Abstract
A novel UV-absorbing and free-radical-catching fluorine–silicone acrylic resin with 2-(4-benzoyl-3-hydroxyphenoxy) ethyl acrylate (BHEA), 2,2,6,6-tetramethyl-4-piperidyl methacrylate (TMPM), dodecafluoroheptyl methacrylate (DFMA) and 3-methacryloxypropyltrimethoxysilane (MPS) as functional monomers was successfully synthesized by solution copolymerization. Based on various investigations, our characterization results for the resin and its coating indicated that the resin exhibits high UV-absorbing and free-radical-catching performances, and the hydrophobicity of the varnish coating was promoted by the actions of fluorine and silicone. In addition, the weatherability improved because of the enduring triple protection of the UV absorbent (BHEA), free-radical-catching agent (TMPM), fluorine (DFMA) and silicone (MPS). After a 1000-h aging test, the color difference (∆E) and rate of loss of gloss (∆G) of varnish coatings were 2.96% and 62%, respectively, and the impact strength and flexibility of color paint coatings were 420 N cm and 2 mm, respectively. Moreover, the chemically bonded free-radical-catching agent (TMPM) showed a more enduring performance for producing nitroxides than the simple blend.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owning to the circulating action of absorbing UV light and releasing the absorbed energy in the form of heat, UV absorbents (UVAs) can effectively reduce the damage caused by UV light.1,2,3 Hindered amine light stabilizers (HALS) can catch free radicals and inhibit the photodegradation process of polymers.4,5,6,7 Therefore, UVAs and HALS, which have a high molecular weight, high-efficiency and multifunction,8,9 are widely used in cosmetics, plastic, and coatings to improve the weather resistance.10,11
Acrylic resin is very popular in coatings and has excellent mechanical properties,12,13 such as flexibility, impact strength, and adhesion ability. However, researchers in recent decades have struggled to improve its light stability for improved weather resistance in harsh environments. Usually, organic fluorine and silicone14,15,16,17,18 are incorporated into an acrylic resin by physical blending or chemical reactions as modifying agents due to their light stability, heat resistance, and low surface energy properties.19,20,21,22 Shin et al.23 synthesized a series of waterborne, fluorinated, polyurethane-acrylate hybrid emulsions with different perfluorodecyl acrylate (PFA) contents as antifouling coatings. They found that the optimum total acrylic monomer/PFA contents were approximately 30 wt%/9 wt% to obtain high-performance water/oil repellent coating materials. Park et al.24 prepared several kinds of UV-curable polyurethane acrylates containing fluorinated acrylic monomers (heptadecafluorodecyl methacrylate, PFA)/vinyltrimethoxysilane (VTMS). The results showed that the UV-curable polyurethane acrylate containing 6 wt% of PFA and 9 wt% of VTMS had strong potential as a coating material for transparent antifouling applications. Kumar et al.25 created UV-resistant and superhydrophobic coatings by introducing silica nanoparticles in a polyvinylidene fluoride matrix (PVDF). The static contact angle (CA) of the composite coating was 154° with a sliding angle of < 2° when the mass ratio of PVDF/SiO2 was 1:1. Meanwhile, both the CA and SA values did not change much before and after UV weathering, showing a high resistance to UV degradation.
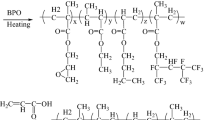
On the basis of the modification of acrylic resin with fluorine and silicone, the modified mechanism and materials should be innovated to further improve the weather resistance. The weathering of polymers is mainly caused by UV light and free radicals, which play a dominant role in the degradation of polymers.26 Therefore, if UV-absorbing monomers and free-radical-catching agents can be grafted onto the chain of an acrylic resin incorporated with organic fluorine and silicone, the UV resistance will be enhanced. In the structure in Fig. 1a, the chemically immobilized functional monomers provide lasting protection for the resin by absorbing UV light and scavenging attacking radicals.
In regard to acrylic resins modified by UV absorbers and HALS, Yaneff et al.27 added reactive UV absorbers (UVA) and hindered amine light stabilizers (HALS) into 2 K urethane coatings to decrease the depletion of stabilizers. The results revealed that the retention degree of the UV stabilizers significantly improved because the agents were covalently bonded to the coating, and the weather durability of the coatings was promoted. Yan et al.28 studied the effects of UV-329, GW-622 and Chinox 101 on the weather resistance of a thermoplastic polyurethane adhesive by physical blending. The results implied that the aging resistance could be greatly improved when the mass ratio of UV-329, GW-622 and Chinox 101 was 3:3:2. However, there are few studies on the modification of acrylic resin by simultaneously covalently reacting the resin with UV absorber, HALS, organic fluorine and silicone.
This paper reports the modification of an acrylic resin with BHEA, TMPM, DFMA, and MPS modifying agents by a solution copolymerization. The chemical structure and properties of the resin were characterized by Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), ultraviolet absorption spectroscopy (UV) and electron paramagnetic resonance analysis (EPR). In addition, the coatings prepared by the resin were investigated by a Xenon lamp accelerated aging test, and their water contact angle (CA), impact strength, and flexibility were determined.
Experimental
Materials
Methyl methacrylate (MMA, 99%), n-butyl acrylate (BA, 99%), styrene (St, 99%), acrylic acid (AA, 99%), hydroxyethyl methacrylate (HEMA, 99.5%), 3-methacryloxypropyltrimethoxysilane (MPS, 99%), dodecafluoroheptyl methacrylate (DFMA, 99%), propylene glycol methyl ether acetate (PMA, 99%), 2,2,6,6-tetramethyl-4-piperidyl methacrylate (TMPM, 99%), xylene (PX, 99%), 2-(4-benzoyl-3-hydroxyphenoxy)ethyl acrylate (BHEA, 98%) and benzoyl peroxide (BPO, 99%) were purchased from Guangdong Weng Jiang Chemical Reagent Co., Ltd., and used without further purification.
Preparation of the resins and coatings
Resin synthesis
Hydroxyl acrylic resin (AS-1), fluorine–silicone acrylic resin (AS-2), and UV-absorbing and free-radical-catching fluorine–silicone acrylic resins (AS-3 and AS-4) were prepared. The formulations of the resins are shown in Table 1.
All the reactions were carried out in a flask equipped with a condenser pipe, magnetic stirrer, and thermometer under a nitrogen atmosphere. Additionally, a peristaltic pump was used to control the drop speed in the synthesis. AS-1, AS-2 and AS-3 were synthesized as follows. First, an appropriate amount of a mixed solution of PX and PMA was poured into the four-necked flask and refluxed at 130°C for 30 min; second, the mixture of monomers and 1.6 g BPO was added dropwise into the flask within 3–4 h under magnetic stirring. The copolymerization continued for another 1 h after the dropwise addition ceased; finally, the rest of the BPO and solvents were well blended and added dropwise into the flask over 40 min. Then, the reaction continued for approximately 2 h until colorless or light-yellow products with a certain viscosity appeared. AS-4 was prepared by the same method except BHEA and TMPM were incorporated into the resin by physical blending at the end of the synthesis.
Coating preparation
Resins, solvents and other additives were well mixed and acted as component A of the varnish; a 60% solution of polyisocyanate (TPA-90SB) diluted by anhydrous butyl acetate was component B (Table 2).
A homogeneous mixture of components A and B was coated on a low-carbon steel plate with a thickness of 30 µm, and then the coatings were completely dried in a baking oven at 80°C for 3 h. The coatings prepared using AS-1, AS-2, AS-3 and AS-4 were designated C-1, C-2, C-3 and C-4, respectively.
In addition to varnishes, several kinds of color paints containing aluminum powder and zinc chrome yellow were prepared (Table 3). All the ingredients of component A were dispersed in a jar for 15 min and then ground to less than 30 µm. The coatings prepared with AS-1, AS-2 and AS-3 were labeled P-1, P-2 and P-3, respectively.
Characterizations
FTIR: Fourier transform infrared spectra in the range from 400 to 4000 cm−1 were obtained by a Nicolet 380 Fourier transform infrared spectrometer. The samples were prepared by pouring the resin solutions into KBr wafers to form films and drying them with an infrared lamp.
XPS: X-ray photoelectron spectra of the resins were recorded by an American PHI5000C ESCA System with a high voltage of 14.0 kV, power of 250 watts, and vacuum less than 10−8 torr. To ensure the results were not influenced by other factors, the resin films were created without any other additives or a curing agent.
UV: Ultraviolet absorption properties were tested by an HP8453 ultraviolet spectrophotometric instrument (American). The UV spectroscopy samples were created by painting the resin solutions onto glass slides to form thin films without adding curing agents or other additives.
EPR: The EPR spectra of the resins and varnish coatings were measured on a Bruker A200 (Germany) instrument under the conditions that the modulation FR was 9.8 GHz, the magnetic field intensity was 3500 ± 50 G, and the mold was 6.3 G.
CA: The water contact angles of the varnish coatings were measured using a DSA100 optical contact angle tester (Germany) at room temperature. Five measurements at different positions on the coatings were averaged to obtain the results for each specimen.
Weather resistance: The weatherability of the varnish coatings was analyzed by a xenon lamp accelerated aging test according to the national standard GB/T1865-2009.29 The irradiance at 340 nm was 1.5 W/m2 in the weathering device, and the average value from 290 to 900 nm was 550 W/m2. The temperature of the blackboard and air in the aging oven was 65 ± 3 and 38 ± 3°C, respectively.
Physical properties: The physical properties of the color paint coatings, such as the flexibility and impact strength, were investigated before and after the aging test according to GB/T1731-93 and GB/T1732-93, respectively.30 In addition, the surface morphology of the coatings was recorded using digital microscopy (RX-600X).
Results and discussion
FTIR analysis
To identify the chemical structure of the resins, FTIR spectra of AS-1, AS-2 and AS-3 were recorded, as shown in Fig. 2.
The absorption peaks at 2978 and 3450 cm−1 appeared in all the curves and were attributed to the stretching vibrations of C–H and –OH, respectively. The spectra of AS-2 and AS-3 were clearly different from the spectrum of AS-1, which was revealed by the broadening of the absorption band from 1000 to 1250 cm−1. This could be ascribed to the overlapping peaks of C–F at 1148 and 1246 cm−1 and Si–O at 1025 and 1092 cm−1, and these peaks proved that the organic fluorine and silicon monomers were bonded in AS-2 and AS-3. In addition, compared with the curve of AS-2, new absorption peaks appeared at 1550 and 1660 cm−1 in the curve of AS-3, which were attributed to the stretching vibrations of C–N from TMPM and C=O from BHEA, respectively. Moreover, there were no peaks of C=C at 1650 cm−1. This suggested that almost all the monomers were chemically bonded into the acrylic resin. These facts showed that the UV-absorbing and free-radical-catching fluorine-silicone acrylic resins were synthesized.
X-ray photoelectron spectroscopy (XPS) analysis
To prove the functional monomers reacted with the other acrylic monomers, the XPS spectra of AS-1, AS-2 and AS-3 are shown in Fig. 3.
The prominent peaks at 285 and 532 eV in all the curves corresponded to C 1s and O 1s, respectively. Compared with the curve of AS-1, binding energies at 688, 168 and 102 eV were detected in the spectra of AS-2 and AS-3 and corresponded to F 1s, Si 2s, and Si 2p. This showed that organic fluorine and silicone were incorporated into the resins. Moreover, the new peak at 400 eV in the spectrum of AS-3 was associated with the N 1s from the free-radical-catching agent (TMPM). These testing results indicated that the functional monomers were bonded to the chain of the acrylic resin.
Ultraviolet absorption properties
The UV-absorbing performance of the resins is very important to their weather resistance. The UV-absorption spectra of AS-1, AS-2 and AS-3 are shown in Fig. 4.
AS-3 had a prominent absorption band from 290 to 380 nm, while AS-1 and AS-2 showed no absorption peaks in the whole ultraviolet region from 200 to 400 nm. This could be explained by the fact that AS-3 was modified by the UV absorbent (BHEA) and exhibited a high UV-absorption property. The hydroxyl and carbonyl groups in BHEA can form an unstable enol-quinone structure when irradiated by UV light, and then they revert to their original structures upon the release of absorbed energy in the form of heat. Thus, the resin polymer obtained enduring protection by converting the UV-light energy into less harmful heat energy. Moreover, no absorption peaks were present below 250 nm in the curves because the glass slide contained many Si=O groups, which can absorb short-wavelength UV light. As the proportion of the short-wavelength UV light in the solar spectrum was very small, we only considered absorbance above 290 nm.
Free-radical-catching performance
The free-radical-catching ability of the resin was assessed by the productivity of nitroxides because radicals, like R· and R′OO·, are easily captured by nitroxides to form a new structure.
As shown in Fig. 5, the curve shows prominent peaks at 3487 G, 3502 G, and 3519 G and is consistent with the EPR spectra of piperidyl-nitroxyl radicals recorded in the literature.31 The results demonstrated that the free-radical-catching agent (TMPM) enabled the resin (AS-3) to produce piperidyl-nitroxyl radicals. Thus, according to the circulation mechanism of Denisov,32 this novel resin modified with TMPM could capture free radicals.
Water contact angle (CA)
The surface energy of the varnish coatings was investigated by the water CA method. The results are shown in Fig. 6.
Clearly, the CAs of deionized water on C-2 and C-3 were above 100°, showing obvious hydrophobic properties, while C-1 displayed hydrophilicity with a contact angle of 73°. There was little difference between the angles of C-2 and C-3, implying that the UV absorber (BHEA) and hindered amine light stabilizer (TMPM) had almost no influence on the surface energy. In other words, the surface energy of the coatings was reduced by DFMA and MPS. This was because organic fluorine and silicone have low surface energies due to their low molecular polarity and weak intermolecular interactions. Moreover, the fluorine- and silicon-containing side chains tend to enrich on the surface in the coating formation process, effectively decreasing the surface energy.
Weather resistance
The color difference (∆E) and rate of loss of gloss (∆G) are usually adopted as indicators of climate resistance because the color and gloss of coatings change with the aging time.
Figure 7a shows the ∆E of C-1 increased very quickly and almost reached the maximum value of 4.94 after 300 h in the weathering test. In contrast, the change rate of ∆E in C-2 clearly slowed down, and the final value was 3.96 after the weathering test. C-3, the UV-absorbing and free-radical-catching fluorine–silicone acrylic resin, exhibited the best color retention ability. The value still stabilized at approximately 2.82 after the 1000 h accelerated aging test. The ∆G values had the same trend as ∆E, and all of the values reached a constant value after rapid growth. As shown in Fig. 7b, the change rate of C-1 was the largest, and the last value of 87.29% was the highest. However, the final ∆G of C-2 was 77.1%, which indicated a better light retention property. Compared to that of C-2, the ∆G of C-3 changed more slowly, and the final value was approximately 62%. This phenomenon indicated that the weatherability of the resins was greatly enhanced by the UV absorber (BHEA), free-radical-catching monomers (TMPM), and organic fluorine (DFMA) and silicon monomers (PMS). Fluorine and silicone with high-energy Si–O and C–F bonds can be degraded by a small amount of UV light. Most of the UV light was absorbed by the bonded BHEA and released in the form of harmless heat. In addition, the free-radical-catching agent (TMPM) in the resin can capture the attacking radicals to inhibit photooxidation. Importantly, the modifying monomers were chemically incorporated into the chain of the resin and not easy to deplete, which had an enduring and positive effect on the weather resistance of the resin. Therefore, under triple protection, the weather resistance of this novel resin reached a new, enhanced level.
Figure 7 shows that C-3 and C-4 exhibited perfect weathering resistance, and their ∆E and ∆G values had almost no differences within the first 200 h. However, the ∆E and ∆G values of C-4 at 700 h were approximately 3.26% and 64%, respectively, which were much higher than those of C-3. This proved that the chemically bonded BHEA and TMPM exhibited a better protection performance than the physically blended mixture. To identify the enduring effects of C-3 on the scavenging of free radicals, the EPR spectra of C-3 and C-4 were recorded before and after a 700-h weathering test.
As shown in Fig. 8, both of the coatings had a high nitroxide production before weathering. Although the property of C-4 was not as good as that of C-3, it was still impressive. However, after the 700-h aging test, the concentration of piperidyl-nitroxyl radicals detected in C-4 decreased by approximately 60%, while C-3 had only a slight decrease and retained a high productivity. This was because the physically blended TMPM in C-4 existed as separate, small molecules, which are easy to lose via volatilization or water spraying. However, this could not occur in C-3 because the chemically bonded TMPM existed as stable, high molecular weight polymers. The phenomenon indicated that chemically reacted TMPM had more lasting effects on photooxidation inhibition than the physically blended mixture.
Mechanical properties
Aging can result in the mechanical deterioration of coatings. To further measure the weather resistance of the resins, the impact strength and flexibility of P-1, P-2 and P-3 were tested.
Figures 9c and 9d shows the impact strength and flexibility of all the coatings were 500 N cm and 1 mm, respectively, before weathering, which indicated excellent mechanical properties. However, after the 1000-h weathering test, the impact strength and flexibility of P-1 decreased to 200 N cm and 4 mm, respectively. Compared with P-1, P-2 showed better mechanical durability with an impact strength and flexibility of 350 N cm and 3 mm, respectively. P-3 performed the best with an impact strength and flexibility of 420 N cm and 2 mm, respectively. After the aging test, the surface morphologies of the coatings after a 300-N cm impaction and bending on a shaft bar with a diameter of 2 mm were recorded and are shown in Figs. 9a and 9b. P-1 peeled off from the low-carbon steel plates, which indicated that the coating film was almost chalked. P-2 and P-3 had cracks, but the cracks in P-3 were smaller than those in P-2 and difficult to observe with the naked eye. In other words, the damage degree of P-2 caused by weathering was greater than that of P-3. As explained in the section on “Weather resistance,” under the enduring protection of the modifying agents, the aging degree of the coatings decreased, and the mechanical durability was enhanced.
Conclusions
A UV-absorbing and free-radical-catching fluorine–silicone acrylic resin was synthesized by solution copolymerization. The modifying monomers, including TMPM, BHEA, DFMA, and MPS, were effectively incorporated into the main chain of the resin. This resin exhibited a high UV-absorbing performance as well as the production of piperidyl-nitroxyl radicals, and the coatings had low surface energies and perfect UV resistance. The CA of deionized water was above 100°, which showed obvious hydrophobic properties. The change rates of the ∆E and ∆G values of the varnish coating clearly decreased with the aging time. The final values of ∆E and ∆G were 2.96% and 62%, respectively, and the values benefited from the enduring protection from BHEA, TMPM, DFMA, and MPS. After the 1000-h aging test, the colored paint coatings retained good mechanical properties with an impact strength and flexibility of 420 N cm and 2 mm, respectively. Moreover, the chemically bonded hindered amine light stabilizer (TMPM) in the acrylic resin showed more enduring effects on restraining photooxidation than the physically blended mixture. This novel kind of super UV-resistant acrylic resin with a low surface energy is expected to have applications for long lifetime protection coatings.
References
Liu, YX, Liu, YY, Lin, JJ, Tan, HF, Zhang, CH, “UV-Protective Treatment for Vectran® Fibers with Hybrid Coatings of TiO2/Organic UV Absorbers.” J. Adhes. Sci. Technol., 28 (18) 1773–1872 (2008)
Lee, JH, Kim, JC, “UV-Absorbing and Emulsifying Property of Cinnamic Acid-Conjugated Gelatin.” J. Adhes. Sci. Technol., 36 (7) 1000–1008 (2015)
Yu, J, Wang, JF, Wang, CP, Liu, YP, Xu, YZ, Tang, CB, Chu, FX, “UV-Absorbent Lignin-Based Multi-Arm Star Thermoplastic Elastomers.” Macromol. Rapid. Comm., 36 (4) 389–404 (2015)
Kaci, M, Cimmino, S, Di Lorenzo, ML, Silvestre, C, Sadoun, T, “Effect of the Thermo-oxidation and Natural Weather on the Structure, Morphology, and Properties of Unstabilized and HALS-stabilized LDPE Films.” J. Macromol. Sci. A, 36 (2) 253–274 (1999)
Lonkar, SP, Kushwaha, OS, Leuteritz, A, Heinrichac, G, Singh, RP, “Self Photostabilizing UV-durable MWCNT/Polymer Nanocomposites.” RSC Adv., 2 (32) 12255–12262 (2012)
You, B, Zhou, DJ, Liu, L, Zen, MQ, Ren, XC, “Preparation of HALS-Functional Core-Shell Nanoparticles and their Application in Poly (Butylene Terephthalate).” Polym. Plast. Technol. Eng., 52 (9) 946–953 (2013)
Hodgson, JL, Coote, ML, “Clarifying the Mechanism of the Denisov Cycle: How do Hindered Amine Light Stabilizers Protect Polymer Coatings from Photo-oxidative Degradation?” Macromolecules, 43 (10) 4573–4583 (2010)
Liu, ZY, Chen, SG, Zhang, G, “Effect of UV Absorbers and Hindered Amine Light Stabilizers on the Photodegradation of Ethylene–octene Copolymer.” J. Appl. Polym. Sci., 127 (2) 1135–1147 (2013)
Parviz, D, Yu, ZN, Hedden, RC, Green, MJ, “Designer Stabilizer for Preparation of Pristine Graphene/Polysiloxane Films and Networks.” Nanoscale, 6 (20) 11722–11731 (2014)
Zhang, G, Li, HX, Antensteiner, M, Mike Chung, TC, “Synthesis of Functional Polypropylene Containing Hindered Phenol Stabilizers and Applications in Metallized Polymer Film Capacitors.” Macromolecules, 48 (9) 2925–2934 (2015)
Wilén, CE, Pfaendner, R, “Improving Weathering Resistance of Flame-Retarded Polymers.” J. Appl. Poly. Sci., 129 (3) 925–944 (2013)
Biswal, T, Samal, R, Sahoo, PK, “Microwave-Assisted Preparation of Poly (2-EHA-co-ST) Copolymer and Poly (2-EHA-co-ST)/MMT Nanocomposite.” J. Appl. Polym. Sci., 125 (2) 1467–1475 (2015)
Mishra, S, Singh, J, Choudhary, V, “Synthesis and Characterization of Butyl Acrylate/Methyl Methacrylate/Glycidyl Methacrylate Latexes.” J. Appl. Poly. Sci., 115 (15) 549–557 (2010)
Yu, S, Zhou, YM, Zhang, T, He, M, “Preparation and Characterization of Acrylate Copolymers Modified by Fluorine and Silicon for Application in Release Films.” Polym. Plast. Technol. Eng., 53 (6) 531–538 (2014)
Kim, DH, Lee, YH, Park, CC, Kim, HD, “Synthesis and Surface Properties of Self-Crosslinking Core-Shell Acrylic Copolymer Emulsions Containing Fluorine/Silicone in the Shell.” Colloid Polym. Sci., 292 173–183 (2014)
Hwang, HD, Kim, HJ, “UV-curable Low Surface Energy Fluorinated Polycarbonate-based Polyurethane Dispersion.” J. Colloid Interface Sci., 362 (2) 274–284 (2011)
Canak, TC, Serhatlı, IE, “Synthesis of Fluorinated Urethane Acrylate Based UV-curable Coatings.” Prog. Org. Coat., 76 (2–3) 388–399 (2013)
Bae, KY, Lim, DH, Park, JW, Kim, HJ, Rafailovich, M, Sokolov, J, “Adhesion Performance and Thermal Stability of Fluorinated PSAs as a Crosslinking System.” J. Adhes. Sci. Technol., 26 (1–3) 361–379 (2012)
Lee, SW, Lee, YH, Park, H, Kim, HD, “Effect of Total Acrylic/Fluorinated Acrylic Monomer Contents on the Properties of Waterborne Polyurethane/Acrylic Hybrid Emulsions.” Macromol. Res., 21 (6) 709–718 (2013)
Glaris, P, Coulon, GF, Dorget, M, Poncin-Epaillard, F, “Fluorinated Epoxy Resin as a Low Adhesive Mould for Composite Material.” Compos. Part B Eng., 63 94–100 (2014)
Wang, Y, Long, J, Bai, YP, Shao, L, Qi, SC, “Thermal Stability and Surface Properties of Acrylic PSAs Modified by Hexafluorobutyl Acrylate.” J. Adhes. Sci. Technol., 30 (3) 300–312 (2016)
Jeon, JH, Park, YG, Lee, YH, Lee, DJ, Kim, HD, “Preparation and Properties of UV-curable Fluorinated Polyurethane Acrylates Containing Crosslinkable Vinyl Methacrylate for Antifouling Coatings.” J. Appl. Polym. Sci., 132 (16) 42168 (2015)
Shin, MS, Lee, YH, Rahman, MM, Kim, HD, “Synthesis and Properties of Waterborne Fluorinated Polyurethane-Acrylate using a Solvent-/Emulsifier-free Method.” Polymer, 54 (18) 4873–4882 (2013)
Park, JM, Jeon, JH, Lee, YH, Lee, DJ, Park, H, Chun, HH, Kim, HD, “Synthesis and Properties of UV-curable Polyurethane Acrylates Containing Fluorinated Acrylic Monomer/Vinyltrimethoxysilane.” Polym. Bull., 72 1921–1936 (2015)
Kumar, D, Li, L, Chen, Z, “Mechanically Robust Polyvinylidene Fluoride (PVDF) based Superhydrophobic Coatings for Self-cleaning Applications.” Prog. Org. Coat., 101 385–390 (2016)
Liu, DL, Coatings Technology. Chemical Industry Publishing House, Beijing (2009)
Yaneff, PV, Adamsons, K, Cliff, N, Kanouni, M, Cindy, P, “Use of Reactable Light Stabilizers to Prevent Migration and to Improve Durability of Coatings On Plastic Substrates.” J. Coat. Technol. Res., 2 (5) 371–387 (2005)
Yan, LM, Song, WS, Tang, YF, “Influence of Light Stabilizer on the Properties of Thermoplastic Polyurethane Adhesive.” Polyurethane Ind., 25 (3) 30–33 (2010), (in chinese)
Yang, F, Zhu, LQ, Li, CY, Wang, XM, Ning, L, “Preparation of Fluorinated and Silicone Acrylic Polyurethane Coatings and their Environmental Behaviors.” Surf. Tech., 44 (2) 19–23 (2015), (in Chinese)
Zhang, YL, “Preparation of Block Fluorinated Polyacrylate Resins and Its Application in Fluorocarbon Coatings.” Master’s thesis, Shanxi University of Science and Technology (2014)
Commereuc, S, Lajoie, P, Verney, V, Lacoste, J, “A New ESR Study of Hindered Amine Stabilizers (HAS) and Their Oxidation Products.” Polym. Int., 52 (4) 576–580 (2003)
Denisov, ET, Shestakov, AF, “Reaction between Radicals and N-Alkoxyamines As Coordinated Cleavage with Fragmentation.” Russ. J. Phys. Chem. A, 89 (8) 1217–1229 (2015)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lei, H., He, D., Hu, J. et al. A fluorine–silicone acrylic resin modified with UV-absorbing monomers and a free radical scavenger. J Coat Technol Res 15, 809–817 (2018). https://doi.org/10.1007/s11998-018-0078-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-018-0078-z