounted for 14% of the total adsorbed cellulase. The cellulase protein recovery rate for the system of 15 IU FPA/(g substrate) and 50 IU FPA/(g substrate) by step 1 were about 1.2 times of those obtained by step 2. For these two systems, the recovered cellulase protein by the first two steps was about 49.1 and 80.1% of the total adsorbed cellulase. This indicated that through washing and shaking with sodium acetate buffer, about 20–86% of the total adsorbed cellulase still remained on the residual substrate. By calculation, the absolute quantities of the remaining cellulase for the systems of 5, 15, and 50 IU FPA/(g substrate) were almost the same, and equal to 13.88, 15.20, and 14.97 mg, respectively. This, to some extent, indicated that some of the cellulase adsorbed to the residual substrate was associated with weak bonds and could be washed by sodium acetate buffer. Some cellulase might be desorbed with slight external force, e.g., shear force caused by shaking. There was still a certain part of cellulase that was intensely attached to the residual substrate, whose absolute quantity could be determined by the characteristics of the residual substrate. This part of cellulase could be released only by more severe conditions. Thus, we used Ca(OH)2 solution in step 3. By this step, the cellulose protein recovery rates reached 84% and 48% in the systems of 5 and 15 IU FPA/(g substrate), and were significantly higher than those by the first two steps. Although it was much lower than that by the first two steps in the system of 50 IU FPA/(g substrate), only 17% of total cellulose protein was recovered by step 3. Through, the stepwise recovery strategy, a satisfactory cellulase protein recovery rate (97–98%) was obtained.
FPA of Recovered Cellulase
The requirement of economic feasibility of cellulase recovery strategy to keep the cellulase activity to the maximum extent was the requirement of economic feasibility of cellulase recovery strategy. By assaying the FPA of the recovered cellulase by the three successive steps above (Table 2), it was apparent that the specific cellulase activity recovered by the first step ranging from 0.93 to 0.96 and approximated to the original cellulase. The value decreased a little by the second step, whereas there was no significantly difference between the first and second steps. But for cellulase recovered by step 3, the specific activity had a significant drop of about 50% as that of the original.
Conclusions
After enzymatic hydrolysis of steam-exploded wheat straw, a portion of cellulase was found to adsorb to the residual substrate. The three successive steps used in this study could avoid the excessive loss of cellulase activity compared to one-step recovery method. This strategy indicates, to some extent, that the adsorption action of cellulase to substrates has many styles. For different adsorption style, it is necessary to develop the suitable recovery method to recover the higher cellulase activity.
In this study, the last step with the weak alkaline solution as described by Otter et al. [9] obtained a higher protein recovery rate, whereas the loss of the cellulase activity could reach about 50%. Further study on the alternative desorbent to desorb cellulase tightly combined to the residual with keeping the activity well will be carried out.
References
Wilke, C. R., Yang, R. D., & von Stocker, V. (1976). Biotechnology and Bioengineering Symposium, 6, 155–175.
Zacchi, G., Skoog, K., & Hahn-Hagerdal, B. (1988). Biotechnology and Bioengineering, 32, 460–466.
Lee, Y. H., & Fan, L. T. (1983). Biotechnology and Bioengineering, 25, 939–966.
Ooshima, H., Burns, D. S., & Converse, A. O. (1990). Biotechnology and Bioengineering, 36, 446–452.
Lee, D., Yu, A. H. C., & Saddler, J. N. (1995). Biotechnology and Bioengineering, 45, 328–336.
Ramos, L. P., Breuil, C., & Saddler, J. N. (1993). Enzyme and Microbial Technology, 15, 19–25.
Mandels, M., Kostick, J., & Parizel, R. (1971). J. Polymer Sci. Part C, 36, 445–459.
Rao, M., Seeta, R., & Deshpande, V. (1982). Biotechnology and Bioengineering, 25, 1863–1871.
Otter, D. E., Munro, P. A., Scott, G. K., & Geddes, R. (1989). Biotechnology and Bioengineering, 34, 291–298.
Deshpande, M. V., & Eriksson, K. E. (1984). Enzyme and Microbial Technology, 6, 338–340.
Sinitsyn, A. P., Bungay, M. L., Clesceri, L. S., & Bungay, H. R. (1983). Applied Biochemistry and Biotechnology, 8, 25–29.
Chen, H. Z. (1998). PhD thesis, Institute of Chemical Metallurgy, Chinese Academy of Sciences, Beijing, China.
Chen, H. Z., Xu, J., & Li, Z. H. (2005). Biochemical Engineering Journal, 23, 117–122.
Miller, G. L. (1959). Analytical Chemistry, 31(3), 426–428.
Ghose, T. K. (1987). Pure and Applied Chemistry, 59, 257–268.
Acknowledgments
Financial support for this study was provided by National Basic Research Program of China (2004CB719700) and the Knowledge Innovation Program of Chinese Academy of Sciences (KJCXZ-SW-206-2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, J., Chen, H. A Novel Stepwise Recovery Strategy of Cellulase Adsorbed to the Residual Substrate after Hydrolysis of Steam Exploded Wheat Straw. Appl Biochem Biotechnol 143, 93–100 (2007). https://doi.org/10.1007/s12010-007-0031-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-0031-6